With the rising tide of obesity worldwide, clinicians and researchers alike find themselves struggling to understand not only what this means to human health, but also how we should best analyze and characterize weight gain (or loss) itself. Obesity as a disease modifier in vascular disease and diabetes has often been simplified to the concept of “risk factor”; yet, as we are learning in lung disease, our understanding must be far more nuanced to capture the complex effects associated with weight change. This is highlighted by the growing number of clinical “paradoxes” associated with obesity in which weight gain appears to confer not only health risks but health benefits, often in an unpredictable fashion (1). The issues that frame these paradoxes typically reflect our inability to explain these divergent associations mechanistically.
Traditionally, our understanding of weight gain and its effects has centered on the concept of obesity as an inflammatory disease (2). However, although useful in the context of vascular disease, this concept often fails to predict the effects of changes in body mass index (BMI) on the lung and lung disease. For example, recent reports have demonstrated that in acute lung injury, obese patients have lower levels of circulating inflammatory cytokines (3) (and improved outcomes [4]) compared with lean patients, and in obese individuals with asthma, surgically induced weight loss is accompanied by evidence of increased airway inflammation (5, 6). These findings speak to a far more complicated relationship between weight change and lung function than can be ascribed solely to proinflammatory effects or even, for that matter, to immune modulation at all, and growing evidence suggests that an interplay of adipokine disarray (6–8), mechanical perturbations (9), and changes in muscle mass (10, 11) is likely to also influence lung disease and its manifestations. In this wider context, let us consider the interesting study by Sood and colleagues (pp. 274–281) in this issue of the Journal (12).
These investigators report an examination of 1,641 current and former smokers at risk for or with mild to moderate chronic obstructive pulmonary disease (COPD), followed for a median of 6 years as part of the Lovelace Smokers’ Cohort in New Mexico, and present both cross-sectional and longitudinal data relating subjects’ BMI to spirometric variables and health status (as assessed by the St. George Respiratory Questionnaire [SGRQ]). Stratifying their cohort by World Health Organization BMI category, they found that at baseline, higher BMI in obese (BMI > 30 kg/m2) smokers was associated with worse health status and lower FEV1, yet in the lean (BMI < 25 kg/m2) smokers, the association between BMI and these variables was reversed. The authors report that these findings delineate a “U-shaped” association between BMI and the extreme weight categories, such that both the morbidly obese and the lean to underweight subsets of their cohort had the lowest FEV1 and worst health status. Even more interestingly, in longitudinal analyses, they found that these opposing relationships appear to be real since they applied to weight gain over the duration of the study as well: rising BMI was associated with worsening health status and falling FEV1 in obese smokers, yet improvement in these outcomes in lean smokers. Interestingly, the authors found no association between IL-6, IL-10, or leptin and outcomes. Do these findings present yet another “paradox” or do they underscore a complex dichotomy between BMI, lung function, and respiratory symptoms in smokers?
Given the methods of analysis, these findings might be more intuitively understood as follows: weight loss is better tolerated by the obese than the lean in their cohort. The authors attempt to dissect weight gain from weight loss in their supplemental data (Table E2), which reveal roughly equal numbers of subjects gaining and losing weight within each BMI category. In the obese cohort, both weight gain and weight loss are strongly associated with inverse changes in FEV1 and SGRQ. However, in the lean cohort, although SGRQ appears similarly bidirectional in it association, only weight loss is significantly associated with FEV1, and in a markedly downward manner. These findings suggest that in smokers without severe COPD, weight change signifies something quite different in lean versus obese patients. But what?
To answer that question, let’s consider the contrast between the Lovelace Smokers’ cohort findings and those of The Copenhagen City Heart Study (13). The latter study found that weight loss was associated with increased COPD-related mortality across the BMI spectrum of their cohort, whereas weight gain was protective. Further examination of this cohort and others suggests that much of this effect may be related to low or decreasing fat-free body mass—presumed here to indicate sarcopenia (10, 14, 15). Further studies by Marquis and colleagues (16) and Rutten and colleagues (11) have gone further to prove this association. It is understandable that loss of muscle mass as a consequence of worsening COPD, inflammation, poor nutrition, or other comorbidities might compromise pulmonary physiology and overall health status. The same effect is seen with falling BMI in the lean (BMI < 25) subset of the Lovelace cohort. How does this explain the findings in the rest of this cohort?
Here it is worth noting that in the previously cited studies, mean BMIs of the cohorts were in the range of 24–25, whereas in the Lovelace cohort, the mean was nearly 28. Furthermore, unlike previous studies, the Lovelace cohort is predominantly women (>70%). Although the ratio of fat mass (FM) to fat-free mass (FFM) generally rises with BMI, it is well known that both sex and age significantly affect FM/FFM balance. For any given BMI, women have a significantly higher percentage of FM compared with men (Figure 1), and FM/FFM ratio rises with age for both sexes (17). Thus, the differences seen between overall BMI/outcome associations in the current study compared with previous studies, including the Copenhagen, are likely to reflect the effects of greater FM on the higher BMI subsets. What are these effects?
Figure 1.
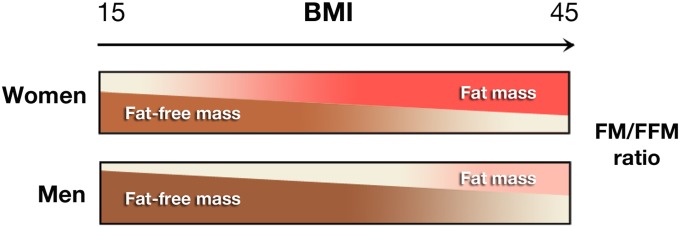
Percentages of total body mass from fat mass (FM) and fat-free mass (FFM) in males and females across the body mass index (BMI) range. Data shown are for subjects 45 years of age (16).
In this study, obese smokers had a markedly lower incidence of Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria–defined COPD (17% vs. 35% in lean smokers) despite having a higher incidence of wheeze and dyspnea and a nearly 20% rate of bronchodilator response at baseline. Although this may in part reflect a notable failure of the GOLD criteria to capture early COPD in the obese (likely due to obesity-driven elevation of the FEV1/FVC ratio), it may also suggest the presence of an additional, discrete syndrome in the obese (female) smoker. This is underscored by the salutary effects of weight loss in this subset—a distinctly different finding than reported for subjects in the Copenhagen Study and others. As Sood and others have shown previously, weight gain and obesity are associated with an increased incidence of airway obstruction and wheezing in the general population, particularly among women, irrespective of smoking status (18, 19), whereas weight loss improves these symptoms, particularly in the nonatopic obese (5). Although generally described as “patients with asthma,” this group of patients has recently been recognized as a distinct phenotype within this syndrome (20). The current work therefore begs the question, what is this form of obese obstructive lung disease that variously appears as difficult-to-treat asthma or the dyspneic, wheezing smoker? As the authors find no association between these findings and a (limited) panel of cytokines and adipokines, it is tempting to invoke the mechanical effects of obesity in this patient subset that hopefully future studies will delineate. The only thing we know for sure is that smoking cessation and weight loss are likely the most effective (and only?) treatments for this rapidly growing population of patients.
Footnotes
This work was supported by grants R01 HL084200 and NCRR P20RR15557 from the National Institutes of Health.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Lainscak M, von Haehling S, Doehner W, Anker SD. The obesity paradox in chronic disease: facts and numbers. J Cachexia Sarcopenia Muscle. 2012;3:1–4. doi: 10.1007/s13539-012-0059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karalis KP, Giannogonas P, Kodela E, Koutmani Y, Zoumakis M, Teli T. Mechanisms of obesity and related pathology: linking immune responses to metabolic stress. FEBS J. 2009;276:5747–5754. doi: 10.1111/j.1742-4658.2009.07304.x. [DOI] [PubMed] [Google Scholar]
- 3.Stapleton RD, Dixon AE, Parsons PE, Ware LB, Suratt BT NHLBI Acute Respiratory Distress Syndrome Network. The association between BMI and plasma cytokine levels in patients with acute lung injury. Chest. 2010;138:568–577. doi: 10.1378/chest.10-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martino JL, Stapleton RD, Wang M, Day AG, Cahill NE, Dixon AE, Suratt BT, Heyland DK. Extreme obesity and outcomes in critically ill patients. Chest. 2011;140:1198–1206. doi: 10.1378/chest.10-3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker-Leclair LA, Griffes LA, Garudathri J, Raymond D, Poynter ME, Bunn JY, Irvin CG.Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation J Allergy Clin Immunol 2011128508–515.e1–e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sideleva O, Suratt BT, Black KE, Tharp WG, Pratley RE, Forgione P, Dienz O, Irvin CG, Dixon AE. Obesity and asthma: an inflammatory disease of adipose tissue not the airway. Am J Respir Crit Care Med. 2012;186:598–605. doi: 10.1164/rccm.201203-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breyer MK, Rutten EP, Locantore NW, Watkins ML, Miller BE, Wouters EF ECLIPSE Investigators (Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints) Dysregulated adipokine metabolism in chronic obstructive pulmonary disease. Eur J Clin Invest. 2012;42:983–991. doi: 10.1111/j.1365-2362.2012.02686.x. [DOI] [PubMed] [Google Scholar]
- 8.Lugogo NL, Hollingsworth JW, Howell DL, Que LG, Francisco D, Church TD, Potts-Kant EN, Ingram JL, Wang Y, Jung SH, et al. Alveolar macrophages from overweight/obese subjects with asthma demonstrate a proinflammatory phenotype. Am J Respir Crit Care Med. 2012;186:404–411. doi: 10.1164/rccm.201109-1671OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Salome CM, King GG, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol (1985) 2010;108:206–211. doi: 10.1152/japplphysiol.00694.2009. [DOI] [PubMed] [Google Scholar]
- 10.Vestbo J, Prescott E, Almdal T, Dahl M, Nordestgaard BG, Andersen T, Sørensen TI, Lange P. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med. 2006;173:79–83. doi: 10.1164/rccm.200506-969OC. [DOI] [PubMed] [Google Scholar]
- 11.Rutten EP, Calverley PM, Casaburi R, Agusti A, Bakke P, Celli B, Coxson HO, Crim C, Lomas DA, Macnee W, et al. Changes in body composition in patients with chronic obstructive pulmonary disease: do they influence patient-related outcomes? Ann Nutr Metab. 2013;63:239–247. doi: 10.1159/000353211. [DOI] [PubMed] [Google Scholar]
- 12.Sood A, Petersen H, Meek P, Tesfaigzi Y. Spirometry and health status worsen with weight gain in obese but improve in normal-weight smokers. Am J Respir Crit Care Med. 2014;189:274–281. doi: 10.1164/rccm.201306-1060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prescott E, Almdal T, Mikkelsen KL, Tofteng CL, Vestbo J, Lange P. Prognostic value of weight change in chronic obstructive pulmonary disease: results from the Copenhagen City Heart Study. Eur Respir J. 2002;20:539–544. doi: 10.1183/09031936.02.00532002. [DOI] [PubMed] [Google Scholar]
- 14.Hopkinson NS, Tennant RC, Dayer MJ, Swallow EB, Hansel TT, Moxham J, Polkey MI. A prospective study of decline in fat free mass and skeletal muscle strength in chronic obstructive pulmonary disease. Respir Res. 2007;8:25. doi: 10.1186/1465-9921-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ischaki E, Papatheodorou G, Gaki E, Papa I, Koulouris N, Loukides S. Body mass and fat-free mass indices in COPD: relation with variables expressing disease severity. Chest. 2007;132:164–169. doi: 10.1378/chest.06-2789. [DOI] [PubMed] [Google Scholar]
- 16.Marquis K, Debigaré R, Lacasse Y, LeBlanc P, Jobin J, Carrier G, Maltais F. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:809–813. doi: 10.1164/rccm.2107031. [DOI] [PubMed] [Google Scholar]
- 17.Meeuwsen S, Horgan GW, Elia M. The relationship between BMI and percent body fat, measured by bioelectrical impedance, in a large adult sample is curvilinear and influenced by age and sex. Clin Nutr. 2010;29:560–566. doi: 10.1016/j.clnu.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661–666. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Assad N, Qualls C, Smith LJ, Arynchyn A, Thyagarajan B, Schuyler M, Jacobs DR, Jr, Sood A. Body mass index is a stronger predictor than the metabolic syndrome for future asthma in women. The longitudinal CARDIA study. Am J Respir Crit Care Med. 2013;188:319–326. doi: 10.1164/rccm.201303-0457OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, Wardlaw AJ, Green RH. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178:218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


