Abstract
Rationale: Chronic hypoxia induces pulmonary vascular remodeling, pulmonary hypertension, and right ventricular hypertrophy. At present, little is known about mechanisms driving these responses. Hypoxia-inducible factor-1α (HIF-1α) is a master regulator of transcription in hypoxic cells, up-regulating genes involved in energy metabolism, proliferation, and extracellular matrix reorganization. Systemic loss of a single HIF-1α allele has been shown to attenuate hypoxic pulmonary hypertension, but the cells contributing to this response have not been identified.
Objectives: We sought to determine the contribution of HIF-1α in smooth muscle on pulmonary vascular and right heart responses to chronic hypoxia.
Methods: We used mice with homozygous conditional deletion of HIF-1α combined with tamoxifen-inducible smooth muscle–specific Cre recombinase expression. Mice received either tamoxifen or vehicle followed by exposure to either normoxia or chronic hypoxia (10% O2) for 30 days before measurement of cardiopulmonary responses.
Measurements and Main Results: Tamoxifen-induced smooth muscle–specific deletion of HIF-1α attenuated pulmonary vascular remodeling and pulmonary hypertension in chronic hypoxia. However, right ventricular hypertrophy was unchanged despite attenuated pulmonary pressures.
Conclusions: These results indicate that HIF-1α in smooth muscle contributes to pulmonary vascular remodeling and pulmonary hypertension in chronic hypoxia. However, loss of HIF-1 function in smooth muscle does not affect hypoxic cardiac remodeling, suggesting that the cardiac hypertrophy response is not directly coupled to the increase in pulmonary artery pressure.
Keywords: pulmonary circulation, right ventricular hypertrophy, animal disease models, knock-out mice
At a Glance Commentary
Scientific Knowledge on the Subject
Hypoxia-induced pulmonary hypertension is a potentially severe and fatal lung disorder that develops in patients with chronic lung disease including chronic obstructive pulmonary disease. Currently, few therapies exist for the treatment of pulmonary hypertension and prevention strategies remain largely unknown. Previous work suggests that hypoxia-inducible factor-1 (HIF-1) contributes to pulmonary vascular remodeling, but the role of vascular smooth muscle HIF-1 in the pulmonary arterial and cardiac remodeling responses is not known.
What This Study Adds to the Field
This study contributes two important new concepts. First, HIF-1α in smooth muscle contributes significantly to the pulmonary vascular remodeling induced by chronic hypoxia. Second, although HIF-1α deletion in smooth muscle significantly attenuates the degree of pulmonary hypertension, it does not attenuate the right ventricular remodeling response to hypoxia. These findings implicate the cell-autonomous role of smooth muscle HIF-1α in the development of hypoxia-induced pulmonary hypertension, and reveal that remodeling of the right ventricle is dissociated from the degree of pulmonary hypertension.
Chronic hypoxia (CH) triggers pulmonary vascular remodeling, leading to pulmonary hypertension and right ventricular (RV) hypertrophy with the ultimate risk of right heart failure. Chronic lung diseases, such as chronic obstructive pulmonary disease, cystic fibrosis, and bronchopulmonary dysplasia, can result in diffuse chronic alveolar hypoxia (1–6). The development of pulmonary hypertension is associated with significant morbidity and mortality in these patients (4, 7, 8). Despite this, few clinical therapies exist for the treatment of pulmonary hypertension and prevention strategies remain largely unknown. A better understanding of the mechanisms underlying hypoxia-induced pulmonary vascular remodeling could potentially lead to the identification of novel therapeutic strategies.
Hypoxia-inducible factors (HIF-1 and HIF-2) play dominant roles in regulating the transcriptional responses to hypoxia. HIF is a heterodimeric transcription factor comprised of an oxygen-regulated α subunit (HIF-1α) and a constitutively expressed β subunit (HIF-1β/ARNT) (9). Under normoxic conditions, degradation of HIF-1α is initiated by hydroxylation of conserved proline residues (10, 11). During hypoxia this process is inhibited allowing HIF-1α to accumulate, permitting dimerization with HIF-1β and activation of hypoxia-specific genes (12). Consistent with its role in tissue oxygen homeostasis, HIF-dependent genes regulate a wide range of processes in vivo including vascular endothelial growth factor (VEGF)-induced vascularization, erythropoiesis, cellular proliferation and migration, and cellular energy and metabolism (13–15). Cells lacking HIF-1α demonstrate impaired up-regulation of cellular proliferation and VEGF expression during hypoxia, and they fail to up-regulate genes involved in glucose transport and energy metabolism (13, 14).
Mice exposed to chronic environmental hypoxia develop pulmonary vascular remodeling, pulmonary hypertension, and RV hypertrophy (16–19). Pulmonary vascular remodeling involves both hypertrophy and hyperplasia of the medial layer, in addition to adventitial and intimal changes in the pulmonary arteries (20–22). Although hypertrophy likely represents a larger contribution to remodeling in proximal vessels, hyperplasia is thought to be the more significant contributor in smaller resistance arteries (20, 21). This structural remodeling of pulmonary arteries represents one key component responsible for alterations in pulmonary vascular resistance (PVR), driving the associated increase in pulmonary artery pressure responsible for hypoxia-induced pulmonary hypertension (22).
In smooth muscle cells, hypoxia-induced proliferation is inhibited by HIF-1α knock-down (23), suggesting that HIF-1 may participate in pulmonary vascular remodeling. Because homozygous deletion of HIF-1α in the mouse induces embryonic lethality arising from abnormal vascular development (14), Shimoda and coworkers (24) studied the effects of CH using heterozygous mice lacking one copy of the gene. Compared with wild-type control animals, heterozygous HIF-1α mice demonstrated impaired lung vascular remodeling in CH and attenuated RV hypertrophic responses (24–27). This was associated with lessened smooth muscle cell hypertrophy, attenuated up-regulation of transient potential receptor proteins and Na+/H+ exchanger-isoform 1, and failure to suppress the expression of plasma membrane K+ channels during CH (24). Collectively, those findings indicate that HIF-1 plays an important role in mediating the lung vascular and RV responses to prolonged hypoxia.
However, systemic loss of HIF activity affects multiple functions that potentially contribute to cardiopulmonary responses to hypoxia. For example, systemic loss of HIF affects endothelium, epithelium, and fibroblasts that participate in the vascular remodeling process, and also affects cardiomyocytes that participate in cardiac remodeling. Systemic HIF depletion could also influence the migration of bone marrow–derived progenitor cells that participate in the remodeling response to hypoxia. Finally, heterozygous HIF-1α mice demonstrate delayed increases in hematocrit (26), and attenuated ventilatory responses to hypoxia arising from altered carotid body function that could affect the degree of alveolar hypoxia for a given inspired oxygen level (25). Hence, assessing the cell-autonomous role of HIF-1 in vascular remodeling requires a selective, cell-specific knock-out. A recent study used mice with a homozygous deletion of HIF-1α in smooth muscle cells (28). However, those mice were deficient in HIF-1α during the critical period of embryologic cardiopulmonary development, which is known to result in compromised vascular development (14).
We hypothesized that HIF-1α in smooth muscle functions as a key factor underlying the development of hypoxia-induced pulmonary hypertension. To determine the role of HIF-1α in these cells, we used mice with smooth muscle–specific conditional deletion of HIF-1α to allow induced deletion in animals with normal cardiopulmonary development and to permit in vivo study of its role in pulmonary vascular remodeling.
Some of the results of these studies have been reported previously in abstract form (29).
Methods
Detailed methods are contained in the online supplement.
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee at Northwestern University, Chicago, Illinois.
Smooth Muscle–Specific Deletion of HIF-1α
Mice with LoxP flanking of exon 2 of the HIF-1α gene (HIF-1α-fl/fl) (30) were bred with mice expressing tamoxifen-inducible Cre recombinase under the control of a smooth muscle–specific promoter (SMM-Cre) (from Dr. Stefan Offermanns, University of Heidelberg, Heidelberg, Germany) (31) to generate HIF-1α-fl/fl–SMM-Cre (HIF-1α–SMM-Cre) mice. At 8 weeks, experimental mice received tamoxifen (10 mg/ml in corn oil vehicle), 2 μg/g body weight × 5 days by intraperitoneal injection; littermate control animals received vehicle alone. A 2-week period was observed after tamoxifen treatment to permit deletion of the HIF-1α gene and degradation of residual message.
Confirmation of Cre Activation by Tamoxifen
To confirm that tamoxifen administration activates Cre recombinase activity in smooth muscle, female reporter mice with a ROSA-targeted LoxP-flanked membrane-targeted td-Tomato (mT) cassette and a membrane-targeted enhanced green fluorescent protein (mG) cassette (mTmG reporter mouse; Jackson Laboratory, Bar Harbor, ME) were bred with the SMM-Cre transgenic mice for subsequent immunofluorescence imaging.
Confirmation of Cell-Specific HIF-1α Deletion
To confirm smooth muscle–specific HIF-1α deletion, HIF-1α mRNA expression in smooth muscle was measured by reverse-transcriptase polymerase chain reaction after treatment with tamoxifen or vehicle. To confirm that cardiac HIF-1α production remained unaltered, HIF-1α mRNA expression in RV tissue was similarly assessed.
Study Design
Experiments involved adult male HIF-1α–SMM-Cre littermates. Mice were housed in normoxia (21% O2) or CH (10% O2) for 30 days (range, 28–35 d). On study completion, mice underwent hemodynamic evaluation before euthanasia for analysis of heart and lung tissue. Pulmonary artery pressure was quantified by two methods: echocardiography (32) using a VisualSonics Vevo-770 echo system (VisualSonics, Toronto, Ontario, Canada), and right heart catheterization with a micromanometer-tipped catheter (Millar Instruments, Houston, TX) using a modification of a previous technique (33).
Pulmonary artery remodeling was quantified by the wall thickness, defined as vessel cross-sectional area (area external - area lumen) divided by the diameter of the lumen (calculated from the perimeter of the lumen; diameter = perimeter/π). Further assessments of remodeling included the muscularization profile of distal arteries, and a proliferation assay using sections of inflation-fixed lungs (4% formaldehyde; Sigma-Aldrich, St. Louis, MO). RV mass was assessed by echocardiography to measure the RV free wall (RVFW) thickness (34), and by measuring weight ratio of the right ventricle to left ventricle and interventricular septum (RV/[LV+S]). Stained sections of formalin-fixed right ventricle were used to quantify the extent of hypertrophy. All analyses were performed by a single observer masked to the experimental grouping. Unless stated otherwise, experimental groups included 6–13 replicate animals.
Statistical Analysis
Student t test was used for analysis between two groups. One-way analysis of variance was used followed by Newman-Keuls post hoc test to identify specific differences among groups. Statistical significance was accepted at P less than 0.05. Data are presented as means ± SEM. Statistical testing was accomplished using Prism 5.0 (GraphPad Software, La Jolla, CA).
Results
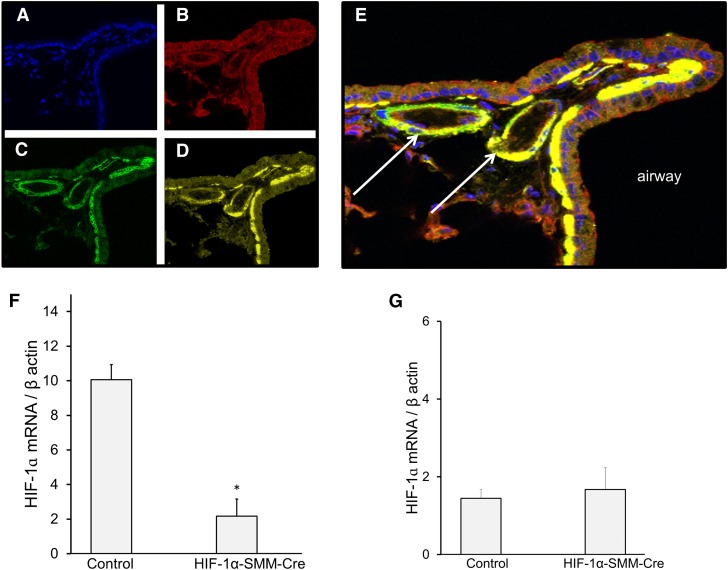
To confirm the activity of the SMM-Cre in smooth muscle, mTmG-reporter-SMM Cre transgenic mice were treated with tamoxifen and lungs were analyzed after 14 days (Figure 1). Many lung cells exhibited constitutive red fluorescence (Figure 1B). Tamoxifen treatment led to Cre activation, causing the appearance of green fluorescence (Figure 1C). Immunostaining for smooth muscle actin identified pulmonary vessels and airways (Figure 1D), which colocalized with the green fluorescence (Figure 1E). These findings confirm successful activation of Cre recombinase in smooth muscle in the lung. To assess this further, mRNA message for HIF-1α was quantified in smooth muscle from the descending aorta of HIF-1α–SMM-Cre mice (Figure 1F). This analysis detected a significant decrease in basal HIF-1α message, consistent with effective gene deletion in smooth muscle. Next, to confirm the specificity of HIF-1α deletion, HIF-1α mRNA message was measured in the right ventricle to verify that cardiomyocyte HIF-1α expression was unaltered in mice undergoing HIF-1α deletion in smooth muscle (Figure 1G). This analysis revealed that mRNA message for HIF-1α in the right ventricle did not differ between control animals and HIF-1α–SMM-Cre mice. These data confirm the specificity of smooth muscle knock-out and demonstrate that expression of HIF-1α in cardiac muscle remains unaffected.
Figure 1.
Smooth muscle–specific hypoxia-inducible factor (HIF)-1α deletion. Tamoxifen induction of Cre recombinase activity in smooth muscle; confocal microscopy images of mouse lung tissue slices at ×40 magnification. (A) DAPI counterstain. (B) Labeled mTmG-reporter constitutively expressing red fluorescence in all cell types. (C) Tamoxifen treatment causes expression to change from red to green fluorescence in cells expressing Cre. (D) Immunofluorescence labeling of smooth muscle actin (yellow) in pulmonary vasculature and airways. (E) Colocalization of yellow and green fluorescence, confirming activation of Cre recombinase in smooth muscle. Arrows identify small pulmonary arteries adjacent to a labeled airway. (F) HIF-1α mRNA message by reverse-transcriptase polymerase chain reaction in normoxic smooth muscle cells after in vivo tamoxifen treatment, confirming effective tissue-specific knock-out. n = 2–3/group; *P < 0.05 versus control. (G) HIF-1α mRNA message by reverse-transcriptase polymerase chain reaction in normoxic right ventricular tissue after in vivo tamoxifen treatment, demonstrating that HIF-1α mRNA expression is retained in cardiomyocytes after HIF-1α deletion in smooth muscle.
At 8 weeks of age, mice were randomized to tamoxifen or vehicle administration followed by normoxia or CH exposure. All mice survived until study completion. During necropsy, no external or gross anatomic differences were observed between HIF-1α–SMM-Cre mice and littermate control animals subjected to the same treatment. Physiologic measurements obtained at study completion are presented in Table 1. The normoxic groups were indistinguishable with respect to weight and hematocrit. Mice exposed to CH weighed less and exhibited higher hematocrits than their normoxic littermate control animals at the time of euthanasia. However, body weight and polycythemia responses in the control and the SMM-HIF-1α deletion groups were not different. To assess systemic cardiovascular control, systemic arterial blood pressure was measured in unanesthetized mice under normoxic conditions. Mean arterial pressure did not differ among experimental groups regardless of SMM–HIF-1α status or environmental exposure.
Table 1:
Endpoint Physiologic Variables
| Body Weight (g) | Hematocrit (%) | Mean Systemic Arterial BP (mm Hg) | |
|---|---|---|---|
| Control | |||
| Normoxia | 28 ± 0.7 | 43 ± 0.8 | 105 ± 4 |
| Chronic hypoxia | 23 ± 0.4* | 62 ± 1.1* | 101 ± 6 |
| HIF-1α–SMM-Cre | |||
| Normoxia | 28 ± 0.7 | 40 ± 1.0 | 115 ± 6 |
| Chronic hypoxia | 23 ± 0.4* | 61 ± 1.3* | 102 ± 9 |
Definition of abbreviations: BP = blood pressure; HIF = hypoxia-inducible factor; SMM-Cre = Cre recombinase smooth muscle–specific promoter.
P < 0.05 versus normoxic control animals.
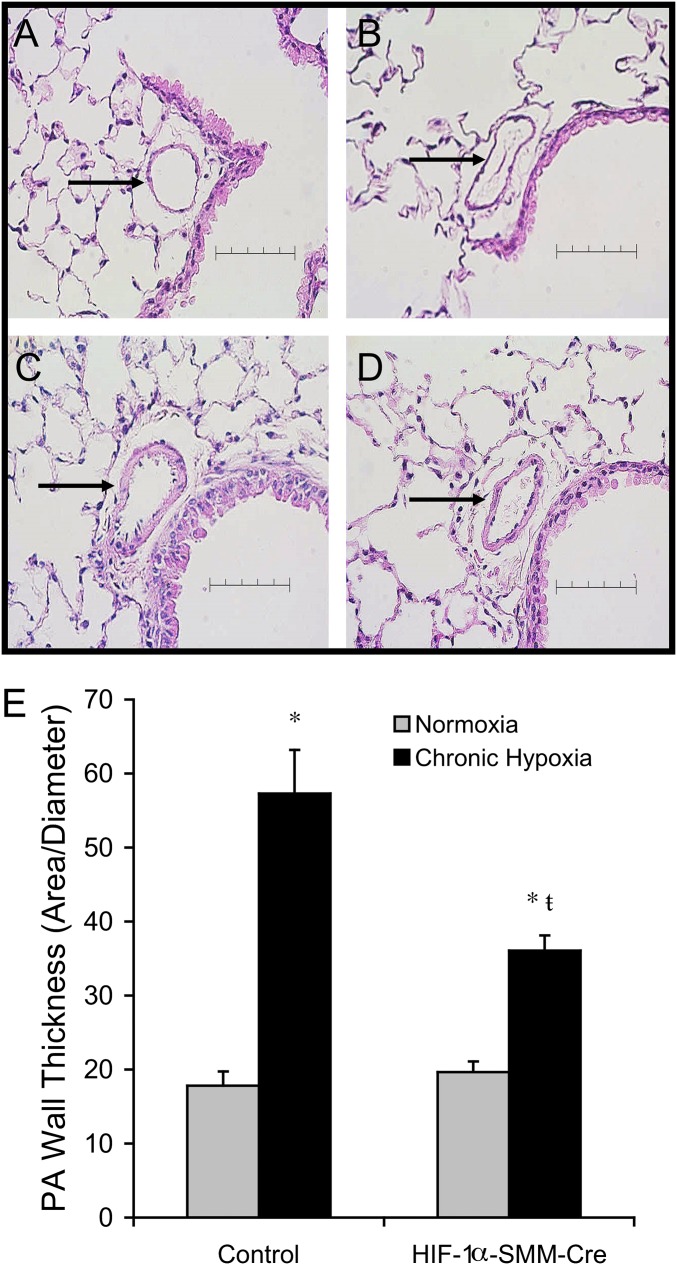
To assess the effect of CH on pulmonary vascular remodeling, wall thickness was measured in small intraparenchymal pulmonary arteries (Figures 2A–2E). The mean lumen diameter of arteries analyzed ranged from 38 to 47 μm (Table 2). CH induced remodeling of pulmonary arteries with thickening of the arterial wall (Figures 2A–2E). Pulmonary artery wall thickness was not different between normoxic groups, demonstrating that HIF-1α deletion in smooth muscle did not induce pulmonary vascular changes under normoxic conditions. During CH, control animals developed a significant increase in pulmonary artery wall thickness, consistent with previous studies (35, 36). By contrast, HIF-1α–SMM-Cre mice exposed to CH exhibited attenuated vascular remodeling compared with hypoxic littermate control animals (Figure 2E).
Figure 2.
Vascular remodeling in small pulmonary arteries after chronic hypoxia exposure. Representative hematoxylin and eosin–stained mouse lung tissue sections. Arrows identify a small pulmonary artery adjacent to a labeled airway. Scale bar = 80 μm. (A) Normoxic control. (B) Normoxic hypoxia-inducible factor (HIF)-1α–Cre recombinase under the control of a smooth muscle–specific promoter (SMM-Cre). (C) Chronic hypoxia. (D) Chronic hypoxia HIF-1α–SMM-Cre. (E) Quantification of pulmonary vascular remodeling in chronic hypoxia by wall thickness demonstrates attenuated remodeling in HIF-1α–SMM-Cre mice. P < 0.05 versus normoxic control (*) or versus hypoxic control (ŧ). PA = pulmonary artery.
Table 2:
Pulmonary Artery Diameter and Cellular Proliferation
| Lumen Diameter (μm) | Ki67-Positive PAs per Lung Slice (Number) | |
|---|---|---|
| Control | ||
| Normoxia | 44.2 ± 1.6 | 2.9 ± 0.6 |
| Chronic hypoxia | 38.1 ± 1.7 | 1.9 ± 0.6 |
| HIF-1α–SMM-Cre | ||
| Normoxia | 41.5 ± 1.8 | 2.4 ± 0.9 |
| Chronic hypoxia | 46.6 ± 2.4 | 4.0 ± 0.7 |
Definition of abbreviations: HIF = hypoxia-inducible factor; PA = pulmonary artery; SMM-Cre = Cre recombinase smooth muscle–specific promoter.
Although the lumen diameter was not statistically different across groups, the average diameter was smallest in the control CH mice, suggesting the greatest degree of pulmonary vascular remodeling. Furthermore, the lumen diameter of vessels from HIF-1α–SMM-Cre mice closely approximated the diameters in the two normoxic groups.
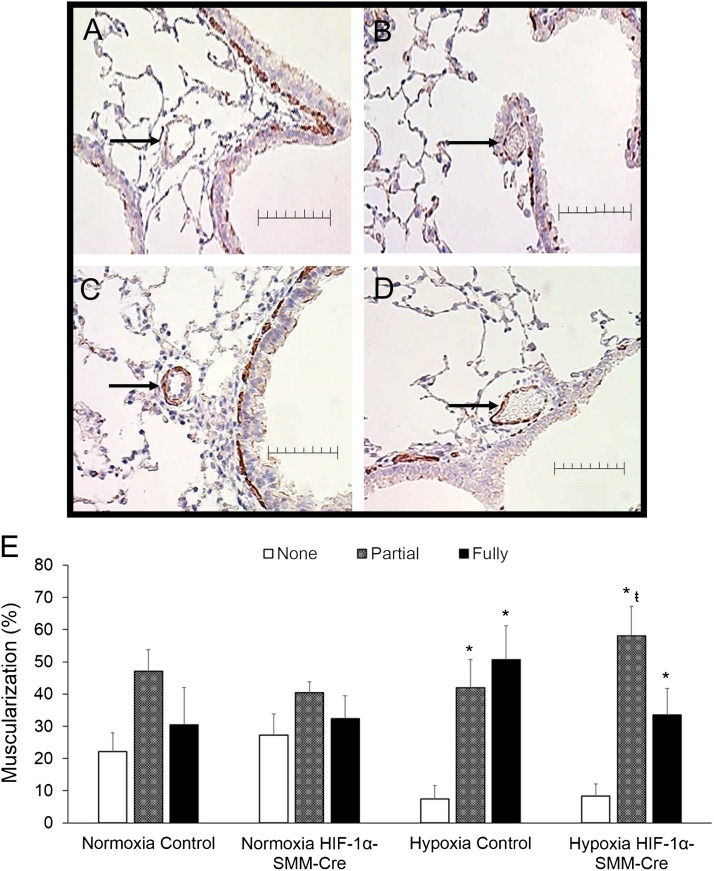
To further evaluate remodeling in more distal pulmonary arteries closer to the level of gas exchange, the muscularization profile of arteries with diameter less than 50 μm was assessed (Figures 3A–3D). In the two normoxic groups, the relative percentages of nonmuscularized, partially muscularized, and fully muscularized vessels were not different. Although CH increased the extent of small vessel muscularization in both groups, the HIF-1α–SMM-Cre mice exposed to CH exhibited fewer vessels with complete circumferential muscularization compared with the CH control mice (Figure 3E). To assess smooth muscle proliferation, lung sections from normoxic and CH groups were stained with Ki67 to detect cells engaged in proliferation. No significant differences between normoxic and hypoxic lung vessels were detected (Table 2).
Figure 3.
Muscularization in distal pulmonary arteries after chronic hypoxia exposure. (A–D) Representative α-smooth muscle actin–stained mouse lung tissue. Arrows identify a pulmonary artery with diameter less than 50 μm. Scale bar = 50 μm. (A) normoxia control. (B) Normoxia hypoxia-inducible factor (HIF)-1α–Cre recombinase under the control of a smooth muscle–specific promoter (SMM-Cre). (C) Chronic hypoxia. (D) Chronic hypoxia HIF-1α–SMM-Cre. (E) Hypoxia-induced muscularization of distal pulmonary arteries (<50-μm diameter) by characterization as nonmuscularized, partially muscularized, and fully muscularized. Chronic hypoxia induced distal muscularization in both groups; however, less full muscularization developed in HIF-1α–SMM-Cre mice relative to chronic hypoxic controls, indicating fewer vessels with complete circumferential muscularization. P < 0.05 versus corresponding group nonmuscularized vessels (*) or versus corresponding group fully muscularized vessels (ŧ).
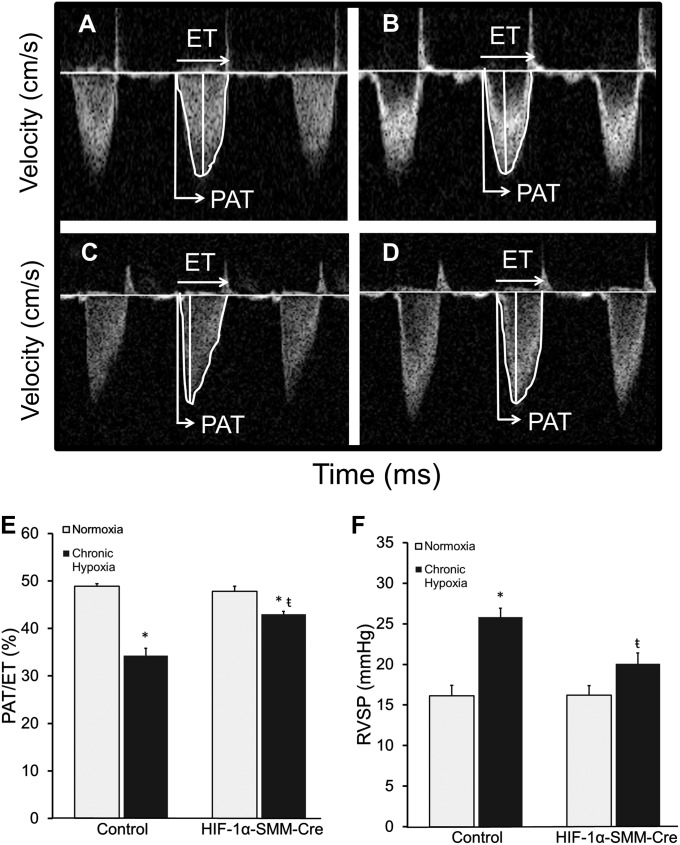
The effects of CH on cardiopulmonary hemodynamics were assessed by echocardiography and RV catheterization during normoxia. Pulmonary hypertension developed in all CH mice. Compared with hypoxic control animals, the HIF-1α–SMM-Cre mice subjected to CH exhibited an attenuated increase in pulmonary artery pressures (Figures 4A–4F). During echocardiographic measurements in sedated mice, heart rates averaged 436 ± 9 beats per minute and were not different across groups. In normoxic mice, control and experimental groups exhibited ratios of pulmonary acceleration time to total pulmonary ejection time (PAT/ET) approaching 50%, consistent with published studies and corresponding to a normal pulmonary artery pressure (Figures 4A, 4B, and 4E) (32). Because the time to peak flow acceleration across the pulmonary valve decreases with increasing pulmonary artery pressures, the ratio of PAT/ET varies inversely with pulmonary artery pressure. CH control mice exhibited a decrease in PAT/ET ratio to 34%, indicating the development of pulmonary hypertension. By contrast, HIF-1α–SMM-Cre mice in CH developed milder pulmonary hypertension as detected by PAT/ET ratio (Figures 4C–4E). We then performed RV catheterization to provide a second, independent measure of pulmonary pressure. During this procedure, heart rates averaged 332 ± 11 beats per minute and were not different among groups. RV systolic pressure (RVSP) measurements in normoxic control and HIF-1α–SMM-Cre mice were not different. However, mice exposed to CH demonstrated an increased RVSP, indicating the development of pulmonary hypertension. However, HIF-1α–SMM-Cre mice exposed to CH exhibited significantly lower RVSP than hypoxic control animals, again consistent with attenuated hypoxia-induced pulmonary hypertension (Figure 4F).
Figure 4.
Pulmonary hypertension after exposure to chronic hypoxia. (A–D) Pulse-wave Doppler images across the pulmonary outflow tract: (A) normoxic control, (B) normoxic hypoxia-inducible factor (HIF)-1α–Cre recombinase under the control of a smooth muscle–specific promoter (SMM-Cre), (C) chronic hypoxia, (D) chronic hypoxia HIF-1α–SMM-Cre. The time to peak flow acceleration across the pulmonary valve decreases with increasing pulmonary artery pressures; the ratio of pulmonary acceleration time to total pulmonary ejection time (PAT/ET) varies inversely with pulmonary artery pressure. (E) Pulmonary hypertension after exposure to chronic hypoxia. PAT/ET demonstrates pulmonary hypertension after chronic hypoxia, which is attenuated in HIF-1α–SMM-Cre mice. (F) Right ventricular systolic pressure (RVSP) after exposure to chronic hypoxia. P < 0.05 versus normoxic control (*) or versus hypoxic control (ŧ).
To evaluate cardiac responses to hypoxia, functional assessments of the right and left ventricle were performed (Table 3). Across all indices, measurements did not differ among groups regardless of SMM–HIF-1α status or exposure. Importantly, because development of pulmonary hypertension did not result in heart failure, the differences we observed in PAT and RVSP were not the result of impaired myocardial function or altered pulmonary blood flow.
Table 3:
Endpoint Cardiac Function
| Left Ventricle |
Right Ventricle |
||||
|---|---|---|---|---|---|
| Fractional Shortening (%) | Ejection Fraction (%) | Fractional Area Change (%) | Tricuspid Annular Plane Systolic Excursion (mm) | Stroke Volume (ml) | |
| Control | |||||
| Normoxia | 30.0 ± 2.2 | 64 ± 32 | 32.6 ± 1.6 | 1.1 ± 0.05 | 0.056 ± 0.05 |
| Chronic hypoxia | 28.5 ± 0.7 | 62 ± 5.3 | 31.9 ± 0.9 | 1.2 ± 0.07 | 0.060 ± 0.07 |
| HIF-1α–SMM-Cre | |||||
| Normoxia | 30.4 ± 2.6 | 62 ± 4.2 | 35.5 ± 1.8 | 1.2 ± 0.07 | 0.058 ± 0.06 |
| Chronic hypoxia | 27.5 ± 2.2 | 60 ± 3.5 | 30.9 ± 1.8 | 1.1 ± 0.05 | 0.042 ± 0.04 |
Definition of abbreviations: HIF = hypoxia-inducible factor; SMM-Cre = Cre recombinase smooth muscle–specific promoter.
n = 4–5 animals per group. Note that stroke volume is proportional to cardiac output because it is derived from the Doppler pulmonary velocity–time integral × pulmonary artery cross-sectional area.
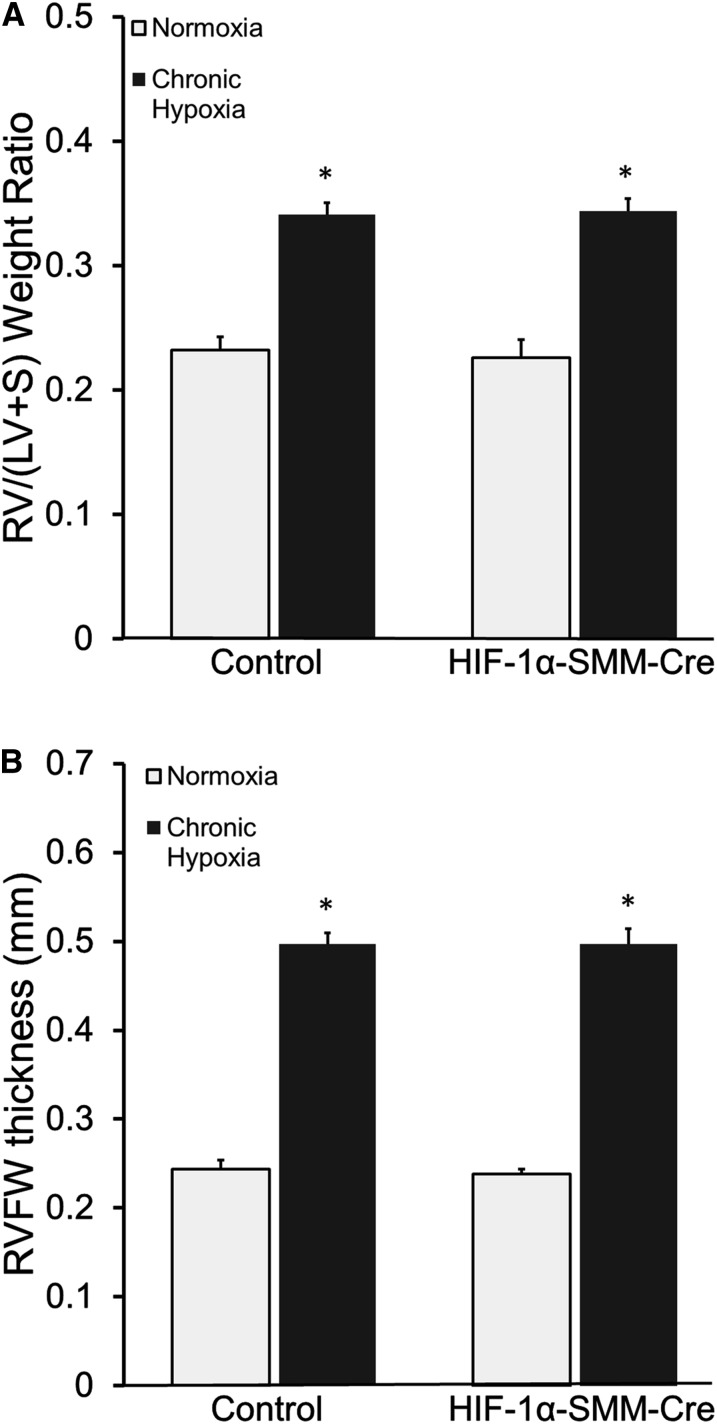
To evaluate the effects of CH on the right ventricle, RV mass was calculated as RV/(LV+Septal) weight, and by echocardiographic assessment of RVFW thickness. RV mass in normoxic control and HIF-1α–SMM-Cre mice was not different. In response to CH, control and HIF-1α–SMM-Cre mice exhibited increases in RV mass (Figure 5A). Likewise, the increase in RVFW thickness in response to CH was not different between control and HIF-1α–SMM-Cre mice (Figure 5B). Thus, although less pulmonary hypertension developed in hypoxic mice with HIF-1α–SMM deletion, the RV remodeling response was not different.
Figure 5.
Cardiac remodeling in chronic hypoxia. (A) Fulton index (RV/[LV+S]) measurement of right ventricular mass. Mice exposed to chronic hypoxia develop increased right heart mass compared with normoxic control littermates. (B) Echocardiographic measurement of right ventricular free wall (RVFW) thickness additionally demonstrates development of right ventricular hypertrophy in all mice after chronic hypoxia. *P < 0.05 versus normoxic control. HIF = hypoxia-inducible factor; LV = left ventricle; RV = right ventricle; S = interventricular septum; SMM-Cre = Cre recombinase under the control of a smooth muscle–specific promoter.
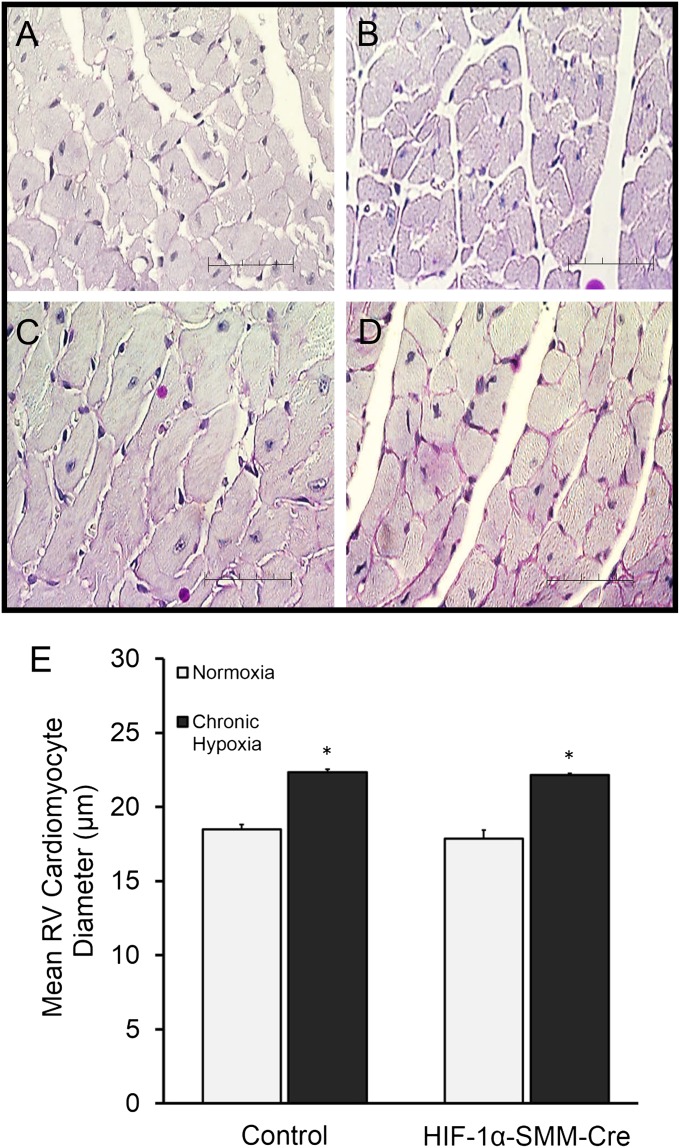
To further investigate the characteristics of the hypoxia-induced cardiac remodeling, we evaluated the degree of cardiomyocyte hypertrophy in the right ventricle (Figures 6A–6D). Cardiomyocyte fiber diameters did not differ in normoxic control and HIF-1α–SMM-Cre mice. During CH, cardiomyocyte diameter increased in control and HIF-1α–SMM-Cre mice (Figure 6E). However, fiber diameters were not different between HIF-1α–SMM-Cre mice and CH control animals.
Figure 6.
Hypertrophy of right ventricular cardiomyocytes in chronic hypoxia. Representative periodic acid Schiff–stained mouse right ventricular tissue sections. (A) Normoxia control. (B) Normoxia hypoxia-inducible factor (HIF)-1α–Cre recombinase under the control of a smooth muscle–specific promoter (SMM-Cre). (C) Hypoxia control. (D) Hypoxia HIF-1α–SMM-Cre. Scale bar = 50 μm. (E) Mice exposed to chronic hypoxia exhibit increased right ventricular fiber diameter compared with normoxic control littermates. Scale bar = 50 μm. RV = right ventricle. *P < 0.05 versus normoxic control.
To determine whether the Cre transgene or tamoxifen might independently affect cardiopulmonary responses, a set of HIF-1α–floxed, Cre-negative mice was treated with tamoxifen or vehicle. After 4 weeks in normoxia, these mice underwent cardiopulmonary assessment. Mice lacking Cre that were administered tamoxifen or vehicle exhibited no baseline differences in comparison with the HIF-1α–SMM-Cre study mice, indicating that tamoxifen and the Cre transgene, by themselves, did not contribute to the observed responses (see online supplement).
Discussion
This study examined the contribution of smooth muscle HIF-1α in hypoxia-induced pulmonary vascular remodeling, pulmonary hypertension, and RV hypertrophy in a murine model. The data reveal two important new concepts. First, HIF-1α in pulmonary vascular smooth muscle contributes significantly to the remodeling events induced by CH in precapillary pulmonary vessels. Second, the data reveal that RV remodeling in CH is not directly linked to the degree of pulmonary hypertension, because the attenuation of the pulmonary artery pressure response in the smooth muscle HIF-1α–deficient animals did not correspond with an attenuation in RV hypertrophy.
HIF-1α is stabilized in many cell types during systemic hypoxia. Our data reveal that the activation of HIF-1 in smooth muscle plays a key role in the vascular remodeling by promoting increased arterial wall thickness and distal muscularization during prolonged hypoxia. Although the importance of HIF-1 in tissue adaptation to hypoxia has been shown previously (13, 23–26), our study demonstrates the importance of the cell-specific role of HIF in the remodeling response driving the development of pulmonary hypertension. We find that loss of HIF-1α in smooth muscle cells significantly attenuates the remodeling of pulmonary arteries and the associated pulmonary hypertension induced by CH.
Although the direct in vivo measurement of PVR is limited by the technical difficulty in measuring left atrial pressure in the mouse, our data strongly suggest that increases in PVR represent an important contribution to the increase in pulmonary artery pressure during CH. First, wall thickness was increased in approximately 40-μm diameter pulmonary arteries from CH mice and lumen diameter tended to be less. This response was attenuated in mice with HIF-1α deletion in smooth muscle. Second, CH increased the degree of muscularization of pulmonary arteries smaller than 50 μm; the degree of complete muscularization was attenuated in the smooth muscle HIF-1α–deleted mice. CH increased both RVSP and the echocardiographic evidence of pulmonary hypertension under normoxic conditions, but this was mitigated by HIF-1α deletion in smooth muscle. Importantly, cardiac function was not significantly affected by CH or by HIF-1α deletion, and the calculated pulmonary blood flow did not change. Collectively, these observations support the conclusion that pulmonary hypertension is attenuated by smooth muscle HIF-1α deletion because the loss of HIF-1 attenuates the increase in PVR during CH.
However, although deletion of HIF-1α in smooth muscle attenuates CH-induced vascular remodeling, its deletion does not completely block this response. Therefore, HIF-1α in smooth muscle is not the sole determinant of vascular remodeling, and other cellular pathways and cell types likely contribute to that response. For example, previous studies have implicated important contributions from endothelial cells and fibroblasts in the pulmonary artery (21, 37, 38). Evidence also suggests that paracrine effects from fibroblasts may promote proliferation in adjacent smooth muscle cells (39–41). It is also possible that HIF activation in migratory bone marrow progenitor cells (41) or other cell types contributes to the remodeling response, or that HIF-independent pathways are also involved (40). Finally, HIF-2 could also be contributing to the remodeling response. In that regard, Hickey and coworkers (42) demonstrated that HIF-2 heterozygosity conferred protection against the development of pulmonary hypertension in a murine model of Chuvash polycythemia.
Pulmonary arteries remodel during CH, leading to the development of pulmonary hypertension that is sustained even if the hypoxic stimulus is discontinued. One mechanism of remodeling involves hypertrophy of arterial smooth muscle, with an associated narrowing of the lumen diameter that augments PVR. A second remodeling mechanism begins with prolonged hypoxic pulmonary vasoconstriction, followed by remodeling of the extracellular matrix such that the arteries become “locked” in a narrowed state. In that case the arteries lose the ability to dilate in response to acute normoxia or vasodilators, even though these might abolish smooth muscle tone. Either of these mechanisms would manifest as an increase in PVR, and possibly as an increase in pulmonary artery wall thickness. Both mechanisms cause a chronic narrowing of pulmonary arteries and an increase in PVR.
Our study demonstrates that HIF-1α deletion in smooth muscle attenuates the increase in PVR during CH. It is possible that HIF-1 is required for smooth muscle hypertrophy and the associated narrowing of lumen diameter in resistance vessels. However, an alternative possibility is that loss of HIF-1 in smooth muscle attenuates the sustained vasoconstrictor response to hypoxia (28, 43, 44). In that case, any extracellular matrix remodeling would have developed while the vessels were less constricted than in the wild-type control animals. Our RVSP and echocardiographic assessments of pulmonary hypertension were made under normoxic conditions, which should have abolished any hypoxia-induced active vascular tone. However, loss of smooth muscle tone after the remodeling was complete would be unlikely to reverse the increase in vascular resistance. Although the decrease in pulmonary artery wall thickness observed in the HIF-1α deletion animals suggests that HIF-1–dependent smooth muscle hypertrophy had occurred, our data do not allow us to definitively identify which mechanism was involved, or whether both contributed.
CH induces pulmonary hypertension and RV hypertrophy in wild-type mice (19, 35, 45). RV remodeling presumably represents an adaptive response to pulmonary hypertension, enhancing the ability of the heart to deal with the increase in afterload. In mice with systemic heterozygous HIF-1α deficiency, Yu and coworkers (26) observed an attenuated RV hypertrophic response to CH in association with an attenuation of pulmonary hypertension. It is important to note that these mice had global heterozygous deficiency and thus decreased HIF-1α levels in cardiac tissue. In the present study, mice had normal HIF-1α production in cardiac tissue. In our mice, pulmonary hypertension was attenuated by HIF-1α deletion in smooth muscle but the degree of RV hypertrophy was unchanged. Collectively, these results suggest that pulmonary artery pressure is not the sole determinant of RV remodeling, and that HIF-1 in the heart may contribute to the cardiac remodeling response. Other studies also suggest that pulmonary artery pressure is not the sole factor regulating cardiac hypertrophy, and that cardiac hypoxia itself may be a stimulus for RV remodeling (45, 46) through the up-regulation of gene expression (47, 48). Animal models of RV pressure overload induced by pulmonary artery banding are not associated with RV failure (49), whereas pressure overload induced by hypoxia is (50, 51). Bogaard and coworkers (46) compared the RV hypertrophic response to VEGF receptor inhibition (using SU5416) with that evoked by pulmonary artery banding in chronically hypoxic rats. During CH, both SU5416 and pulmonary artery banding elicited similar increases in RVSP, but the SU5416 + hypoxia animals exhibited exaggerated RV hypertrophic responses. Those findings suggest that hypoxia per se may amplify RV remodeling independently from its effects on pulmonary hypertension. Interestingly, the RV levels of HIF-1α were greater in the SU5416 + hypoxia animals, suggesting that the direct effect of hypoxia on the RV remodeling may be mediated by cardiac HIF-1α (46). Increased capillary density and sustained VEGF expression that is limited to the right ventricle has also been observed in rats with hypoxia-induced pulmonary hypertension (52), underscoring the importance of HIF-1α in RV remodeling and indicating that the hypoxic response may be ventricle-specific. Further supporting the role of HIF-1α in RV remodeling, Bohuslavová and coworkers (45) noted a ventricle-specific pattern of gene expression, with most HIF-1α–regulated pathways increased in the right ventricle. In their study, CH triggered the transcriptional activation of multiple HIF-1α downstream targets specifically in the right ventricle. Finally, Shimoda and coworkers (24, 27) noted that HIF-1α heterozygous mice exhibited attenuated pulmonary hypertension and attenuated RV hypertrophy during CH, again consistent with the idea that HIF-1α contributes to pulmonary hypertension and RV remodeling.
Some limitations to this study should be noted. First, our analysis of vessel remodeling was restricted to approximately 40-μm pulmonary arteries located adjacent to airways. Smaller intraparenchymal arteries likely contribute to PVR, but these were excluded because of the difficulty distinguishing them from pulmonary veins. Second, we attribute the results of this study to the deletion of HIF-1α from smooth muscle cells. However, Cre recombinase can exert effects that are independent of the intended genetic recombination event (53), so we cannot exclude the possibility that nonspecific effects of Cre influenced these results.
Collectively, our results demonstrate that HIF-1α in smooth muscle plays an important role in hypoxia-induced pulmonary vascular remodeling and pulmonary hypertension. Although hypoxia-induced pulmonary hypertension contributes to the development of RV hypertrophy and potentially to right heart failure, our results suggest that hypoxia-induced factors other than pulmonary hypertension also contribute to the cardiac remodeling. A better understanding of the direct effects of hypoxia on cardiac tissue could guide the development of cardiac-specific interventions aimed to minimize cardiac damage leading to right ventricle failure, the major cause of mortality in this population.
Acknowledgments
Acknowledgment
The authors thank Stephan Offermanns, Ph.D., for providing the SMMHC-Cre mice.
Footnotes
Supported by grants from the National Institutes of Health, including HL35440 and HL079650 (P.T.S.), T32 HL76139-7 (M.K.B.), HL054705 and HL102235 (R.H.S.), and HL107577 (S.J.S.); the Northwestern University Center for Genetic Medicine Transgenic and Targeted Mutagenesis Laboratory; the Northwestern University Mouse Histology and Phenotyping Laboratory; and a Cancer Center Support Grant (NCI CA060553).
Author Contributions: M.K.B., hypothesis generation, study design, data acquisition, analysis and interpretation, and article writing. G.B.W., P.T.M., J.M.N., and R.H.S., study design and data interpretation. R.W.D., study design and mouse breeding design. L.C., S.K.B., and S.J.S., acquisition of data. V.J.D. and L.B., acquisition of data and data analysis. P.T.S., study conception, hypothesis delineation, data interpretation, and article writing and revision.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201302-0302OC on November 19, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Strange C, Highland KB. Pulmonary hypertension in interstitial lung disease. Curr Opin Pulm Med. 2005;11:452–455. doi: 10.1097/01.mcp.0000174250.38188.6d. [DOI] [PubMed] [Google Scholar]
- 2.Roy R, Couriel JM. Secondary pulmonary hypertension. Paediatr Respir Rev. 2006;7:36–44. doi: 10.1016/j.prrv.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Stenmark KR, Abman SH. Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Annu Rev Physiol. 2005;67:623–661. doi: 10.1146/annurev.physiol.67.040403.102229. [DOI] [PubMed] [Google Scholar]
- 4.Naeije R. Pulmonary hypertension and right heart failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:20–22. doi: 10.1513/pats.200407-037MS. [DOI] [PubMed] [Google Scholar]
- 5.Stern RC, Borkat G, Hirschfeld SS, Boat TF, Matthews LW, Liebman J, Doershuk CF. Heart failure in cystic fibrosis. Treatment and prognosis of cor pulmonale with failure of the right side of the heart. Am J Dis Child. 1980;134:267–272. doi: 10.1001/archpedi.1980.02130150025007. [DOI] [PubMed] [Google Scholar]
- 6.Vera KB, Moore D, Flack E, Liske M, Summar M. Significant differences in markers of oxidant injury between idiopathic and bronchopulmonary-dysplasia-associated pulmonary hypertension in children. Pulm Med. 2012;2012:301475. doi: 10.1155/2012/301475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, Mullen MP. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics. 2007;120:1260–1269. doi: 10.1542/peds.2007-0971. [DOI] [PubMed] [Google Scholar]
- 8.Kim GB. Pulmonary hypertension in infants with bronchopulmonary dysplasia. Korean J Pediatr. 2010;53:688–693. doi: 10.3345/kjp.2010.53.6.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semenza GL, Nejfelt MK, Chi SM, Antonarakis SE. Hypoxia-inducible nuclear factors bind to an enhancer element located 3′ to the human erythropoietin gene. Proc Natl Acad Sci USA. 1991;88:5680–5684. doi: 10.1073/pnas.88.13.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 11.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 12.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 14.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gleadle JM, Ratcliffe PJ. Induction of hypoxia-inducible factor-1, erythropoietin, vascular endothelial growth factor, and glucose transporter-1 by hypoxia: evidence against a regulatory role for Src kinase. Blood. 1997;89:503–509. [PubMed] [Google Scholar]
- 16.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006;99:675–691. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 17.Fagan KA. Selected contribution: pulmonary hypertension in mice following intermittent hypoxia. J Appl Physiol (1985) 2001;90:2502–2507. doi: 10.1152/jappl.2001.90.6.2502. [DOI] [PubMed] [Google Scholar]
- 18.Hales CA, Kradin RL, Brandstetter RD, Zhu YJ. Impairment of hypoxic pulmonary artery remodeling by heparin in mice. Am Rev Respir Dis. 1983;128:747–751. doi: 10.1164/arrd.1983.128.4.747. [DOI] [PubMed] [Google Scholar]
- 19.Abud EM, Maylor J, Undem C, Punjabi A, Zaiman AL, Myers AC, Sylvester JT, Semenza GL, Shimoda LA. Digoxin inhibits development of hypoxic pulmonary hypertension in mice. Proc Natl Acad Sci USA. 2012;109:1239–1244. doi: 10.1073/pnas.1120385109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyrick B, Reid L. Hypoxia-induced structural changes in the media and adventitia of the rat hilar pulmonary artery and their regression. Am J Pathol. 1980;100:151–178. [PMC free article] [PubMed] [Google Scholar]
- 21.McKenzie JC, Clancy J, Jr, Klein RM. Autoradiographic analysis of cell proliferation and protein synthesis in the pulmonary trunk of rats during the early development of hypoxia-induced pulmonary hypertension. Blood Vessels. 1984;21:80–89. [PubMed] [Google Scholar]
- 22.Pak O, Aldashev A, Welsh D, Peacock A. The effects of hypoxia on the cells of the pulmonary vasculature. Eur Respir J. 2007;30:364–372. doi: 10.1183/09031936.00128706. [DOI] [PubMed] [Google Scholar]
- 23.Schultz K, Fanburg BL, Beasley D. Hypoxia and hypoxia-inducible factor-1alpha promote growth factor-induced proliferation of human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2006;290:H2528–H2534. doi: 10.1152/ajpheart.01077.2005. [DOI] [PubMed] [Google Scholar]
- 24.Shimoda LA, Manalo DJ, Sham JSK, Semenza GL, Sylvester JT. Partial HIF-1alpha deficiency impairs pulmonary arterial myocyte electrophysiological responses to hypoxia. Am J Physiol Lung Cell Mol Physiol. 2001;281:L202–L208. doi: 10.1152/ajplung.2001.281.1.L202. [DOI] [PubMed] [Google Scholar]
- 25.Kline DD, Peng YJ, Manalo DJ, Semenza GL, Prabhakar NR. Defective carotid body function and impaired ventilatory responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1 alpha. Proc Natl Acad Sci USA. 2002;99:821–826. doi: 10.1073/pnas.022634199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, et al. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest. 1999;103:691–696. doi: 10.1172/JCI5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimoda LA, Fallon M, Pisarcik S, Wang J, Semenza GL. HIF-1 regulates hypoxic induction of NHE1 expression and alkalinization of intracellular pH in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol. 2006;291:L941–L949. doi: 10.1152/ajplung.00528.2005. [DOI] [PubMed] [Google Scholar]
- 28.Kim YM, Barnes EA, Alvira CM, Ying L, Reddy S, Cornfield DN. Hypoxia-inducible factor-1α in pulmonary artery smooth muscle cells lowers vascular tone by decreasing myosin light chain phosphorylation. Circ Res. 2013;112:1230–1233. doi: 10.1161/CIRCRESAHA.112.300646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ball MK, Schumacker PT. HIF-1alpha in vascular smooth muscle regulated pulmonary hypertension in chronic hypoxia. Am J Respir Crit Care Med. 2013;187:A5390. [Google Scholar]
- 30.Ryan HE, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit JM, Johnson RS. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res. 2000;60:4010–4015. [PubMed] [Google Scholar]
- 31.Wirth A, Benyó Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horváth B, Maser-Gluth C, Greiner E, et al. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14:64–68. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- 32.Thibault HB, Kurtz B, Raher MJ, Shaik RS, Waxman A, Derumeaux G, Halpern EF, Bloch KD, Scherrer-Crosbie M. Noninvasive assessment of murine pulmonary arterial pressure: validation and application to models of pulmonary hypertension. Circ Cardiovasc Imaging. 2010;3:157–163. doi: 10.1161/CIRCIMAGING.109.887109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pacher P, Nagayama T, Mukhopadhyay P, Bátkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc. 2008;3:1422–1434. doi: 10.1038/nprot.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nisbet RE, Graves AS, Kleinhenz DJ, Rupnow HL, Reed AL, Fan TH, Mitchell PO, Sutliff RL, Hart CM. The role of NADPH oxidase in chronic intermittent hypoxia-induced pulmonary hypertension in mice. Am J Respir Cell Mol Biol. 2009;40:601–609. doi: 10.1165/rcmb.2008-0145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ochoa CD, Yu L, Al-Ansari E, Hales CA, Quinn DA. Thrombospondin-1 null mice are resistant to hypoxia-induced pulmonary hypertension. J Cardiothorac Surg. 2010;5:32. doi: 10.1186/1749-8090-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young KC, Hussein SM, Dadiz R, deMello D, Devia C, Hehre D, Suguihara C. Toll-like receptor 4-deficient mice are resistant to chronic hypoxia-induced pulmonary hypertension. Exp Lung Res. 2010;36:111–119. doi: 10.3109/01902140903171610. [DOI] [PubMed] [Google Scholar]
- 37.Taraseviciene-Stewart L, Nicolls MR, Kraskauskas D, Scerbavicius R, Burns N, Cool C, Wood K, Parr JE, Boackle SA, Voelkel NF. Absence of T cells confers increased pulmonary arterial hypertension and vascular remodeling. Am J Respir Crit Care Med. 2007;175:1280–1289. doi: 10.1164/rccm.200608-1189OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stenmark KR, Gerasimovskaya E, Nemenoff RA, Das M. Hypoxic activation of adventitial fibroblasts: role in vascular remodeling. Chest. 2002;122(Suppl 6):326S–334S. doi: 10.1378/chest.122.6_suppl.326s. [DOI] [PubMed] [Google Scholar]
- 39.Davie NJ, Gerasimovskaya EV, Hofmeister SE, Richman AP, Jones PL, Reeves JT, Stenmark KR. Pulmonary artery adventitial fibroblasts cooperate with vasa vasorum endothelial cells to regulate vasa vasorum neovascularization: a process mediated by hypoxia and endothelin-1. Am J Pathol. 2006;168:1793–1807. doi: 10.2353/ajpath.2006.050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rose F, Grimminger F, Appel J, Heller M, Pies V, Weissmann N, Fink L, Schmidt S, Krick S, Camenisch G, et al. Hypoxic pulmonary artery fibroblasts trigger proliferation of vascular smooth muscle cells: role of hypoxia-inducible transcription factors. FASEB J. 2002;16:1660–1661. doi: 10.1096/fj.02-0420fje. [DOI] [PubMed] [Google Scholar]
- 41.Hayashida K, Fujita J, Miyake Y, Kawada H, Ando K, Ogawa S, Fukuda K. Bone marrow-derived cells contribute to pulmonary vascular remodeling in hypoxia-induced pulmonary hypertension. Chest. 2005;127:1793–1798. doi: 10.1378/chest.127.5.1793. [DOI] [PubMed] [Google Scholar]
- 42.Hickey MM, Richardson T, Wang T, Mosqueira M, Arguiri E, Yu H, Yu QC, Solomides CC, Morrisey EE, Khurana TS, et al. The von Hippel-Lindau Chuvash mutation promotes pulmonary hypertension and fibrosis in mice. J Clin Invest. 2010;120:827–839. doi: 10.1172/JCI36362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hyvelin JM, Howell K, Nichol A, Costello CM, Preston RJ, McLoughlin P. Inhibition of Rho-kinase attenuates hypoxia-induced angiogenesis in the pulmonary circulation. Circ Res. 2005;97:185–191. doi: 10.1161/01.RES.0000174287.17953.83. [DOI] [PubMed] [Google Scholar]
- 44.Nagaoka T, Morio Y, Casanova N, Bauer N, Gebb S, McMurtry I, Oka M. Rho/Rho kinase signaling mediates increased basal pulmonary vascular tone in chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol. 2004;287:L665–L672. doi: 10.1152/ajplung.00050.2003. [DOI] [PubMed] [Google Scholar]
- 45.Bohuslavová R, Kolář F, Kuthanová L, Neckář J, Tichopád A, Pavlinkova G. Gene expression profiling of sex differences in HIF1-dependent adaptive cardiac responses to chronic hypoxia. J Appl Physiol (1985) 2010;109:1195–1202. doi: 10.1152/japplphysiol.00366.2010. [DOI] [PubMed] [Google Scholar]
- 46.Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, Ockaili R, McCord JM, Voelkel NF. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation. 2009;120:1951–1960. doi: 10.1161/CIRCULATIONAHA.109.883843. [DOI] [PubMed] [Google Scholar]
- 47.Fan C, Iacobas DA, Zhou D, Chen Q, Lai JK, Gavrialov O, Haddad GG. Gene expression and phenotypic characterization of mouse heart after chronic constant or intermittent hypoxia. Physiol Genomics. 2005;22:292–307. doi: 10.1152/physiolgenomics.00217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iacobas DA, Fan C, Iacobas S, Haddad GG. Integrated transcriptomic response to cardiac chronic hypoxia: translation regulators and response to stress in cell survival. Funct Integr Genomics. 2008;8:265–275. doi: 10.1007/s10142-008-0082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Faber MJ, Dalinghaus M, Lankhuizen IM, Steendijk P, Hop WC, Schoemaker RG, Duncker DJ, Lamers JM, Helbing WA. Right and left ventricular function after chronic pulmonary artery banding in rats assessed with biventricular pressure-volume loops. Am J Physiol Heart Circ Physiol. 2006;291:H1580–H1586. doi: 10.1152/ajpheart.00286.2006. [DOI] [PubMed] [Google Scholar]
- 50.Cruz JA, Bauer EM, Rodriguez AI, Gangopadhyay A, Zeineh NS, Wang Y, Shiva S, Champion HC, Bauer PM. Chronic hypoxia induces right heart failure in caveolin-1-/- mice. Am J Physiol Heart Circ Physiol. 2012;302:H2518–H2527. doi: 10.1152/ajpheart.01140.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J. 2001;15:427–438. doi: 10.1096/fj.00-0343com. [DOI] [PubMed] [Google Scholar]
- 52.Partovian C, Adnot S, Eddahibi S, Teiger E, Levame M, Dreyfus P, Raffestin B, Frelin C. Heart and lung VEGF mRNA expression in rats with monocrotaline- or hypoxia-induced pulmonary hypertension. Am J Physiol. 1998;275:H1948–H1956. doi: 10.1152/ajpheart.1998.275.6.H1948. [DOI] [PubMed] [Google Scholar]
- 53.Rawlins EL, Perl AK. The a “MAZE”ing world of lung-specific transgenic mice. Am J Respir Cell Mol Biol. 2012;46:269–282. doi: 10.1165/rcmb.2011-0372PS. [DOI] [PMC free article] [PubMed] [Google Scholar]