It is imperative that organisms sense and respond to changes in oxygen concentration for their survival. Hypoxia-inducible factor-1 (HIF-1) is the highly conserved global master regulator of oxygen homeostasis, and genes regulated at the transcriptional level by HIF-1 are involved in a multitude of processes, including angiogenesis, vascular reactivity and remodeling, vasomotor control, glucose and energy metabolism, erythropoiesis, and cell proliferation and viability (1). In the lung, elevated levels of HIF-1 are associated with poor prognosis for lung cancer (2), and stabilization of HIF-1 by hypoxia-induced reactive oxygen species derived from mitochondria has been shown to contribute to pulmonary fibrosis in patients with acute lung injury (3–5).
Chronic exposure to alveolar hypoxia results in sustained pulmonary vasoconstriction and pulmonary vascular remodeling leading to the development and progression of hypoxia-induced pulmonary hypertension (HPH). Patients with a variety of chronic lung diseases, such as chronic obstructive pulmonary disease, cystic fibrosis, bronchopulmonary dysplasia, and idiopathic pulmonary fibrosis, as well as residents living at high altitude experience persistent alveolar hypoxia. This leads to pulmonary hypertension, for which there are few clinical treatments available. Patients may eventually develop right ventricular hypertrophy leading to right heart failure and death. Therefore, understanding the molecular mechanisms underlying the development of HPH is critical for the improvement of therapeutic strategies.
In this issue of the Journal, Ball and colleagues (pp. 314–324) demonstrate the role of HIF-1α in the development of HPH using a smooth muscle–specific conditional HIF-1α knockout (HIF-1α-SMM-Cre) mouse (6). The authors identify two important concepts in this study. First, Ball and colleagues show that the right ventricular systolic pressure (RVSP) in HIF-1α-SMM-Cre mice exposed to hypoxia is significantly less than the RVSP in the hypoxic control mice. These results demonstrate that HIF-1α in the pulmonary arterial smooth muscle cells (PASMC) contributes to increased RVSP and pulmonary vascular remodeling induced by chronic hypoxia. This is consistent with previous studies using HIF-1α+/− mice that demonstrate that HIF-1α deficiency significantly delays the development of pulmonary vascular remodeling, pulmonary hypertension, and right ventricular failure in HIF-1α+/− mice exposed to chronic hypoxia in comparison to wild-type littermates (7, 8). The authors indicate that the attenuation of pulmonary hypertension may be due to decreased pulmonary vasoconstriction in response to hypoxia in the HIF-1α-SMM-Cre mice. In PASMC, HIF-1α is required for increased cell capacitance, membrane depolarization, decreased K+ current density, enhanced store-operated Ca2+ entry, up-regulated canonical transient receptor potential 1 (TRPC1) and TRPC6 expression, and augmented Na+/H+ exchanger expression in response to chronic hypoxia (7, 9, 10). A rise in cytosolic free Ca2+ concentration ([Ca2+]cyt) in PASMC is a major trigger for pulmonary vasoconstriction and an important stimulus for PASMC proliferation and migration, which contribute to pulmonary vascular remodeling. One possible explanation is that in hypoxia, HIF-1α causes membrane depolarization and up-regulates Ca2+ channel expression leading to increased pulmonary vascular remodeling (Figure 1). Whereas previous studies have used mouse models to demonstrate a general role for (or an association of ) HIF-1α, the current study identifies that HIF-1α is specifically required in PASMC for the development of HPH.
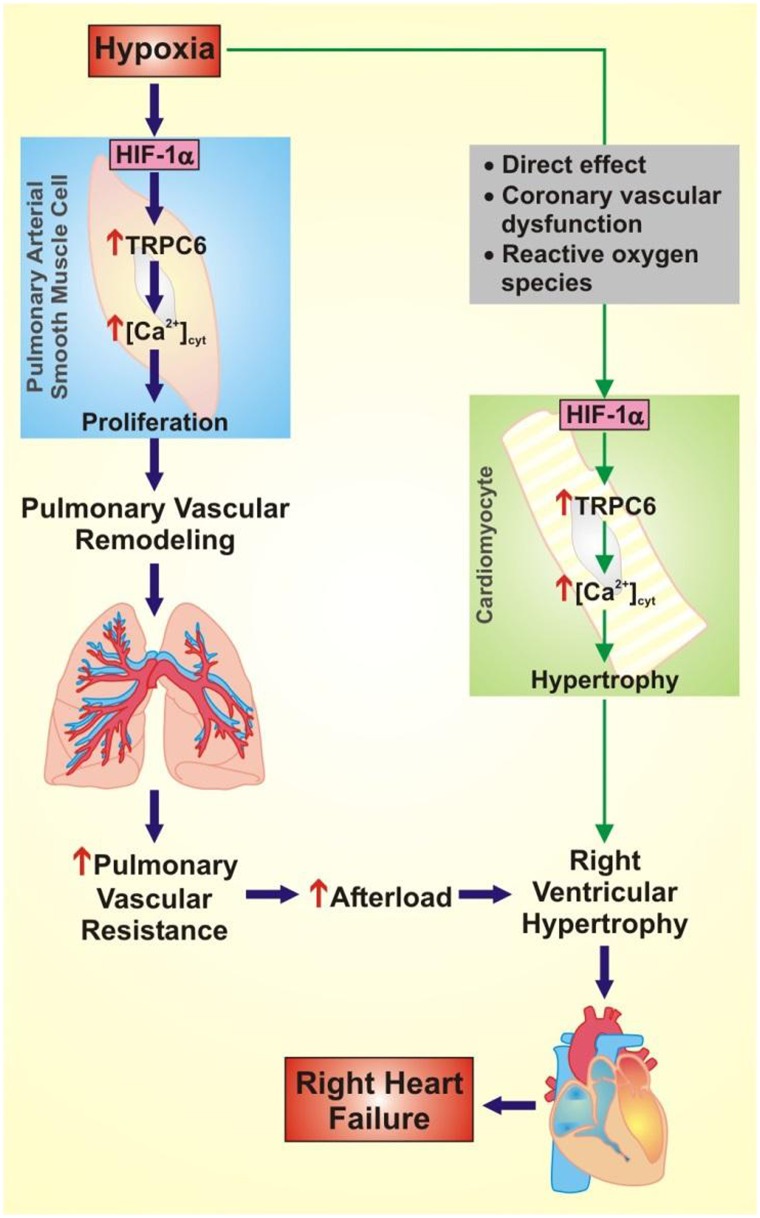
Figure 1.
Proposed role of hypoxia-induced hypoxia-inducible factor-1α (HIF-1α) in pulmonary hypertension and right heart failure. Hypoxia causes increased expression of HIF-1α in pulmonary arterial smooth muscle cells (PASMC), which results in increased expression of canonical transient receptor potential 6 (TRPC6), increased cytoplasmic free Ca2+ concentration ([Ca2+]cyt), and PASMC proliferation. Increased PASMC proliferation contributes to pulmonary vascular remodeling, increased pulmonary vascular resistance, and increased afterload on the right ventricle. In cardiomyocytes, the hypoxia-induced increase in HIF-1α may be a direct effect or due to coronary vascular dysfunction or reactive oxygen species. Increased expression of HIF-1α in cardiomyocytes results in increased expression of TRPC6, increased [Ca2+]cyt, and cardiomyocyte hypertrophy. Increased afterload and cardiomyocyte hypertrophy lead to right ventricular hypertrophy and right heart failure.
The second important finding of this study is that although deletion of HIF-1α in SMC attenuates the hypoxia-induced increase in RVSP, it does not attenuate right ventricular remodeling in response to chronic hypoxia. This finding is important and suggests that something other than pressure overload contributes to right ventricular remodeling in HPH. These data are consistent with a previous report demonstrating that increased pulmonary arterial pressure is insufficient to explain right heart failure (11). It is likely that, in addition to its many known effects, HIF-1α has a direct role in the response of cardiomyocytes to hypoxia (Figure 1). Overexpression of HIF-1α specifically in cardiomyocytes results in cardiomyopathy with contractile dysfunction resulting from reduced expression of the sarco(endo)plasmic reticulum Ca2+-ATPase (12). Additionally, hypoxia induces increased TRPC6 expression in cardiomyocytes via HIF-1α, and increased TRPC6 expression contributes to pathogenic cardiac remodeling (13, 14). There are several studies that suggest HIF-1α has cardioprotective effects in ischemic preconditioning, where exposure of the heart to short periods of ischemia and reperfusion increases HIF-1α and protects against further damage upon exposure to longer episodes of ischemic stress (15). However, studies using constitutive expression of HIF-1α in the heart suggest that the metabolic preconditioning induced by increased HIF-1α via increasing gene expression and glucose metabolism is beneficial initially; however, sustained HIF-1α expression may contribute to heart failure (16).
The current study by Ball and colleagues highlights the role of HIF-1α in PASMC in the development of HPH, and reveals that in HPH, increased pulmonary arterial pressure does not necessarily correlate with right ventricular remodeling. It would be interesting to determine if a similar concept can be applied to other forms of pulmonary hypertension, such as idiopathic pulmonary hypertension, where the initial trigger is unknown. Additionally, more studies are needed to determine the multiple molecular mechanisms by which hypoxia affects cardiomyocytes and cardiac function. The effect of hypoxia on cardiomyocytes may be direct, or it may be the result of coronary vascular dysfunction due to increased HIF-1α in the coronary arteries or the result of increased reactive oxygen species in the cardiomyocytes. This study indicates that hypoxia-induced factors other than increased afterload due to pulmonary vascular remodeling contribute to cardiac remodeling (e.g. TRPC6 and/or other HIF-1α sensitive proteins). Therefore, more studies are needed to provide a better understanding of the effects of hypoxia on cardiac remodeling in order to develop cardiac-specific therapies for the treatment of HPH.
Footnotes
This work was supported, in part, by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (HL066012 and HL098053).
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Maxwell PH, Ratcliffe PJ. Oxygen sensors and angiogenesis. Semin Cell Dev Biol. 2002;13:29–37. doi: 10.1006/scdb.2001.0287. [DOI] [PubMed] [Google Scholar]
- 2.Giatromanolaki A, Koukourakis MI, Sivridis E, Turley H, Talks K, Pezzella F, Gatter KC, Harris AL. Relation of hypoxia inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer. 2001;85:881–890. doi: 10.1054/bjoc.2001.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schroedl C, McClintock DS, Budinger GR, Chandel NS. Hypoxic but not anoxic stabilization of HIF-1alpha requires mitochondrial reactive oxygen species. Am J Physiol Lung Cell Mol Physiol. 2002;283:L922–L931. doi: 10.1152/ajplung.00014.2002. [DOI] [PubMed] [Google Scholar]
- 5.Zhou G, Dada LA, Wu M, Kelly A, Trejo H, Zhou Q, Varga J, Sznajder JI. Hypoxia-induced alveolar epithelial-mesenchymal transition requires mitochondrial ROS and hypoxia-inducible factor 1. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1120–L1130. doi: 10.1152/ajplung.00007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ball MK, Waypa GB, Mungai PT, Nielsen JM, Czech L, Dudley VJ, Beussink L, Dettman RW, Berkelhamer SK, Steinhorn RH, et al. Regulation of hypoxia-induced pulmonary hypertension by vascular smooth muscle HIF-1α. Am J Respir Crit Care Med. 2014;189:314–324. doi: 10.1164/rccm.201302-0302OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimoda LA, Manalo DJ, Sham JS, Semenza GL, Sylvester JT. Partial HIF-1alpha deficiency impairs pulmonary arterial myocyte electrophysiological responses to hypoxia. Am J Physiol Lung Cell Mol Physiol. 2001;281:L202–L208. doi: 10.1152/ajplung.2001.281.1.L202. [DOI] [PubMed] [Google Scholar]
- 8.Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, et al. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest. 1999;103:691–696. doi: 10.1172/JCI5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimoda LA, Fallon M, Pisarcik S, Wang J, Semenza GL. HIF-1 regulates hypoxic induction of NHE1 expression and alkalinization of intracellular pH in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol. 2006;291:L941–L949. doi: 10.1152/ajplung.00528.2005. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res. 2006;98:1528–1537. doi: 10.1161/01.RES.0000227551.68124.98. [DOI] [PubMed] [Google Scholar]
- 11.Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, Ockaili R, McCord JM, Voelkel NF. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation. 2009;120:1951–1960. doi: 10.1161/CIRCULATIONAHA.109.883843. [DOI] [PubMed] [Google Scholar]
- 12.Bekeredjian R, Walton CB, MacCannell KA, Ecker J, Kruse F, Outten JT, Sutcliffe D, Gerard RD, Bruick RK, Shohet RV. Conditional HIF-1alpha expression produces a reversible cardiomyopathy. PLoS ONE. 2010;5:e11693. doi: 10.1371/journal.pone.0011693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu W, Wan L, Zhao D, Qu X, Cai F, Huo R, Wang N, Zhu J, Zhang C, Zheng F, et al. Mild hypoxia-induced cardiomyocyte hypertrophy via up-regulation of HIF-1α-mediated TRPC signalling. J Cell Mol Med. 2012;16:2022–2034. doi: 10.1111/j.1582-4934.2011.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, Hill JA, Olson EN. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest. 2006;116:3114–3126. doi: 10.1172/JCI27702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu J, Chen P, Li Y, Ardell C, Der T, Shohet R, Chen M, Wright GL. HIF-1α in heart: protective mechanisms. Am J Physiol Heart Circ Physiol. 2013;305:H821–H828. doi: 10.1152/ajpheart.00140.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hölscher M, Schäfer K, Krull S, Farhat K, Hesse A, Silter M, Lin Y, Pichler BJ, Thistlethwaite P, El-Armouche A, et al. Unfavourable consequences of chronic cardiac HIF-1α stabilization. Cardiovasc Res. 2012;94:77–86. doi: 10.1093/cvr/cvs014. [DOI] [PubMed] [Google Scholar]