Abstract
Objective
Apelin and its cognate receptor Aplnr/Apj are essential for diverse biological processes. However, the function of Apelin signaling in lymphatic development remains to be identified, despite the preferential expression of Apelin and Aplnr within developing blood (BECs) and lymphatic endothelial cells (LECs) in vertebrates. In this report, we aim to delineate the functions of Apelin signaling during lymphatic development.
Approaches and Results
We investigated the functions of Apelin signaling during lymphatic development using zebrafish embryos, and found that attenuation of Apelin signaling substantially decreased the formation of the parachordal vessel (PaCV) and the number of LECs within the developing thoracic duct, indicating an essential role of Apelin signaling during the early phase of lymphatic development. Mechanistically, we found that abrogation of Apelin signaling selectively attenuates lymphatic endothelial AKT1/2 phosphorylation without affecting the phosphorylation status of ERK1/2. Moreover, lymphatic abnormalities caused by the reduction of Apelin signaling were significantly exacerbated by the concomitant partial inhibition of AKT signaling. Apelin and Vascular Endothelial Growth Factor-C (VEGF-C) signaling provide a non-redundant activation of AKT during lymphatic development, as over-expression of VEGF-C or apelin was unable to rescue the lymphatic defects caused by the lack of Apelin or VEGF-C, respectively.
Conclusions
Taken together, our data present compelling evidence suggesting that Apelin signaling regulates lymphatic development by promoting AKT activity in a VEGF-C/VEGFR3 independent manner during zebrafish embryogenesis.
Keywords: Apelin signaling, Lymphatic development, AKT, Zebrafish
INTRODUCTION
Lymphatic vessels have essential roles in maintaining the homeostasis of interstitial body fluid and facilitating immune responses in vertebrates 1–3. In addition, lymphatic vessels have been associated with progression of diverse diseases in humans, including tumor metastasis and obesity 4, 5. Malformation or obstruction of lymphatic vessels cause an accumulation of interstitial fluid resulting in the swelling of extremities, pathological conditions collectively categorized as lymphedema, which affect more than 140 million people worldwide 6–9. During development, lymphatic endothelial cells (LECs), the main component of lymphatic vessels, first appear around E10 in mice, embryonic week 6 to 7 in humans, and 3 days post-fertilization (dpf) in zebrafish 10–13. Lineage tracing and in vivo time lapse analyses in mouse and zebrafish support the model proposed by Florence Sabin, that the LECs may originate from endothelial cells (ECs) in the cardinal vein 12, 14, 15.
Apelin and its receptor Aplnr/Apj regulate a wide range of developmental and physiological processes 16–19. While Apelin is widely expressed during development, the expression of Aplnr/Apj is more restricted 16. In mouse, Apj is strongly expressed within the cardiovascular system as early as E8.0 20. Similarly, aplnra and b are specifically expressed in developing ECs and cardiomyocytes in zebrafish 17, 19. Consistent with the expression pattern, Apj knockout mice display various cardiovascular defects and are embryonic lethal in certain genetic background 20–22. Zebrafish have two Apelin receptors, aplnra and aplnrb 17, 19. While aplnra and b are broadly expressed in areas including heart primordial cells and lateral plate mesoderm during early development, their expression gradually become restricted to blood vessel in late development 19, 23. After 24 hours post-fertilization (hpf), only venous endothelial cells in the trunk region express detectable levels of aplnra 23, suggesting that Apelin signaling may be essential for blood (BECs) and lymphatic endothelial cells (LECs). Mutations in aplnrb cause similar developmental defects in zebrafish 17, 19. Despite its proposed role in cardiovascular development, current analyses on Apelin signaling predominantly aim to address its role in vascular physiology 16, 24–26, and consequently, the function of Apelin signaling in the development of lymphatic vessels remains largely unknown.
In this report, we examined the function of Apelin signaling in developing LECs, and found that Apelin signaling provides a non-redundant function to induce AKT activation, therefore, serving as an essential signaling input to promote lymphatic development. Abrogation of Apelin signaling caused severe defects of the lymphatic structure in zebrafish embryos by substantially decreasing the phosphorylation of AKT1/2, independent of VEGF-C signaling. Our data presented here support the idea that Apelin signaling is essential for proper lymphatic development.
Materials and Methods
Please see supplemental file.
RESULTS
Apelin signaling modulates the proper lymphatic vessel formation during zebrafish development
To delineate the roles of Apelin signaling in vascular development of zebrafish, we first attenuated the level of Aplnra by morpholino (MO) mediated knock-down of aplnra (Figure IA-D in the online-only Data Supplement). Since proper circulation is essential for lymphatic development, a suboptimal dose of aplnra MO was injected (1.8ng/embryos) to avoid the early cardiac abnormalities that have been previously reported 17, 19 and assess the role of Apelin signaling specifically in lymphatic development (Fig. S1D). The general morphology and the rate of heartbeat at 4dpf in embryos injected with 1.8ng of aplnra MO were comparable to those of control MO-injected embryos (Fig. 1A, Figure IB and IC in the online-only Data Supplement). In addition, patterning of inter-segmental vessels (ISVs), the dorsal aorta, and the cardinal vein were not affected in 4dpf embryos injected with 1.8ng of aplnra MO (Fig. 1A).
Fig. 1. Lack of aplnra activity causes lymphatic vessel defects.
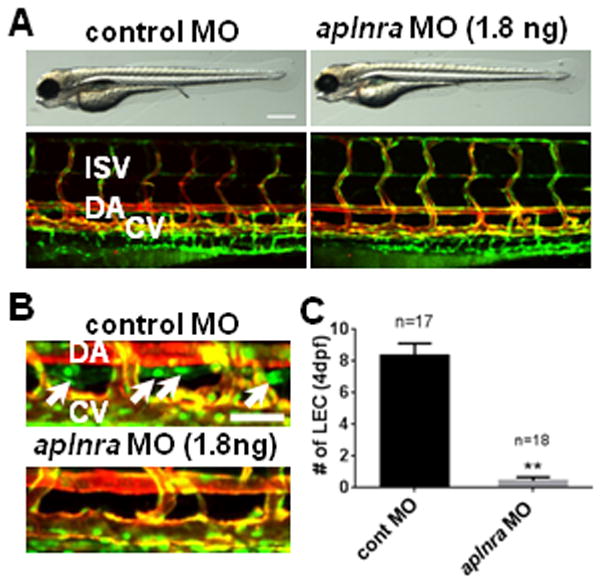
(A) Gross morphology (top panels) and vascular structures (bottom panels) in 4dpf aplnra MO-injected embryos. (B) The loss of LECs in thoracic duct (white arrows) in 4dpf aplnra MO-injected embryos. (C) Quantification of the number of LECs in control and aplnra MO-injected embryos. LECs within the thoracic duct between 8th and 15th somites were counted. All embryos shown have Tg(fli1a:nEGFP);Tg(kdrl:mCherry) double transgenic background to visualize BECs (shown as yellow) and LECs (shown as green). DA, dorsal aorta; CV, cardinal vein; ISV, inter-segmental vessel. Scale bars are 400μm (A) and 50μm (B).
Although the vasculature was unaffected in aplnra MO-injected embryos, these embryos did contain profound defects in lymphatic vessels (Fig. 1B and 1C). To visualize developing LECs, we utilized Tg(fli1a:nEGFP);Tg(kdrl:mCherry) double transgenic lines, which contain nEGFP+/mCherry+ BECs (appearing as yellow in the merged figures) and nEGFP+/mCherry− LECs. While the developing thoracic duct, located between the dorsal aorta and the cardinal vein, are clearly visible in 4dpf control MO-injected embryos, similar structures were absent in aplnra MO-injected embryos (Fig. 1B). In addition, the number of nEGFP+/mCherry− LECs within the thoracic duct in aplnra MO-injected embryos was greatly decreased (Fig. 1C), suggesting that LECs are more sensitive to attenuated level of Apelin/Aplnr signaling activity. Similarly, the number of VEGFR3+ LECs in the diaphragm of Apj−/− mice was significantly reduced (Figure IE and IF in the online-only Data Supplement), suggesting that the role of Apelin/Aplnr signaling on lymphatic development may be conserved within vertebrate species.
We next determined whether the attenuation of the cognate ligand of Aplnr, Apelin, would cause comparable defects in lymphatic development. To ensure that the lymphatic defects found in apln MO-injected embryos are not secondary to the previously reported cardiac defects 17, 19, the amount of MO was titrated. Injection of 2.7 or 5.4ng per embryo effectively blocked the normal splicing of apln transcript (Figure IIA in the online-only Data Supplement) without causing obvious morphological defects in axis formation, rate of heartbeat, or blood vessel formation (Figure IIB and IIC in the online-only Data Supplement). In contrast, the formation of parachordal vessels (arrows; PaCV) which give rise to the presumptive LECs, were severely affected by partial knock-down of apln (Fig. 2A and 2B). At 3dpf, embryos injected with 2.7ng of apln MO completely lacked PaCV. Consistent with the defects in PaCVs, we found that the number of LECs in 4dpf apln MO-injected embryos was decreased in a dose-dependent manner (Fig. 2C and 2D). Consistent with these findings, micro-lymphangiography demonstrated that embryos with a reduced level of Apelin signaling do not develop proper lymphatic vessels (Figure III in the online-only Data Supplement).
Fig. 2. Apelin is required for zebrafish lymphatic vessel formation.
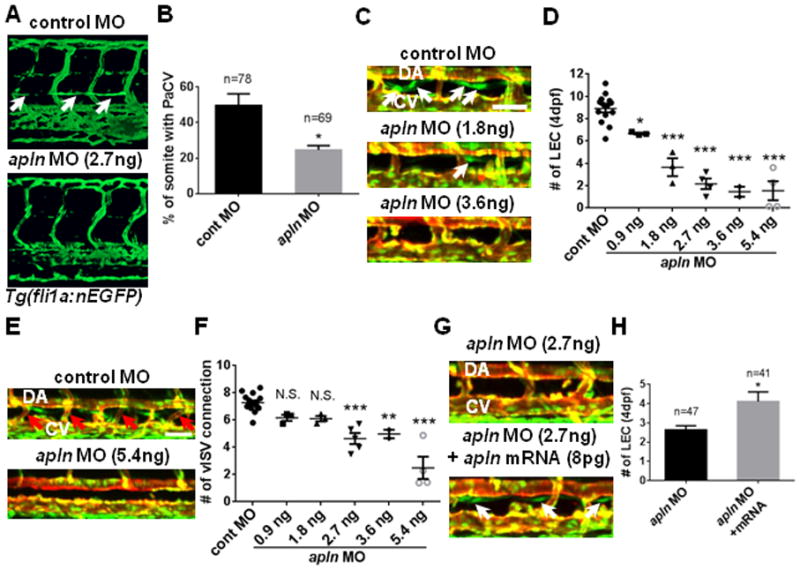
(A) The loss of parachordal vessel (white arrows) in 3dpf apln MO-injected embryos in Tg(fli1a:nEGFP) transgenic background. (B) Quantification of the formation of parachordal vessel (PaCV) at 3dpf. (C) Representative images of 4dpf apln MO-injected embryos in the trunk region. White arrows point to LECs within the thoracic duct. The deficit in the number of LECs progressively worsened as the dose of apln MO increases. (D) Quantification of the number of LECs in control and apln MO-injected embryos at 4dpf. LECs located between 8th and 15th somites were counted. Each dot represents an individual set of experiments. Total number of embryos are; control MO=96, apln MO 0.9ng=26, 1.8ng=30, 2.7ng=37, 3.6ng=19, and 5.4ng=29. (E) The loss of venous ISVs (red arrows; vISV) in 4dpf embryos injected with a high dose of apln MO. (F) Quantification of the number of vISVs in control and apln MO-injected embryos. Each dot represents an individual set of experiments. Total number of embryos are; cont MO=103, apln MO 0.9ng=26, 1.8ng=30, 2.7ng=48, 3.6ng=19, and 5.4ng=27. (G) Over-expression of synthetic apln mRNA can rescue lymphatic defects in apln MO-injected embryos. White arrows point to LECs in embryos co-injected with synthetic apln mRNA and apln MO. (H) Quantification of the number of LECs at 4dpf in embryos injected with apln MO alone and in embryos co-injected with apln MO and synthetic apln mRNA. DA, dorsal aorta; CV, cardinal vein. Scale bars are 50μm.
Since LECs emerge from venous ISVs 12, it is possible that a decrease in Apelin signaling activity may limit the number of forming venous ISVs and indirectly influence the number of LECs. To evaluate this possibility, we examined the effects of Apelin signaling on the formation of venous ISVs by counting the number of ISVs that were directly connected to the cardinal vein (venous ISV connections) at 4dpf. While the number of LECs was substantially decreased by injecting a low concentration of apln MO (0.9 and 1.8ng/embryo), the number of venous ISV connections was largely unaffected (Fig. 2E and 2F), suggesting that Apelin signaling is likely to directly regulate lymphatic development in zebrafish embryos.
To further confirm that the lymphatic defects in apln MO injected embryos is directly caused by the attenuation of Apelin signaling, we attempted a phenotypic rescue of apln MO-injected embryos by injecting synthetic apln mRNA. Since apln mRNA injection can cause cardiac defects 17, the amount of mRNA was titrated. While a high dose of apln mRNA caused severe cardiac defects, injection of 8pg apln mRNA per embryo did not cause any phenotypic defects (Figure IVA and IVB in the online-only Data Supplement). When co-injected, 8pg apln mRNA successfully restored the number of LECs in apln MO injected embryos (Fig. 2G and 2H), suggesting that the reduction in the number of LECs in apln MO injected embryos was not a secondary effect of general developmental delays caused by MO injection. Taken together, our data suggest that proper activity of Apelin/Aplnra signaling is essential for lymphatic development in zebrafish embryos.
Apelin signaling modulates AKT activity in human and zebrafish LECs
It has been reported that Apelin promotes the migration of BECs and LECs in cell culture 25. Consistent with the previous report, knockdown of APLNR in human LECs (hLECs) adversely affected the migration of human LECs (hLECs). In a scratch wound assay, APLNR siRNA-treated hLECs displayed a significantly attenuated migratory behavior compared to control siRNA-treated hLECs (Fig. 3A and B). The migration defect of APLNR siRNA-treated hLECs was likely caused by a reduced level of Apelin signaling since addition of exogenous Apelin 13 was not able to alleviate the migration defects in APLNR siRNA-treated hLECs (Fig. 3A and B).
Fig. 3. Apelin signaling is essential for hLEC migration and maintenance of phospho-AKT1/2.
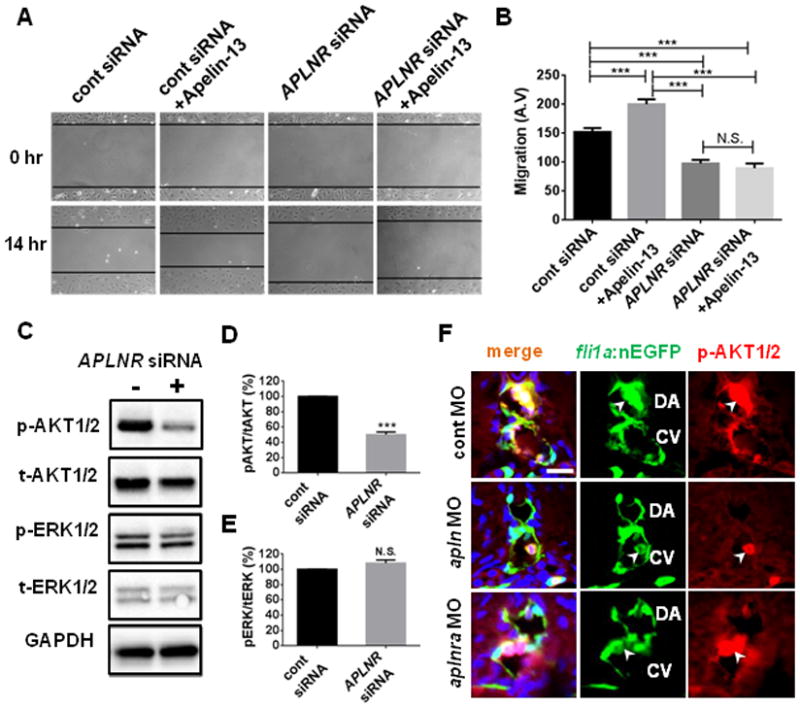
(A) Representative images of scratch wound healing assay using human LEC (hLEC). Black lines demarcate the boundaries of scratch wounds. The recovery of the wound was substantially attenuated in APLNR siRNA-treated hLECs compared to control siRNA-treated hLECs. (B) Quantification of the migration of hLECs. (C) Lack of Apelin signaling selectively attenuated the level of phospho-AKT1/2 in hLECs grown in complete growth medium 3 days after siRNA treatment. Experiments were performed as triplicates. (D) Quantification of the relative levels of phospho-AKT1/2 and total AKT in control or APLNR siRNA-treated hLECs. (E) Quantification of the levels of phospho-ERK1/2 and total ERK1/2 in control or APLNR siRNA-treated hLECs. (F) Confocal image showing a transverse section of 48hpf control MO (top), apln MO (middle), or aplnra MO (bottom) injected Tg(fli1a:nGFP) embryos. Developing endothelial cells (EC) within DA and CV contain a high level of phospho-AKT1/2 (arrowheads). Lack of Apelin signaling selectively abrogated the presence of phospho-AKT1/2 in ECs without affecting the phospho-AKT1/2 in nearby blood cells (arrowhead). Scale bar is 10μm.
To identify the downstream effectors of Apelin signaling in LECs, we examined the level of phospho-AKT1/2 and phospho-ERK1/2 in APLNR siRNA-treated hLECs (Fig. 3C). Since it has been reported that ERK1/2 and AKT, which function as downstream effectors for several G-protein coupled receptors including APLNR 16, 27, 28, are essential for modulating the development of lymphatic vessels 1, 3, it is tempting to speculate that ERK1/2 and AKT may be involved downstream of Apelin signaling during lymphatic development. While the level of phospho-ERK1/2 did not change in APLNR siRNA-treated hLECs compared to control siRNA-treated cells, the level of phospho-AKT1/2 was significantly decreased upon APLNR depravation (Fig. 3C–3E). Moreover, stimulation with Apelin ligand in hLECs led to an increased level of phospho-AKT1/2 in a dose dependent manner (Figure V in the online-only Data Supplement). Similarly, attenuation of Apelin signaling by MO injection drastically reduced the level of phospho-AKT1/2 in developing zebrafish. While phospho-AKT1/2 is strongly detected within ECs in the dorsal aorta and cardinal vein of 48hpf control MO-injected embryos, it is largely absent in apln MO-injected embryos (Fig. 3F). Therefore, it appears that Apelin signaling may function as a major stimulus for AKT1/2 phosphorylation in both cell culture and in vivo. Previously, Aplnrs have been reported to function independent of Apelin ligand 18. Therefore, we also examined the level of phospho-AKT1/2 in apln MO-injected embryos, and found that phospho-AKT1/2 was substantially reduced in these embryos, suggesting that phosphorylation of AKT1/2 is dependent on Apelin/Aplnr signaling (Fig. 3F).
Apelin and AKT activity functionally cooperate for zebrafish lymphatic vessel formation
To further substantiate the link between AKT activity and Apelin signaling in LECs, we manipulated the level of AKT activity in zebrafish embryos and examined the effects on developing LECs. Since manipulation of AKT activity at earlier developmental stages may compromise the specification of arterial and venous ECs 29–31, we utilized previously reported chemical antagonists of AKT to induce temporal AKT inhibition. Treatment with either 10μM LY294002 (Phosphoinositide 3-Kinase (PI3K) inhibitor) 32 or 2μM Torin1 (mTORC1/2 inhibitor) 33, both of which inhibit AKT phosphorylation, drastically reduced the number of LECs in 4dpf zebrafish embryos (Fig. 4A–4D). Moreover, the lymphatic defects caused by a partial reduction of Apelin signaling activity was significantly exacerbated by administering a suboptimal dose of the aforementioned chemical antagonists of AKT (Fig. 4E–4G). While embryos injected with 0.9ng of apln MO or treated with 5μM of LY294002 contained an average of 5.6±0.65 and 6.2±0.79 LECs respectively, embryos that were injected with 0.9ng of apln MO and treated with 5μM of LY294002 had significantly fewer LECs (3.8±1.11), representing a further ~40% reduction the in number of LECs compared to single manipulations (Fig. 4E and 4F). Similar effects were also observed in embryos that were injected with 0.9ng of apln MO and treated with 1μM of Torin1 (~40% reduction; Fig. 4E and 4G).
Fig. 4. Apelin and AKT cooperate to promote lymphatic development in zebrafish.
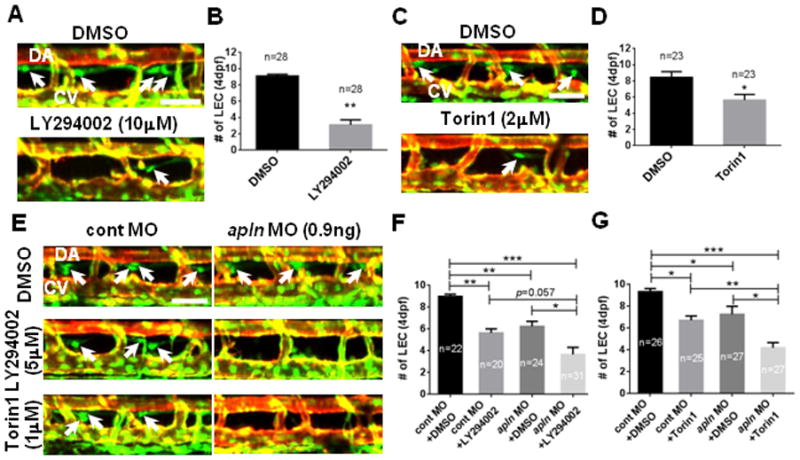
(A) The number of LECs (white arrows) was substantially decreased in embryos treated with a chemical inhibitor of PI3K (LY294002) between 48hpf and 4dpf, therefore, with an attenuated level of AKT activity. (B) Quantification of the number of LECs in 4dpf DMSO or 10μM LY294002 treated embryos. (C) The number of LECs (white arrows) in embryos treated with Torin1 between 48hpf and 4dpf, a chemical antagonist against another upstream regulator of AKT, mTORC1/2, was significantly decreased. (D) Quantification of the number of LECs in 4dpf DMSO or 2μM Torin1 treated embryos. (E) Apelin and AKT may function within the same pathway. Treating embryos injected with a low dose of apln MO with a suboptimal dose of LY294002 (5μM) or Torin1 (1μM) exacerbated the effects of apln MO on the number of LECs (arrows). (F) Quantification of the number of LECs in control or apln MO injected embryos treated with either DMSO or LY294002. (G) Quantification of the number of LECs in control or apln MO injected embryos treated with either DMSO or Torin1. DA, dorsal aorta; CV, cardinal vein. Scale bars are 50μm.
Apelin and VEGF-C signal independently in zebrafish lymphatic development
Previously, it has been reported that AKT activity within ECs can be induced by VEGF-C signaling 34–36, which is the key stimulus for lymphatic development 37, 38. We next tested whether Apelin and VEGF-C signaling converge at the level of AKT or function redundantly to promote differentiation and/or maintenance of LECs. Attenuation of Apelin signaling did not influence the expression of VEGF-C signaling components, including expression of vegfc or its receptor flt4 (Figure VIA in the online-only Data Supplement). Similarly, inhibition of VEGF-C signaling activity did not affect the expression of apln, aplnra, or aplnrb (Figure VIB in the online-only Data Supplement). However, co-injection of apln and vegfc MO with suboptimal doses exacerbated the lymphatic phenotypes (Fig. 5A and 5B), indicating that Apelin and VEGF-C signaling may synergistically promote lymphatic development. We next examined whether Apelin signaling is sufficient to compensate for the loss of VEGF-C signaling. Ectopic activation of Apelin signaling by synthetic mRNA injection was unable to alleviate the lymphatic defects in vegfc MO injected embryos (Fig. 5C and 5D). In a similar manner, ectopic expression of Vegfc by synthetic mRNA injection was unable to restore the lymphatic abnormalities caused by reduced Apelin signaling (Fig. 5E and 5F). The inability of apln mRNA to alleviate the defects of vegfc MO-injected embryos and vegfc mRNA to apln MO-injected embryos collectively suggest that Apelin and Vegfc signaling may function in a non-redundant manner in lymphatic development.
Fig. 5. Apelin and Vegfc signaling are not redundant but functionally synergize to facilitate lymphatic development in zebrafish.
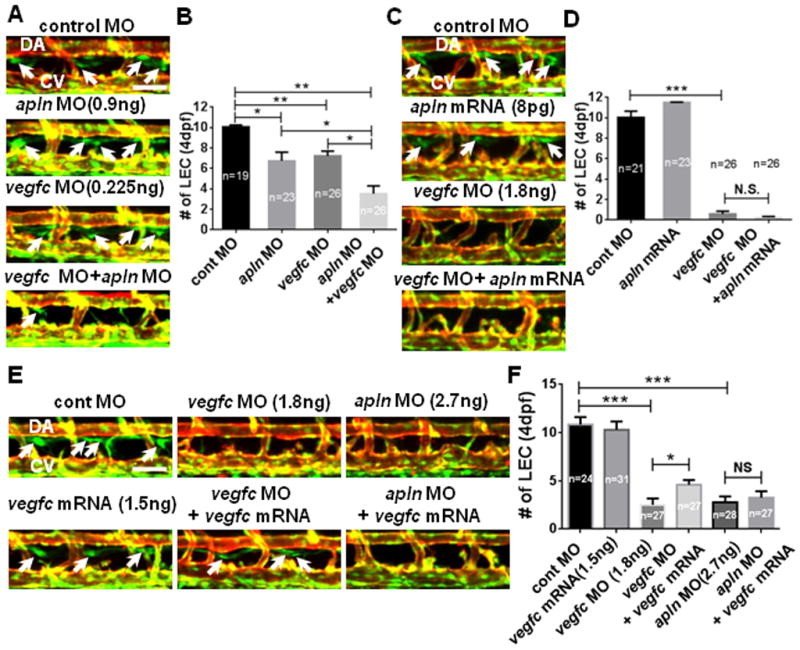
(A) Apelin and Vegfc signaling may synergistically promote lymphatic development. 4dpf embryos co-injected with low doses of apln and vegfc MOs contained a substantially decreased number of LECs (arrows) compared to control or single MO injected embryos. (B) Quantification of the number of LECs. (C) Apelin signaling may have a non-redundant role with Vegfc signaling during LEC development. Over-expression of Apelin by mRNA injection did not alleviate lymphatic defects in 4dpf vegfc MO-injected embryos. (D) Quantification of the number of LECs. (E) Representative images for the lymphatic vessel phenotype in 4dpf Tg(fli1a:nEGFP);Tg(kdrl:mCherry) embryos injected with apln and vegfc MOs and vegfc mRNA. vegfc mRNA injection at 1–2 cell stage only rescues the defects caused by Vegfc knockdown, but not Apln knock-down. (F) Quantification of LECs. DA, dorsal aorta; CV, cardinal vein. Scale bars are 50μm.
Considering that both Apelin and Vegfc signaling can activate AKT to induce lymphatic development, but appear to have non-redundant functions, it is possible that Vegfc and Apelin signaling may have distinct functions; while Vegfc may potentiate presumptive LECs within venous vascular beds, Apelin signaling may successively promote differentiation of LECs in later developmental stages. Alternatively, it is possible that Apelin and Vegfc signaling may induce AKT activity in a temporally distinct manner. To examine this possibility, we analyzed the expression of apln, aplnra, vegfc, and vegfr3 within the first five days of development in zebrafish embryos (Fig. 6A). While the expression level of vegfc gradually increased and then stabilized, the expression level of vegfr3 precipitously dropped by 2dpf, indicating that the activity of Vegfc-Vegfr3 signaling may reduce to the basal level when LECs emerge from venous ISVs (Fig. 6A). In contrast, the expression of apln and aplnra continuously increased during the first five days of development (Fig. 6A and Figure VIC in the online-only Data Supplement), suggesting functions of Apelin signaling may be required later in development than Vegfc signaling.
Fig. 6. Apelin and Vegfc signaling is temporally separated for lymphatic vessel formation in zebrafish.
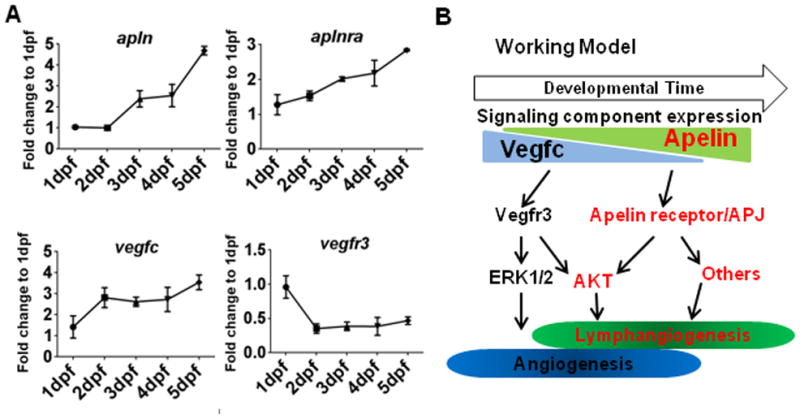
(A) Expression profiles of apln, aplnra, vegfc, and vegfr3 during the first five days of zebrafish development, normalized to actb1. The expression of apln and aplnra gradually increased over time, while the expression of vegfc and vegfr3 is either constant or attenuated. (B) Schematic working model. Apelin and Vegfc signaling appear to have non-overlapping functions during lymphatic development. While functionally redundant in activating AKT, Apelin and Vegfc signaling may be temporally separated and seem to activate specific sets of downstream effectors.
DISCUSSION
Considering the expression pattern of Apelin signaling components during development, and their role during lymphatic regeneration 16, 25, 26, it is likely that Apelin signaling provides key regulation during lymphatic development. In this report, we have demonstrated that Apelin signaling induced AKT activity is essential for differentiation of LECs during development. AKT activity has been implicated in both developmental and pathological lymphangiogenesis 34, 37, 38. For instance, targeted deletion of Akt1, Akt2, or Akt3 in mouse caused a significant reduction in the number of developing LECs, and led to defects in lymphatic valve formation 37, 39. In addition, Kaposi’s sarcoma associated herpesvirus (KSHV) activates AKT to induce ectopic lymphatic structures 40, 41. Although AKT can be activated by diverse signaling inputs 42, 43, attenuating Apelin signaling substantially decreased the phosphorylation of AKT in LECs, both in cell culture and developing zebrafish embryos. Therefore, it appears that Apelin signaling may function as a main inducer of AKT activity within LECs. Moreover, our data suggest that Apelin signaling may coordinate with VEGF-C signaling, an essential pro-lymphangiogenic signaling pathway 34, 36, 39, to maintain the proper level of AKT activity in LECs. During development, components of Apelin and Vegfc signaling appear to be expressed at distinct stages, suggesting that these two pro-lymphangiogenic signaling pathways may activate the same downstream effectors, but their activity may be temporally separated during development (Fig. 6B).
Although Apelin signaling is required for AKT activation in LECs and the disruption of the Apelin-AKT signaling cascade drastically reduced the number of LECs in zebrafish, ectopic AKT activation by chemical agonists (data not shown) and ectopic expression on Vegfc (Fig. 5E) were not sufficient to restore the number of LECs in zebrafish embryos with compromised Apelin signaling. Therefore, it is likely that the pro-lymphangiogenic role of Apelin signaling may be transduced by additional effectors. ERK1/2, which are known to be activated by Vegfc signaling in LECs 35, 36, do not seem to mediate Apelin signaling in LECs, since a lack of Apelin signaling did not affect the phosphorylation status of ERK1/2 in hLECs (Fig. 3C). Ongoing work in the lab is examining the role of other serine threonine kinases, including ERK5, Raf, and PKA as potential downstream mediators of Apelin signaling in LECs. Convergence of Apelin and VEGF-C at AKT, as well as divergent signaling pathways activated by these distinct signaling cascades, likely provide the necessary cues that ensure proper lymphatic development.
Supplementary Material
SIGNIFICANCE.
Despite importance of Apelin/APJ signaling in diverse biological processes, its role during lymphatic development is relatively unknown. We find that Apelin/APJ signaling is essential to induce and maintain phospho-AKT1/2 level in lymphatic endothelial cells therefore, positively regulates lymphatic development. Moreover, Apelin/APJ signaling may synergize with VEGF-C signaling to promote lymphatic fate.
Acknowledgments
We thank Drs. Victoria Bautch, Anne Eichmann, and Mike Simons for their discussion and critical reading of the manuscript, Drs. Elke A. Ober and Ian C. Scott for providing transgenic lines, members of Jin and Chun labs for helpful discussion, Jose Cardona-Costa for excellent fish care, and Tyler Ross for image analyses. We also thank the Korea Zebrafish Organogenesis Mutant Bank (ZOMB) for providing zebrafish lines.
SOURCES OF FUNDING
This work has been supported by grants from the NIH to S.-W.J. (HL090960 and HL119345), and to H.J.C. (HL095654 and HL113005), and an American Heart Association post-doctoral fellowship to J.-D.K (11POST7440010).
Non-standard Abbreviations
- AKT
Serine–threonine kinase Akt/Protein kinase B
- BEC
Blood vessel endothelial cell
- dpf
days post-fertilization
- ERK
Extracellular signal regulated kinase
- HM
Horizontal myoseptum
- hLEC
Human lymphatic endothelial cell
- LEC
Lymphatic endothelial cell
- MO
Morpholino
- mTORC1/2
mammalian target of rapamycin complex 1 and 2
- PaCV
Parachordal vessel
- PI3K
Phosphoinositide 3-kinase
Footnotes
DISCLOSURES
None
References
- 1.Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 2.Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17:1371–1380. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- 3.Schulte-Merker S, Sabine A, Petrova TV. Lymphatic vascular morphogenesis in development, physiology, and disease. J Cell Biol. 2011;193:607–618. doi: 10.1083/jcb.201012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Achen MG, McColl BK, Stacker SA. Focus on lymphangiogenesis in tumor metastasis. Cancer Cell. 2005;7:121–127. doi: 10.1016/j.ccr.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murdaca G, Cagnati P, Gulli R, Spanò F, Puppo F, Campisi C, Boccardo F. Current views on diagnostic approach and treatment of lymphedema. Am J Med. 2012;125:134–140. doi: 10.1016/j.amjmed.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 7.Witte MH, Dellinger MT, Papendieck CM, Boccardo F. Overlapping biomarkers, pathways, processes and syndromes in lymphatic development, growth and neoplasia. Clin Exp Metastasis. 2012;29:707–727. doi: 10.1007/s10585-012-9493-1. [DOI] [PubMed] [Google Scholar]
- 8.Rockson SG, Rivera KK. Estimating the population burden of lymphedema. Ann N Y Acad Sci. 2008;1131:147–154. doi: 10.1196/annals.1413.014. [DOI] [PubMed] [Google Scholar]
- 9.Brorson H, Ohlin K, Olsson G, Svensson B, Svensson H. Controlled compression and liposuction treatment for lower extremity lymphedema. Lymphology. 2008;41:52–63. [PubMed] [Google Scholar]
- 10.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 11.van der Putte SC. The development of the lymphatic system in man. Adv Anat Embryol Cell Biol. 1975;51:3–60. [PubMed] [Google Scholar]
- 12.Yaniv K, Isogai S, Castranova D, Dye L, Hitomi J, Weinstein BM. Live imaging of lymphatic development in the zebrafish. Nat Med. 2006;12:711–716. doi: 10.1038/nm1427. [DOI] [PubMed] [Google Scholar]
- 13.Kuchler AM, Gjini E, Peterson-Maduro J, Cancilla B, Wolburg H, Schulte-Merker S. Development of the zebrafish lymphatic system requires vegfc signaling. Curr Biol. 2006;16:1244–1248. doi: 10.1016/j.cub.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 14.Francois M, Harvey NL, Hogan BM. The transcriptional control of lymphatic vascular development. Physiology (Bethesda) 2011;26:146–155. doi: 10.1152/physiol.00053.2010. [DOI] [PubMed] [Google Scholar]
- 15.Ny A, Koch M, Schneider M, Neven E, Tong RT, Maity S, Fischer C, Plaisance S, Lambrechts D, Héligon C, Terclavers S, Ciesiolka M, Kälin R, Man WY, Senn I, Wyns S, Lupu F, Brändli A, Vleminckx K, Collen D, Dewerchin M, Conway EM, Moons L, Jain RK, Carmeliet P. A genetic xenopus laevis tadpole model to study lymphangiogenesis. Nat Med. 2005;11:998–1004. doi: 10.1038/nm1285. [DOI] [PubMed] [Google Scholar]
- 16.Kidoya H, Takakura N. Biology of the apelin-apj axis in vascular formation. J Biochem. 2012;152:125–131. doi: 10.1093/jb/mvs071. [DOI] [PubMed] [Google Scholar]
- 17.Zeng XX, Wilm TP, Sepich DS, Solnica-Krezel L. Apelin and its receptor control heart field formation during zebrafish gastrulation. Dev Cell. 2007;12:391–402. doi: 10.1016/j.devcel.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Scimia MC, Hurtado C, Ray S, Metzler S, Wei K, Wang J, Woods CE, Purcell NH, Catalucci D, Akasaka T, Bueno OF, Vlasuk GP, Kaliman P, Bodmer R, Smith LH, Ashley E, Mercola M, Brown JH, Ruiz-Lozano P. Apj acts as a dual receptor in cardiac hypertrophy. Nature. 2012;488:394–398. doi: 10.1038/nature11263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott IC, Masri B, D’Amico LA, Jin SW, Jungblut B, Wehman AM, Baier H, Audigier Y, Stainier DY. The g protein-coupled receptor agtrl1b regulates early development of myocardial progenitors. Dev Cell. 2007;12:403–413. doi: 10.1016/j.devcel.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Sheikh AY, Chun HJ, Glassford AJ, Kundu RK, Kutschka I, Ardigo D, Hendry SL, Wagner RA, Chen MM, Ali ZA, Yue P, Huynh DT, Connolly AJ, Pelletier MP, Tsao PS, Robbins RC, Quertermous T. In vivo genetic profiling and cellular localization of apelin reveals a hypoxia-sensitive, endothelial-centered pathway activated in ischemic heart failure. American journal of physiology. Heart and circulatory physiology. 2008;294:H88–98. doi: 10.1152/ajpheart.00935.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charo DN, Ho M, Fajardo G, Kawana M, Kundu RK, Sheikh AY, Finsterbach TP, Leeper NJ, Ernst KV, Chen MM, Ho YD, Chun HJ, Bernstein D, Ashley EA, Quertermous T. Endogenous regulation of cardiovascular function by apelin-apj. American journal of physiology. Heart and circulatory physiology. 2009;297:H1904–1913. doi: 10.1152/ajpheart.00686.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang Y, Kim J, Anderson JP, Wu J, Gleim SR, Kundu R, McLean DL, Kim JD, Park H, Jin SW, Hwa J, Quertermous T, Chun HJ. Apelin-apj signaling is a critical regulator of endothelial mef2 activation in cardiovascular development. Circ Res. 2013 doi: 10.1161/CIRCRESAHA.113.301324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tucker B, Hepperle C, Kortschak D, Rainbird B, Wells S, Oates AC, Lardelli M. Zebrafish angiotensin ii receptor-like 1a (agtrl1a) is expressed in migrating hypoblast, vasculature, and in multiple embryonic epithelia. Gene Expr Patterns. 2007;7:258–265. doi: 10.1016/j.modgep.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 24.McLean DL, Kim J, Kang Y, Shi H, Atkins GB, Jain MK, Chun HJ. Apelin/apj signaling is a critical regulator of statin effects in vascular endothelial cells--brief report. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:2640–2643. doi: 10.1161/ATVBAHA.112.300317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawane M, Kidoya H, Muramatsu F, Takakura N, Kajiya K. Apelin attenuates uvb-induced edema and inflammation by promoting vessel function. Am J Pathol. 2011;179:2691–2697. doi: 10.1016/j.ajpath.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sawane M, Kajiya K, Kidoya H, Takagi M, Muramatsu F, Takakura N. Apelin inhibits diet-induced obesity by enhancing lymphatic and blood vessel integrity. Diabetes. 2013 doi: 10.2337/db12-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Chen J, Bai B, Du H, Liu Y, Liu H. Heterodimerization of human apelin and kappa opioid receptors: Roles in signal transduction. Cell Signal. 2012;24:991–1001. doi: 10.1016/j.cellsig.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Hashimoto Y, Ishida J, Yamamoto R, Fujiwara K, Asada S, Kasuya Y, Mochizuki N, Fukamizu A. G protein-coupled apj receptor signaling induces focal adhesion formation and cell motility. Int J Mol Med. 2005;16:787–792. [PubMed] [Google Scholar]
- 29.Hong CC, Peterson QP, Hong JY, Peterson RT. Artery/vein specification is governed by opposing phosphatidylinositol-3 kinase and map kinase/erk signaling. Curr Biol. 2006;16:1366–1372. doi: 10.1016/j.cub.2006.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong CC, Kume T, Peterson RT. Role of crosstalk between phosphatidylinositol 3-kinase and extracellular signal-regulated kinase/mitogen-activated protein kinase pathways in artery-vein specification. Circ Res. 2008;103:573–579. doi: 10.1161/CIRCRESAHA.108.180745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren B, Deng Y, Mukhopadhyay A, Lanahan AA, Zhuang ZW, Moodie KL, Mulligan-Kehoe MJ, Byzova TV, Peterson RT, Simons M. Erk1/2-akt1 crosstalk regulates arteriogenesis in mice and zebrafish. J Clin Invest. 2010;120:1217–1228. doi: 10.1172/JCI39837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gharbi SI, Zvelebil MJ, Shuttleworth SJ, Hancox T, Saghir N, Timms JF, Waterfield MD. Exploring the specificity of the pi3k family inhibitor ly294002. Biochem J. 2007;404:15–21. doi: 10.1042/BJ20061489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An atp-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mtorc1. J Biol Chem. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Lüthi U, Barberis A, Benjamin LE, Mäkinen T, Nobes CD, Adams RH. Ephrin-b2 controls vegf-induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- 35.Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res. 2001;49:568–581. doi: 10.1016/s0008-6363(00)00268-6. [DOI] [PubMed] [Google Scholar]
- 36.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. Vegf receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 37.Zhou F, Chang Z, Zhang L, Hong YK, Shen B, Wang B, Zhang F, Lu G, Tvorogov D, Alitalo K, Hemmings BA, Yang Z, He Y. Akt/protein kinase b is required for lymphatic network formation, remodeling, and valve development. Am J Pathol. 2010;177:2124–2133. doi: 10.2353/ajpath.2010.091301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morello F, Perino A, Hirsch E. Phosphoinositide 3-kinase signalling in the vascular system. Cardiovasc Res. 2009;82:261–271. doi: 10.1093/cvr/cvn325. [DOI] [PubMed] [Google Scholar]
- 39.Coso S, Zeng Y, Opeskin K, Williams ED. Vascular endothelial growth factor receptor-3 directly interacts with phosphatidylinositol 3-kinase to regulate lymphangiogenesis. PLoS One. 2012;7:e39558. doi: 10.1371/journal.pone.0039558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aguilar B, Choi I, Choi D, Chung HK, Lee S, Yoo J, Lee YS, Maeng YS, Lee HN, Park E, Kim KE, Kim NY, Baik JM, Jung JU, Koh CJ, Hong YK. Lymphatic reprogramming by kaposi sarcoma herpes virus promotes the oncogenic activity of the virus-encoded g-protein-coupled receptor. Cancer Res. 2012;72:5833–5842. doi: 10.1158/0008-5472.CAN-12-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morris VA, Punjabi AS, Lagunoff M. Activation of akt through gp130 receptor signaling is required for kaposi’s sarcoma-associated herpesvirus-induced lymphatic reprogramming of endothelial cells. J Virol. 2008;82:8771–8779. doi: 10.1128/JVI.00766-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hers I, Vincent EE, Tavaré JM. Akt signalling in health and disease. Cell Signal. 2011;23:1515–1527. doi: 10.1016/j.cellsig.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Hemmings BA, Restuccia DF. Pi3k-pkb/akt pathway. Cold Spring Harb Perspect Biol. 2012;4:a011189. doi: 10.1101/cshperspect.a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


