Abstract
Although amphibian and fish models of heart regeneration have existed for decades, a mammalian equivalent has long remained elusive. Our discovery of a brief postnatal window for heart regeneration in neonatal mice has led to the establishment of surgical models for cardiac regenerative studies in mammals for the first time. This protocol describes a 10-min surgical procedure to induce cardiac injury in 1-d-old neonatal mice. This allows for the analysis of cardiac regeneration after surgical amputation of the left ventricle (LV) (apical resection) and coronary artery occlusion (myocardial infarction (MI)). A comparative analysis of neonatal and adult responses to myocardial injury should enable identification of the key differences between regenerative and nonregenerative responses to cardiac injury. This protocol can also be adapted to the growing repertoire of genetic models available in the mouse, and it provides a valuable tool for unlocking the molecular mechanisms that guide mammalian heart regeneration during early postnatal life.
INTRODUCTION
Human advancement in medicine and industry has revolutionized our way of living, and one major outcome has been the prolongation of the human life span1,2. This has led to the emergence of new challenges to human physiology, causing a paradigm shift in the burden of human diseases. Diseases of aging and lifestyle now represent the commonest causes of morbidity and mortality in the developed world, with cardiovascular diseases leading the way2. Ischemic heart disease, caused by the narrowing or occlusion of coronary arteries, results in a decrease in myocardial perfusion, leading to myocyte dysfunction and death3. Other causes of myocyte loss include end-stage cardiac hypertrophy, myocarditis, valvular heart disease, infiltrative disease and others, all of which can lead to loss of the contractile ability of the myocardium, which is the basis of systolic heart failure4. The human heart has little capacity to replenish these lost cardiomyocytes, which are subsequently replaced by fibrous tissue4–6.
Models of cardiac regeneration
In contrast to adult mammals, vertebrates such as urodele amphibians and teleost fish retain an extraordinary capacity for cardiac regeneration throughout life7–9, and this appears to be mediated by proliferation-competent cardiomyocytes, which remain mononucleated and can enter the cell cycle throughout the life span of these species10,11. Until recently, experimental models of heart regeneration were restricted to the lower vertebrates, and studies in the genetically tractable zebrafish model over the past decade have provided unprecedented insight into the key molecular and cellular events required for heart regeneration in these species9.
We recently sought to investigate whether the mammalian heart retains regenerative potential during a developmental time window when its cardiomyocytes are still proliferative. These studies showed that the neonatal mouse heart is capable of regeneration after injury at postnatal day 1 (P1), in response to either surgical amputation of the cardiac ventricles (apical resection)12 or after MI13. In addition, a report of partial regeneration after neonatal heart cryoinjury has been described14. In the report, the authors describe a nonischemic cryoinjury model in neonatal hearts, which appears to be followed by incomplete regeneration. Although the current protocol outlines apical resection and ischemic MI models in the neonate, the cryoinjury model may be a useful tool to shed light on the differential regenerative response after various types of injury. Although the cryoinjury model may offer a more reproducible injury compared with ischemic infarction, the type of injury may have important implications on the subsequent regenerative response. For example, cryoinjury in the zebrafish model is associated with fibrosis and a protracted regenerative response15. Thus, mechanistic insights may be gained from the correlation between the type of injury and the subsequent regenerative response.
As in vertebrates, lineage-tracing analysis, which was performed in the apical resection and the ischemic MI mouse models, showed that this process is predominantly mediated through the proliferation of pre-existing cardiomyocytes (Box 1)12. Thus, the apical resection and MI models in neonatal mice provide an opportunity to further investigate the mechanisms responsible for the age-dependent decline in cardiac regenerative capacity9.
Box 1 | Lineage tracing ● TIMING 1–2 h, 21 d after injury.
Identifying the cell of origin of the newly formed myocardium is essential for revealing the therapeutic target for heart regeneration. In order to determine the cellular origin of the de novo cardiomyocytes, a tamoxifen-inducible cardiomyocyte-specific reporter line can be created and used for the protocol. To do this, the following adaptations to the procedure need to be made:
Cross the Myh6-MerCreMer line23 with the Rosa26-lacZ reporter mice24. The temporal expression of the α-myosin heavy chain (MHC) promoter, which turns on permanently around birth, helps minimize, but does not eliminate, the possibility of leakiness before the tamoxifen injection.
At birth (P0), inject the neonates with a single s.c. dose of 2 mg of tamoxifen (Sigma-Aldrich) dissolved in 90% sesame oil and 10% ethanol (vol/vol). Nearly 70% of cardiomyocytes should become lacZ positive. On the following day (P1), perform the surgery (apical resection or MI) as described in Steps 1–15 of the PROCEDURE.
At 21 d after injury, collect the hearts and embed them in tissue-freezing medium, and then flash-freeze them in 2-methylbutane cooled on liquid nitrogen.
For apical resection, cut cryosections (8 µm) of the heart in a four-chamber view. For MI, cut the sections in a two-chamber view below the ligature.
Fix the sections with 2% (vol/vol) glutaraldehyde in 2% (vol/vol) PBS.
Wash the fixed sections in 1% (wt/vol) sodium deoxycholate and 0.2% (vol/vol) NP40 substitute in PBS three times for 5 min.
Stain the cryosections with X-gal staining solution (4 mM potassium ferricyanide, 4 mM potassium ferrocyanide trihydrate, 0.4 mM magnesium chloride, 0.1% sodium deoxycholate, 0.2% NP40 and 1 mg ml−1 X-gal in PBS) for 48 h at 37 °C to detect lacZ activity. Rinse the sections in PBS twice for 5 min.
Counterstain the sections with nuclear fast red.
Quantify the lacZ staining by using Image J software (NIH). The percentage of lacZ-expressing cardiomyocytes should be similar in injured and sham mice, indicating that the majority of the newly formed myocardium is derived from the proliferation of pre-existing cardiomyocytes.
Δ CRITICAL STEP Owing to the limitations of the labeling efficiency (the single tamoxifen pulse labels 70–80% of myocytes), a limited contribution of a progenitor population cannot be excluded. An additional inherent limitation of this model and the fate mapping strategy is the narrow postnatal regenerative window, which may introduce an error secondary to the overlap of recombination and repair. Importantly, it is difficult to assess the lineage contribution of progenitor cells in the absence of fate mapping strategies that irreversibly labels these populations.
Experimental design
Injury of the neonatal mouse heart is an appealing technique to study the regenerative response of the mammalian heart. One technical hurdle to this approach was that endotracheal intubation and mechanical ventilation of a 1-d-old neonatal mouse is not technically feasible. Intriguingly, thoracotomy of neonatal mice without mechanical ventilation did not result in lung collapse, and it was associated with normal postoperative recovery12. The ability to perform thoracotomy without intubation in neonatal mice allowed for surgical intervention and the development of a cardiac injury model at a young age. Here we describe two different models of injury of the neonatal mouse heart. The first model involves surgical amputation of the ventricular apex (apical resection, Step 8A), similar to that described in adult zebrafish7. The second model (Step 8B) is of more relevance to human ischemic heart disease, and it involves permanent ligation of the left anterior descending coronary artery (LAD)16 to induce MI13. P1 mice can regenerate their hearts after both apical resection and MI injuries; however, this regenerative capacity is mostly lost by postnatal day 7 (P7).
Applications of the neonatal mouse model for cardiac regeneration
Owing to the inability of the adult mammalian heart to regenerate after injury, the development of a model that serves as a platform for mammalian heart regeneration was essential. Previously described cardiac surgical models have led to important insights in understanding the pathological remodeling of the heart after injury, as well as the contribution of multiple exogenous stem cell populations to the adult heart after injury17–19. However, these models used adult mammals, which do not possess an endogenous cardiac regenerative capacity. The neonatal mouse heart shares similar characteristics with that of some other vertebrates, which have a high capacity to regenerate their hearts after injury7,10,11. The advantage of having a model mammalian heart that can regenerate after injury has tremendous implications for dissecting the molecular networks that regulate this process. The wealth of genetic mouse models available provides us with the platform required for a rigorous analysis of the genetic control of mammalian heart regeneration.
MATERIALS
REAGENTS
Mice
We have used neonatal mice at postnatal day 1 (P1) or day 7 (P7) delivered from timed-pregnant ICR/CD-1 mice (Charles River Laboratories) previously; however, this protocol can be used on any mouse strain. The day of birth is considered P0, and the surgeries were performed the following day (P1). If you wish to perform lineage tracing, use a tamoxifen-inducible cardiomyocyte-specific reporter mouse line, as described in Box 1 ! CAUTION All experiments involving rodents must conform to relevant institutional and governmental regulations. This protocol was approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center.
Skin glue (Vetclose, cat. no. 031477, Butler Schein)
Methylene blue
2,3,5-Triphenyltetrazolium chloride (TTC; Sigma-Aldrich, cat. no. 298-96-4)
Sodium phosphate dibasic (Na2HPO4, Sigma-Aldrich, cat. no. 255793)
Sodium phosphate monobasic (NaH2PO4, Sigma-Aldrich, cat. no. S3139)
d-Mannitol (Sigma-Aldrich, cat. no. M4125)
Formaldehyde (Sigma-Aldrich, cat. no. F8775)
Paraformaldehyde (EMS, PA, cat. no. 15710)
Glutaraldehyde (Sigma-Aldrich, cat. no. 340855)
Potassium ferricyanide (K3Fe(CN)6, Sigma-Aldrich, cat. no. 702587)
Potassium ferrocyanide trihydrate (K4Fe(CN)6·3H2O, Sigma-Aldrich, cat. no. P9387)
Sodium deoxycholate (Sigma-Aldrich, cat. no. D6750)
NP40 substitute (Sigma-Aldrich, cat. no. 74385)
Normal horse serum (Vector Labs, cat. no. S-2000)
10× Antigen Retrieval Solution (Dako, cat. no. S1699)
Low-melting-point agarose (Invitrogen, cat. no. 16520050)
5-BrdU (Millipore, cat. no. 203806) ! CAUTION It is harmful on inhalation, on swallowing and when in contact with skin. It may cause genetic damage and it is teratogenic.
Tamoxifen (Sigma-Aldrich, cat. no. T5648) ! CAUTION It may be harmful when swallowed and when in contact with skin.
Sesame oil (Sigma-Aldrich, cat. no. S3547)
X-gal (5-bromo-4-chloro-3-indolyl-β-d-galactoside, Sigma-Aldrich, cat. no. B4252)
Saline, 0.9% (wt/vol) (Sigma-Aldrich)
Anti-bromodeoxyuridine antibody (Roche, cat. no. 11170376001)
Anti-Nkx2.5 antibody (R&D Systems, cat. no. AF2444)
Alexa Fluor 488 donkey anti-mouse (Invitrogen, cat. no. A-21202)
Alexa Fluor 555 donkey anti-goat (Invitrogen, cat. no. A-21432)
Vectashield mounting medium with DAPI (Vector Labs, cat. no. H-1200)
2-Methylbutane (Sigma-Aldrich, cat. no. M32631)
EQUIPMENT
Ice bed
Blunt scissors (Olsen-Hegar needle holders with scissors, 1.5 mm, Fine Science Tools, cat. no. 12002-12)
Hemostat (Crile hemostat, Fine Science Tools, cat. no. 13004-14)
Iridectomy scissors (Vannas spring scissors, 2 mm cutting edge, Fine Science Tools, cat. no. 15000-03)
6-0 Prolene sutures (Ethicon, cat. no. 8889H)
Sterilizer (Germinator 500 dry bead sterilizer, Cellpoint, cat. no. 5-1450)
Heating lamp or heating pad
Vevo 2100 small animal echocardiography system (VisualSonics)
ImageJ software (freely available from the US National Institutes of Health (NIH))
Microtome
Vibratome
REAGENT SETUP
TTC solution
Dissolve TTC (Sigma-Aldrich; 3 mM TTC) in 100 ml of buffer containing 0.8 M Na2HPO4, 2 mM NaH2PO4 and 0.14 M d-Mannitol (pH 7.8).
Δ CRITICAL The solution must be prepared immediately before use and placed at 37 °C.
PROCEDURE
Surgery ● TIMING 10–15 min per mouse pup
-
1|
Remove all the pups from the nursing mother and place them in a different cage. Keep the mother’s cage away.
Δ CRITICAL STEP All pups must be removed from the mother directly before surgery. It is important to minimize the time that the pups are away from the nursing mother.
-
2|
Anesthetize the neonatal mice by hypothermia on ice for ~3–5 min (Fig. 1a). Avoid direct contact with the ice by placing gauze below the pup to avoid frostbite. Hypothermia is accompanied by both apnea and asystole20, thus preventing excessive blood loss during surgery.
Δ CRITICAL STEP Owing to the ineffectiveness of anesthetics on neonatal mice, as well as the ability of neonatal rodents (up to 1 week old) to tolerate low temperatures21, hypothermia is the most appropriate way to anesthetize neonatal mice.
Δ CRITICAL STEP Prolonged hypothermia can increase the mortality of the neonates. Check the neonates frequently while on ice until the pups are akinetic and apneic.
? TROUBLESHOOTING
-
3|
Remove the pup from the ice bed, and transfer it to the surgical area.
-
4|
Place the mouse pup in the supine position and tape the arms and tail for immobilization.
-
5|
Gently wipe the chest with a Betadine swab.
-
6|
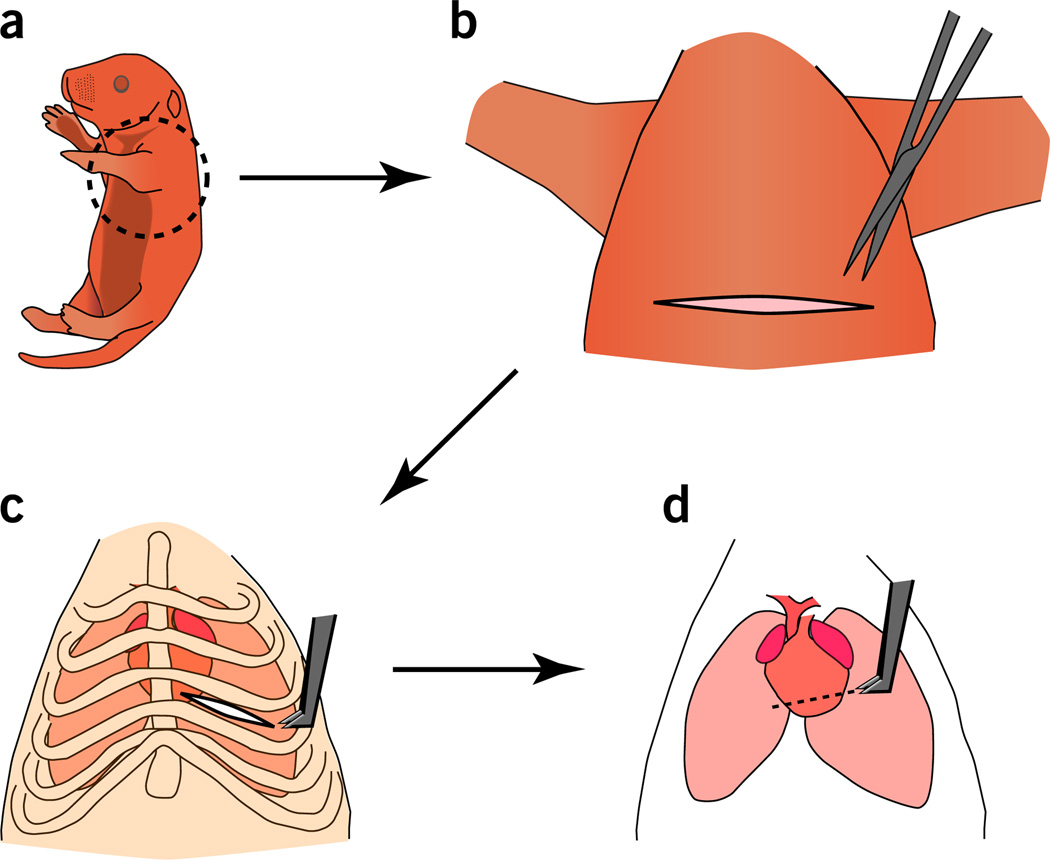
Perform a transverse skin incision across the lower half of the chest by using small scissors. Gently separate the skin from underlying muscle by using blunt dissection. A wider skin incision will enhance the surgical field markedly and facilitate visualization of the site of the intercostal incision (Fig. 1b).
-
7|
Perform lateral thoracotomy by making a small incision at the fourth intercostal space. Separate the intercostal muscles by blunt dissection of the intercostal muscles (Fig. 1c).
Δ CRITICAL STEP Choosing the correct site of entry into the chest cavity is essential for the success of the procedure.
Adequate visualization of the heart will allow the surgery to be performed rapidly, thus decreasing mortality. If the incision is too high, the heart will not be adequately exposed for apical resection.
-
8|
If you are performing apical resection, follow option A. If you are performing MI, follow option B. If you are creating a sham-operated control for either procedure, proceed directly to Step 9.
Figure 1.
Apical resection of the neonatal mouse heart. (a) A neonatal mouse pup to be placed in ice to induce hypothermia. (b) After immobilization of the pup in the supine position using tape, skin incision is performed transversely along the chest cavity. (c) Access to the heart through intercostal muscle separation at the fourth intercostal space. (d) Apical resection of 15% of the heart is performed (piece-meal resection of small portions until the chamber is exposed). Exposing the LV chamber represents a landmark for efficient myocardial resection and surgical reproducibility.
(A) Apical resection
Exteriorize the heart outside the chest cavity by applying a steady pressure on the abdomen. Use the iridectomy scissors to resect the apex of P1 hearts (Fig. 1d).
-
Gradually resect the ventricular apex over several incisions, removing the smallest amount of tissue possible each time until the left ventricular chamber is exposed (Fig. 1d). The removal of 15% of the LV is the optimal amount to ensure injury without increasing surgical mortality.
Δ CRITICAL STEP The amount of myocardium resected affects the survival rate of the neonates. Resection of larger portions of the myocardium can prevent rapid formation of an apical hematoma, which can lead to exsanguination and death. Our surgical protocol using chamber exposure as a landmark for the limit of resection ensures reproducibility of the procedure and minimizes surgical lethality.
? TROUBLESHOOTING
(B) MI
-
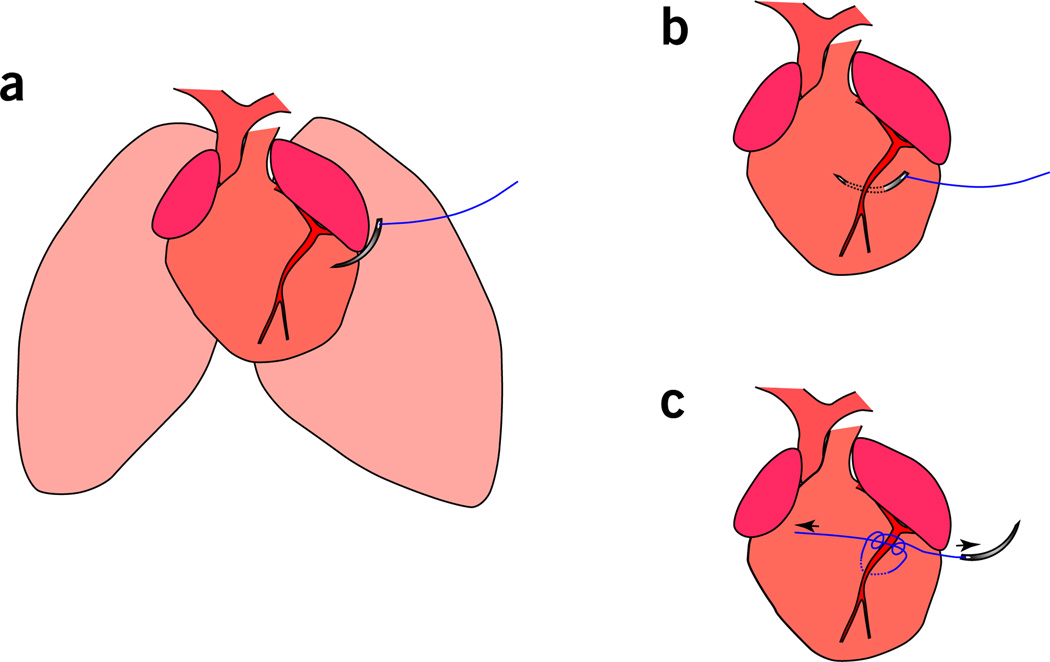
Ligate the LAD by passing a C1 tapered needle attached to a 6-0 Prolene suture through the mid-ventricle below the LAD and tie off to induce infarction. The needle should be inserted through the left ventricular wall to ensure ligation of the LAD (Fig. 2a,b). Note that visualization of the LAD is not possible; therefore, the ligature should depend on the anatomical location, and it should include the myocardium adjacent to the coronary vessel.
Δ CRITICAL STEP The neonatal mouse heart is very fragile, and thus if the ligature is too tight the experimenter risks severing the LAD, which will result in death.
? TROUBLESHOOTING
Check whether there is blanching of the myocardium below the ligation. If so, this is indicative of ischemia (Fig. 2c). To further confirm efficient ligation, methylene blue can be injected into the ventricular chamber through the apex after LAD ligation. The absence of methylene blue staining to the territory supplied by the LAD indicates proper ligation and lack of perfusion.
Figure 2.
MI of the neonatal mouse heart. (a) A C-1 tapered needle attached to a 6-0 Prolene suture is used for LAD ligation after thoracotomy. (b) The needle is passed out of the LV for ligation at a depth of 2–3 mm (deeper insertion of the needle is also acceptable). (c) Ligation of the LAD coronary artery. Note: this should result in blanching of the myocardium below the ligation, which is indicative of proper induction of ischemia.
-
9|
Use a 6-0 nonabsorbable Prolene suture to suture the ribs together and seal the chest wall incisions. Use skin glue to join the skin back together.
-
10|
Warm the neonates rapidly. Place the neonates in your hand while warming them under a heat lamp for several minutes until recovery. Do not warm the neonates in close proximity to the heat lamp, or for long durations, as this can cause increased mortality.
Δ CRITICAL STEP Make sure that the pups are warmed immediately after the surgery is done. Extended hypothermia will lower the chance of the pups properly recovering. Warming the pups should be a rapid process rather than gradual warming, which might prevent the pups from recovering.
? TROUBLESHOOTING
-
11|
Remove the blood and skin-glue traces from the pups by cleaning the injury site with alcohol wipes. This reduces the chances of cannibalization.
Δ CRITICAL STEP Steps 2–11 should take <10 min.
-
12|
Return the pups that had surgery to their littermates as soon as possible. This stimulates recovery and enhances the survival of neonates after surgery.
Δ CRITICAL STEP Owing to the absence of centralized pain reflexes at early postnatal age, no pain relief medications are required for P1 and P7 neonatal mice after surgery.
-
13|
Once all the surgeries required for an individual litter have been completed, mix the pups with the bedding from the mother’s cage and then re-introduce the pups to their mother.
? TROUBLESHOOTING
-
14|
Sterilize the surgical instruments in the dry bead sterilizer after each surgery.
-
15|
The day after surgery, monitor the mice. Those that underwent apical resection should be indistinguishable from sham-operated mice.
Analysis of consequences of injury ● TIMING 1–2 h, 21 d after injury
-
16|
If you performed MI (Step 8B), successful surgery can be confirmed by measuring left ventricular systolic function by echocardiography 3–4 d after MI (option A) and/or by confirming cardiomyocyte death via TTC staining (option B), which distinguishes between viable (red) and nonviable22 myocardium. Cardiomyocyte labeling and lineage tracing after either injury (Step 8A and Step 8B) can be performed by detecting the formation of new cardiomyocytes by performing a BrdU pulse-chase experiment, which detects newly formed DNA (Step 8C and Box 1). BrdU incorporation is detected by co-staining BrdU and Nkx2.5 to quantify the BrdU-positive cardiomyocytes.
(A) Echocardiography to confirm a successful MI surgery
-
Restrain the conscious mouse pup in your hand, and use very gentle pressure to apply a 40-MHz mouse ultrasound probe from the Visual Sonics Vevo 2100 echocardiography machine to the pup’s chest.
Δ CRITICAL STEP The probe gel cools the body temperature of the pups. Make sure to keep the pup warm while performing the echo to avoid a vagal response. Prewarming the echo gel is advised.
Obtain a parasternal short-axis view of the LV, and record M-mode echocardiography images. It may be possible to obtain images at more than one level, which will increase the accuracy of LV function assessment, although this may not be technically feasible in very young neonates.
Analyze the images by measuring the left ventricular internal diameter at end diastole, as well as the left ventricular internal diameter at end systole. Calculate the ejection fraction and fractional shortening. The data for sham-operated mice should show normal contractile function, whereas 3 d after MI depressed LV function should be detected.
If desired, proceed to option B or option C.
(B) TTC staining
Prepare the TTC solution as described in Reagent Setup and place it at 37 °C.
Collect the hearts either at 3 or 21 d after MI.
Heat low-melt agar and pour the agar into tissue blocks.
Place the hearts into individual low-melt agar blocks on ice until the agar solidifies.
Once the agar has solidified, glue each heart block on the vibratome platform.
Slice sections about 350 µm thick through the block by using a vibratome.
Incubate the sections in TTC solution at 37 °C for 5 min.
Fix the sections in 10% (vol/vol) formaldehyde for 30 min at room temperature (20–25 °C).
Quantify the percentage of viable (red) and nonviable22 myocardium by using ImageJ software.
(C) Cardiomyocyte labeling and lineage tracing after injury
-
Inject BrdU s.c. into the mice on days 1, 7 and 14 after surgery with 35 mg kg−1 of BrdU dissolved in sterile 0.9% (wt/vol) saline solution.
Δ CRITICAL STEP To avoid false-positive labeling of binucleating cardiomyocytes and to only detect dividing cells, inject the first BrdU injection before cardiomyocyte binucleation, which takes place before P7 in mice12.
At 21 d post injury, collect the hearts and fix them in 4% (vol/vol) PFA overnight, followed by paraffin embedding.
Slice 5-µm-thick sections through the paraffin block with a microtome.
Deparaffinize the slides and boil them in antigen retrieval solution twice for 5 min. Block with horse serum for 30 min, and then incubate with primary antibodies against BrdU (1:25 dilution) and Nkx2.5 (1:10 dilution) for 2 h at 37 °C.
Wash the slides three times in PBS, and then incubate them with donkey anti-mouse and anti-goat secondary antibodies conjugated to Alexa Fluor 488 or 555 (1:400 dilution) for 1 h at room temperature.
Wash the slides three times in PBS and mount in Vectashield containing DAPI.
-
Quantify the number of BrdU-positive cardiomyocytes in sham- and surgery-operated mice.
? TROUBLESHOOTING
Steps 2 and 10, temperature control
Warming the pups is an essential step after surgery for recovery from hypothermia. Make sure that the pups are warm and motile before returning them to the foster mother. P7 pups are less resistant to hypothermia than P1, which are remarkably resilient with respect to body temperatures. Careful monitoring of the temperature before, during and after the surgery is crucial to ensure survival of the P7 pups.
Step 8A(ii), reproducibility of apical resection
To ensure the reproducibility of apical resection in P1 mice, an anatomical landmark of chamber exposition is required. Small amounts of apex can be resected until chamber exposition ensures about 15% of the apex being resected.
Surgical lethality is much higher in P7 pups than in P1 pups. This is due to several reasons. The first reason is the inefficiency of P7 pups in forming an effective blood clot to seal the apex after apical resection. Because of this, a smaller proportion of the apex has to be resected in P7 pups, with minimal chamber exposition. Second, P7 pups are less resistant to hypothermia than their P1 counterparts. The time that P7 pups are under hypothermia should be closely controlled and it should be as short as possible, and the surgery should be done rapidly as well. A major advantage of the MI model, compared with the apical resection model, is the increased survival of mice after surgery at P7. Furthermore, MI can be performed beyond P7 (e.g. P14 and adult), allowing for relevant comparisons between regenerating and nonregenerating hearts at neonatal and adult time points in the same species.
Step 8B(i), reproducibility of MI
Maintaining a reproducible size of injury by LAD ligation of the coronary artery is challenging because of the surgical variability of the location of the ligature. To ensure reproducibility of the size of injury, enter the anterior wall of the LV midway through the line extending down from the middle of the left atrial appendage. Exit with the needle along the line at the medial end of the appendage. After MI, assessment of the infarct size of cohorts of hearts by TTC staining is required to ensure reproducibility and accuracy with respect to the surgeon. Anytime the operator changes, similar analysis of the technique and size of the infarct is necessary to avoid changes that result from operator experience.
Step 13, maternal cannibalization
Survival rates of surgery of P1 neonates range from 70 to 90 percent, with the majority of mortality occurring during, or a few hours after, the surgery. However, maternal cannibalization poses a major technical hurdle and reduces the overall survival rates to 60–70%.
The choice of a suitable foster mother is crucial. ICR/CD-1 mice are good foster mothers; they have a higher tendency to take care of and nurture their newborns. When you are using a genetic model that has a different background with poor fostering instinct, such as C57BL/6, place the pups with a CD-1 foster mother at birth (P0), and perform the surgeries the following day (P1).
Another factor that affects maternal cannibalization rates is the number of pups returned to the mother after surgery. The final number of pups introduced to the foster mother after surgery affects the cannibalization instinct of the mother. The higher the number of pups introduced, the less likely they are to be cannibalized.
Experienced foster mothers (i.e., those that had one or two previous litters) are more likely to take care of the pups after injury. First-time mothers are inexperienced and have a higher tendency to cannibalize their pups. This is particularly problematic in mouse strains with poor fostering instincts, such as C57BL/6.
If the foster mother is given a mix of pups that had surgery and sham controls, which are clearly healthier, it increases the mortality rate as the foster mother can detect the difference and neglects the less active pups. A full litter should be dedicated either for surgery or sham. To decrease the maternal instinct for cannibalization, all pups are wiped with ethanol to clear all remaining traces of blood and skin glue.
● TIMING
Steps 1–15, surgery: 10–15 min per mouse pup
Step 16, analysis of consequence of injury: 21 d after injury, takes 1–2 h
Box 1, lineage tracing: 21 d after injury, takes 1–2 h
ANTICIPATED RESULTS
This protocol describes two different models of injury of the neonatal mouse heart. This protocol is reproducible for inducing apical resection and MI in the neonatal mouse heart, followed by complete regeneration in 3 weeks.
7 d after injury, a global proliferative response is detected in the myocardium. Proliferation and de-differentiation of cardiomyocytes are detected in injury, border and remote zones. This gradually decreases until complete regeneration of the myocardium takes place in 21 d (ref. 12).
The advantage of the MI injury is our ability to detect myocyte death and decline in heart function. After MI, nearly 75% of the cardiomyocytes are lost below the ligation. This is translated into a drop of the fractional shortening to nearly 30%. The percentage of viable myocardium and fractional shortening is restored to normal levels within 3 weeks. It is noteworthy that, in a model using cryoinjury for neonatal heart regeneration, activation of a c-kit reporter line in the heart after cryoinjury was demonstrated, although the lack of fate mapping analysis of c-kit cells precludes definitive assessment of the role of these cells in neonatal heart regeneration. Interestingly, the authors describe partial regeneration after cryoinjury, which is in contrast to the robust regeneration seen after resection and ischemic infarction injury14.
Examples of typical results seen after Step 16A can be found in Figure 1 of ref. 12. Typical results seen after 16B can be found in Figure 1 of ref. 13. Typical results seen after Step 16C can be found in Figure 3 of ref. 12.
Our protocol provides researchers with a mammalian heart regeneration model to gain insight for translating these mechanisms into potential therapeutic strategies for cardiac injury in adult humans.
ACKNOWLEDGMENTS
We thank J. Cabrera for graphical assistance. This work was supported by the National Health and Medical Research Council and National Heart Foundation of Australia (E.R.P.), a cardiovascular research scholar award from Gilead Sciences, a Grant-in-Aid award from the American Heart Association and an NIH R01 grant (1R01HL115275-01; H.A.S.).
Footnotes
AUTHOR CONTRIBUTIONS A.I.M., E.R.P., E.N.O. and H.A.S. designed the experiments. A.I.M. and E.R.P. performed the experiments. A.I.M., E.R.P., E.N.O. and H.A.S. analyzed the data. A.I.M., W.K. and H.A.S. made the figures. A.I.M., E.R.P. and H.A.S. wrote the manuscript. All authors approved the manuscript.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
References
- 1.Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93:1137–1146. doi: 10.1136/hrt.2003.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jessup M, Brozena S. Heart failure. New Engl. J. Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 3.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 4.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murry CE, Reinecke H, Pabon LM. Regeneration gaps: observations on stem cells and cardiac repair. J. Am. Coll. Cardiol. 2006;47:1777–1785. doi: 10.1016/j.jacc.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Laflamme MA, Murry CE. Regenerating the heart. Nat. Biotechnol. 2005;23:845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 7.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 8.Oberpriller JO, Oberpriller JC. Response of the adult newt ventricle to injury. J. Exp. Zool. 1974;187:249–253. doi: 10.1002/jez.1401870208. [DOI] [PubMed] [Google Scholar]
- 9.Mahmoud AI, Porrello ER. Turning back the cardiac regenerative clock: Lessons from the neonate. Trends Cardiovasc. Med. 2012;22:128–133. doi: 10.1016/j.tcm.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Jopling C, et al. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464:606–609. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kikuchi K, et al. Primary contribution to zebrafish heart regeneration by gata4+ cardiomyocytes. Nature. 2010;464:601–605. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porrello ER, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porrello ER, et al. Regulation of neonatal and adult mammalian heart regeneration by the mir-15 family. Proc. Natl. Acad. Sci. USA. 2013;110:187–192. doi: 10.1073/pnas.1208863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jesty SA, et al. C-kit+ precursors support postinfarction myogenesis in the neonatal, but not adult, heart. Proc. Natl. Acad. Sci. USA. 2012;109:13380–13385. doi: 10.1073/pnas.1208114109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez-Rosa JM, Martin V, Peralta M, Torres M, Mercader N. Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development. 2011;138:1663–1674. doi: 10.1242/dev.060897. [DOI] [PubMed] [Google Scholar]
- 16.Egger B, Ladurner P, Nimeth K, Gschwentner R, Rieger R. The regeneration capacity of the flatworm Macrostomum lignano—on repeated regeneration, rejuvenation, and the minimal size needed for regeneration. Dev. Genes Evol. 2006;216:565–577. doi: 10.1007/s00427-006-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang NF, et al. A rodent model of myocardial infarction for testing the efficacy of cells and polymers for myocardial reconstruction. Nat. Protoc. 2006;1:1596–1609. doi: 10.1038/nprot.2006.188. [DOI] [PubMed] [Google Scholar]
- 18.McCall FC, et al. Myocardial infarction and intramyocardial injection models in swine. Nat. Protoc. 2012;7:1479–1496. doi: 10.1038/nprot.2012.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Laake LW, et al. Monitoring of cell therapy and assessment of cardiac function using magnetic resonance imaging in a mouse model of myocardial infarction. Nat. Protoc. 2007;2:2551–2567. doi: 10.1038/nprot.2007.371. [DOI] [PubMed] [Google Scholar]
- 20.Christensen G, Minamisawa S, Gruber PJ, Wang Y, Chien KR. High-efficiency, long-term cardiac expression of foreign genes in living mouse embryos and neonates. Circulation. 2000;101:178–184. doi: 10.1161/01.cir.101.2.178. [DOI] [PubMed] [Google Scholar]
- 21.Phifer CB, Terry LM. Use of hypothermia for general anesthesia in preweanling rodents. Physiol. Behav. 1986;38:887–890. doi: 10.1016/0031-9384(86)90058-2. [DOI] [PubMed] [Google Scholar]
- 22.Kulandavelu S, et al. Embryonic and neonatal phenotyping of genetically engineered mice. ILAR J. 2006;47:103–117. doi: 10.1093/ilar.47.2.103. [DOI] [PubMed] [Google Scholar]
- 23.Sohal DS, et al. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circulation Res. 2001;89:20–25. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- 24.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]