Abstract
In the present study, we used microelectrode recordings of multiunit responses to evaluate patterns of the reactivation of somatosensory cortex after sensory loss produced by spinal cord lesions in the common marmoset (Callithrix jacchus). These New World monkeys have become a popular model in studies of cortical organization and function. Primary somatosensory cortex and adjoining somatosensory areas can become extensively deactivated by lesions of somatosensory afferents as they ascend in the dorsal columns of the cervical spinal cord. Six to 7 weeks after complete lesions of the cuneate fasciculus subserving the forelimb at cervical levels 5–6, the hand region in contralateral areas 3b and 1 was reactivated by inputs from the forelimb, but excluded representations of some or all digits. In a similar manner, recording sites from the forelimb region of areas 2–5 responded to parts of the forelimb but not to digits after an extensive lesion of the contralateral cuneate fasciculus at C5–C6. Lesions that damaged only the gracile fasciculus or a small percentage of the cuneate fasciculus did not produce changes in the gross hand representation in contralateral areas 3b, 3a, 1, and 2. Finally, a complete but lower lesion of the cuneate fasciculus at C8 produced some abnormalities in the reactivation, but the digits were represented. The results indicate that areas 3a, 3b, 1, and 2–5 of the somatosensory cortex are extensively reactivated after large, apparently complete lesions of the contralateral cuneate fasciculus, but afferents from the digits may not contribute to their reactivation.
Keywords: cortical plasticity, New World monkey, somatosensory
After a loss of inputs from cutaneous afferents in primates and other mammals, primary somatosensory cortex may become unresponsive to somatosensory stimuli on the deprived skin for a period of days to months, depending on the extent of the sensory loss. Typically, much of this unresponsive cortex recovers and becomes responsive to preserved sensory inputs (Jain et al., 1997; Kaas et al., 2008). After an extensive loss of inputs from the forelimb, the hand and forearm portions of the deactivated contralateral hand and forelimb portion of primary somatosensory cortex may even become responsive to cutaneous afferents from the chin and lower face, but this reactivation takes 6–8 months to develop (Jain et al., 1997). When the sensory loss is incomplete, preserved inputs from the hand allow a more rapid reactivation over weeks to months, and the reactivation of hand cortex is correlated with a recovery of the use of the hand in food retrieval tasks (Darian-Smith and Ciferri, 2006; Qi et al., 2011).
Lesions of the cutaneous afferents in the dorsal columns of the spinal cord have been one method to experimentally study reactivation and recovery in monkeys. The low-threshold mechanoreceptor afferents from the hand and other parts of the forelimb enter the spinal cord at lower cervical and upper thoracic levels and branch, with one branch terminating in the dorsal horn and the other ascending in the cuneate fasciculus to terminate in the cuneate nucleus of the upper spinal cord and lower brainstem (Florence et al., 1988, 1991). Other sensory inputs, including those that mediate pain and temperature as well as crude touch, also terminate on dorsal horn neurons (Willis and Coggeshall, 2004). The dorsal horn neurons provide a secondary pathway that preserves many sensory abilities, and some of the secondary neurons project to the cuneate nucleus (Dykes and Craig, 1998; Rustioni et al., 1979) where they may contribute to the reactivation of contralateral somatosensory cortex. Until recently, the reactivation of the hand portion of somatosensory cortex after lesions of the dorsal columns in monkeys has been thought to depend completely on branches of primary afferents that have been preserved in the dorsal columns after lesions. However, considerable reactivation of hand cortex is now known to occur even after an estimated 97–99% of afferent branches from the digits have been cut (Bowes et al., 2012; Qi et al., 2011). These results suggest that secondary neurons in the dorsal horn of the spinal cord contribute to the reactivation and behavioral recovery.
In the present study, unilateral dorsal column lesions were made at the cervical 5–6 level in adult marmosets. Complete lesions at this level included afferents from all of the digits and other parts of the hand. Marmosets are small New World monkeys with no central fissure, and thus provide advantages in microelectrode mapping studies of somatosensory cortex. In addition, their smaller brains facilitate histological processing, especially when brain sections are cut parallel to the surface of cortex that has been recorded from and flattened after perfusion. Tracers were injected into the digits so that preserved inputs to the cuneate nucleus were labeled and the effectiveness of lesions could be evaluated. The results indicate that after complete lesions of the cuneate fasciculus at levels that involve all ascending branches of the hand, the hand regions of contralateral somatosensory cortex are extensively reactivated by inputs from the hand and other parts of the forelimb, but with reduced or no reactivation from the digits. Lesions that preserve inputs from the hand resulted in a more complete reactivation of cortex, and spinal cord lesions that did not involve the cuneate fasciculus did not alter the somatotopy of somatosensory cortex.
MATERIALS AND METHODS
In the present study, somatosensory cortex organization was mapped with microelectrode recordings after weeks of recovery following lesions of the contralateral dorsal columns of the spinal cord in adult common marmosets (Callithrix jacchus). All methods were part of a protocol approved by the Vanderbilt Animal Care and Use Committee, and followed the National Institutes of Health (NIH) guidelines. Three of the marmosets were a generous gift from Dr. Afonso Silva of the NIH.
Spinal cord lesions
In preparation for surgery, nine adult male common marmosets were anesthetized with a mixture of ketamine (8–10 mg/kg) and xylazine (0.4 mg/kg). Appropriate dosages of dexamethasone (1mg/kg, IM; Phoenix Scientific, St. Joseph, MO), Robinul (0.015 mg/kg, IM; Baxter Healthcare, Deerfield, IL), and the antibiotic ceftiofur (2.2 mg/kg, IM; Pfizer, New York, NY) were administered. Each marmoset was then intubated. Two drops of lidocaine were applied in each ear canal to provide local anesthesia and the marmoset was placed in a stereotaxic instrument. Anesthesia was maintained with 0.5–3.0% isoflurane for the duration of the experiment, and the heart rate, respiratory rate, and body temperature were monitored. In each case, an incision was made above the cervical vertebrae, and the layers of the superficial and deep back muscles were separated from the vertebrae along the midline. A laminectomy was performed with fine rongeurs, wherein the spiny process and lamina of one cervical vertebra were removed. Subsequent to removal of the dura and visualization of the dorsal root entry zones, a depth-marked needle was inserted at midline and lateral to that at the dorsal root entry zone. This was done to minimize distortion of the spinal cord prior to confirming the desired mediolateral extent of the lesion. Small depth-marked iris scissors were then inserted into these borders, and the lesion was completed. The depth-marked needle was then reinserted into the lesion and swept across its extent to ensure no fibers were left intact. In four cases, lesions were instead placed in different rostrocaudal or mediolateral locations in the spinal cord, leaving the ascending cuneate fasciculi largely intact. These cases served as controls.
Gelfilm faux-dura was used to bridge the vertebra that had been removed, and water-insoluble hemostatic gelfoam (Baxter Healthcare, Hayward, Ca) held this in place and staunched bleeding. The muscles were sutured back into place with absorbable thread, and the skin was stapled. The animal was then allowed to recover from anesthesia, and monitored carefully over the next few days. To alleviate any possible pain and distress, buprenorphine (0.005–0.01 mg/kg, IM/SC; Reckitt & Colman Pharmaceuticals, Richmond, VA) was administered twice daily for 3 days postoperatively.
CTB injections
Five to 6 weeks later, and 4–7 days prior to microelectrode mapping, the marmosets were lightly anesthetized with ketamine (8–10 mg/kg), and injections of 1% anterograde tracer cholera toxin B-subunit (CTB) were made in the digits of the left and right hand. This was achieved with multiple injections of ~5–10 μl each in the glabrous digits via a Hamilton syringe. The animal was then allowed to recover from anesthesia. CTB immunoreactivity within the cuneate nucleus was later used in addition to spinal cord section reconstruction to assess the extent of the dorsal column lesion.
Electrophysiological recording
Marmosets were initially anesthetized with a mixture of ketamine (8–10 mg/kg) and xylazine (0.4 mg/kg). Appropriate dosages of dexamethasone (1mg/kg, IM), Robinul (0.015 mg/kg, IM), and the antibiotic ceftiofur (2.2 mg/kg, IM) were then administered. The marmosets were intubated, and two drops of lidocaine were applied within each ear canal to facilitate placing the animal within the ear bars of the stereotaxic instrument. Anesthesia was maintained with ketamine administered intravenously via an infusion pump at 0.25–1.53 ml/hr for the duration of the experiment, supplemented with xylazine (0.2 mg/kg) every 2– hours. The heart rate, respiratory rate, and temperature were monitored throughout.
A craniotomy contralateral to the spinal cord lesion was made to expose the somatosensory cortex. A section of the dura was removed to expose the region of interest, and the brain surface was coated with silicone to prevent drying of the cortex. A high-resolution picture of the brain was used to mark the placement of the electrode penetrations. A low-impedance tungsten microelectrode (1 mΩ at 1 kHz; Microprobe, Potomac, MD) was advanced perpendicular to the brain surface with a hydraulic microdrive, and recordings were made at multiple sites within a grid across area 3b and adjoining areas 3a and 1. Electrode penetrations were approximately 0.25 mm apart, with small deviations to avoid blood vessels. Multineuron responses were elicited with cotton-tipped brushes and fine-tipped pliable probes. Receptive fields were determined with near threshold stimuli. After the recording session, several electrolytic lesions (10 μA) were made within the cortex at strategic sites so that recording sites could be related to cortical architecture.
Tissue processing and anatomical reconstruction
After terminal recording sessions, marmosets were given a lethal dose of Euthasol (50–90 mg/kg; Virbac, Fort Worth, TX) and perfused transcardially with buffered 4% paraformaldehyde (PFA), and then with 4% PFA with 10% sucrose.
Cortex
After perfusion, the mapped hemisphere was separated from the rest of the brain, and in some instances blocked, flattened, and cut parallel to the cortical surface at 40-μm intervals. One series of every fourth section was processed for metabolic-indicative cytochrome oxidase, and another series was processed for myelin to delineate architectonic boundaries and myelin-dense somatosensory area 3b. Architectonic borders of each section were identified, and each section was aligned with the others via landmarks including the lateral sulcus, the blood vessels throughout, and the electrolytic lesions made during mapping. Images of these sections were then imported into Adobe Illustrator (Adobe Systems, San Jose, CA), in which realignment was done using the opacity function.
Spinal cord
Before the spinal cord was removed, the level of the spinal cord lesion was identified and marked with pins contralateral to the lesion, one just rostral and the other just caudal. This enabled us to reliably identify the lesion in the processed sections, and also served as alignment landmarks during reconstruction. The perilesioned spinal cord was sectioned horizontally. An alternating series of horizontal sections was reacted for cytochrome oxidase, enabling clear demarcation of the gray and white matter. Another series was incubated with primary goat anti-CTB (1:4000; List Biological Laboratories, Campbell, CA) diluted in Tris-buffered saline containing 2% Triton X-100 (Sigma, St. Louis, MO) and 2.5% normal rabbit serum (Millipore, Billerica, MA) to visualize the forelimb primary afferents and terminal fields that were labeled by CTB injections into the digits.
CTB section images were later placed in Adobe Photoshop, and the same contrast and brightness level tools were applied bilaterally to heighten contrast. An image of each horizontal section was placed in a separate layer in Adobe Illustrator. They were aligned with the use of program guidelines that horizontally itercepted the two pinpoints and ran vertically along the spinal cord midline. This enabled a precise alignment of sections for reconstruction of the lesion in the coronal plane.
Brainstem
Once removed from the brain, the brainstem and adjacent spinal cord were separated, blocked and left in 30% sucrose overnight. Sections were then cut coronally at 40-μm intervals. One series of alternating sections was processed for cytochrome oxidase to maximally visualize the cuneate nucleus, and the other processed for CTB immunoreactivity. These sections were incubated with primary goat anti-CTB (1:4,000; List Biological Laboratories) diluted in Tris-buffered saline containing 2% Triton X-100 and 2.5% normal rabbit serum to visualize the forelimb primary afferents and terminal fields. They were subsequently placed in Adobe Photoshop, and the same contrast and brightness level tools were applied bilaterally to heighten contrast.
RESULTS
In the present study, we determined the effects of unilateral lesions of sensory afferents in the spinal cord of adult marmosets on the somatotopy of contralateral somatosensory cortex 6-7 weeks after the lesion. The unilateral spinal cord lesions varied from complete at cervical 5-6 level of the cuneate fasciculus to those that were partial or only damaged other parts of the spinal cord. The microelectrode recording results are related to lesion characteristics below.
Areas 3a, 3b, and 1 in cases with a complete or extensive C5/C6 spinal cord lesion
Case 9-28
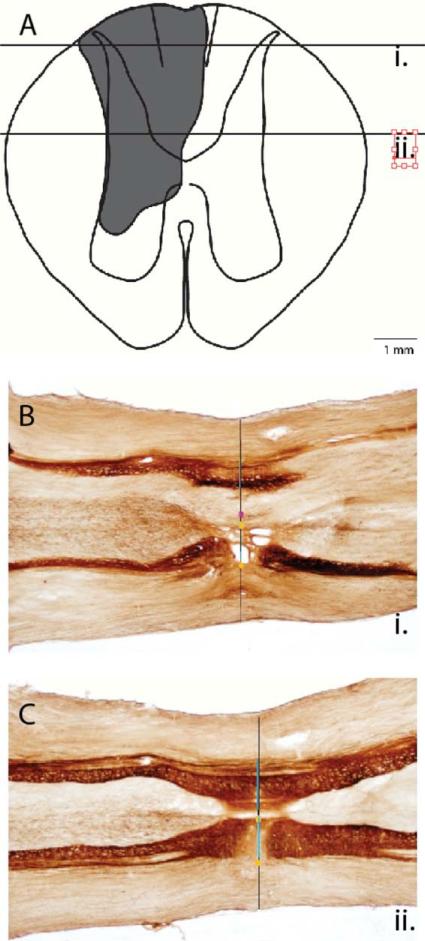
In marmoset case 9-28, the lesion of the cuneate fasciculus at the C5/C6 level on the left side of the spinal cord was complete, and the deprived portions of contralateral hand cortex were extensively reactivated after 6 weeks of recovery. The spinal cord lesion included all of the cuneate fasciculus and the gracile fasciculus, and more ventral parts of the spinal cord (Fig. 1A). The extent of the lesion was reconstructed from the damage apparent in a series of horizontal sections through the cervical spinal cord from more dorsal (Fig. 1B) to more ventral levels (Fig. 1C). Although there was extensive damage to the dorsal and ventral horns of the spinal cord, the lesion was restricted to the C5–6 level. Thus, branches of afferents from the hand and forelimb that terminated in the dorsal horn at higher levels were preserved.
Figure 1.
A reconstruction of the spinal cord lesion at the cervical C5/C6 level in marmoset 9-28. A: The extent of the lesion (shading) as reconstructed from serial sections cut in the horizontal plane and processed for cytochrome oxidase (CO). The horizontal lines mark the locations of sections shown in B and C. The illustrated lesion includes both missing tissue and the glial scar. B,C: Horizontal sections through the spinal cord stained for CO. Each section is aligned with guidelines (not shown) that intersect the pinpoints and the midline; then the spinal cord width, gray matter, and lesion/glial scar borders are demarcated. The reconstructed spinal cord in this case indicated that a complete lesion of the dorsal columns had been made. Scale bar = 1 mm in B (applies to B,C). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The effectiveness of the spinal cord lesion was further evaluated by using CTB as an anterograde tracer to label primary afferents from the digits of both hands that terminated in the cuneate nucleus of both sides of the brainstem (Fig. 2A). As expected, on the normal side of the brainstem where there was no spinal cord lesion, there were multiple patches of labeled terminals throughout the rostrocaudal extent of the cuneate nucleus in a pattern that reflects the normal distribution of terminations from the digits in normal monkeys (Florence et al., 1989, 1991). In contrast, on the lesioned side, there was no detectable terminal label from afferents from the digits in the cuneate nucleus. Thus, the lesion of the cuneate fasciculus was complete at the C5–6 level, and a lesion at this level intercepted the cuneate fasciculus afferents from the digits of the hand.
Figure 2.
Labeling of the cuneate nucleus by CTB injection into the digits after unilateral dorsal column lesions. A: The distribution of CTB-labeled axon terminals in the cuneate nucleus of marmoset 9-28 after the dorsal columns were sectioned at the C5/C6 level on the left side. All five digit tips were injected in both hands. A lack of label in the left cuneate nucleus, compared with the dense patches of label in the right nucleus, indicated that the lesion successfully interrupted afferents from all five digits. B: Pattern of label after injections of CTB in digits of both hands in four other cases with C5/C6 unilateral dorsal column lesions. Sparse label in the cuneate nucleus on the lesioned side in cases 10-34 and 9-26 indicates that the lesions were large, but incomplete. Scale bar = 0.5 mm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The hand region of somatosensory cortex contralateral to the spinal cord lesion was explored with microelectrode recordings 6–7 weeks after the spinal cord lesion. As marmosets have no central fissure, most of area 3b is exposed for recording on the brain surface, as are associated somatosensory areas 1 and 3a (Fig. 3). The location of the hand and forearm representation in area 3b and adjoining cortex (Fig. 3) has been established in previous microelectrode mapping experiments (Huffman and Krubitzer, 2001), and the location of the lateral border of the hand representation with the face representation is marked by a narrow myelin-light septum (Jain et al., 1998; Krubitzer and Kaas, 1990). This septum was identified in our myelin-stained brain sections, and it continued to mark the medial border of the face representation in our experimental monkeys, as sensory inputs from the face were not lesioned.
Figure 3.
A lateral view of a marmoset brain with the locations of the representation of the hand indicated in somatosensory areas 3a, 3b, and 1. For reference, the expected locations of the second somatosensory area (S2) and the parietal ventral somatosensory area (PV), as well as primary and secondary visual areas (17 and 18) are included. Primary motor cortex (M) is rostral to area 3a, and area 2 is caudal to area 1. The lateral sulcus (ls) and a shallow superior temporal sulcus (sts) are identified.
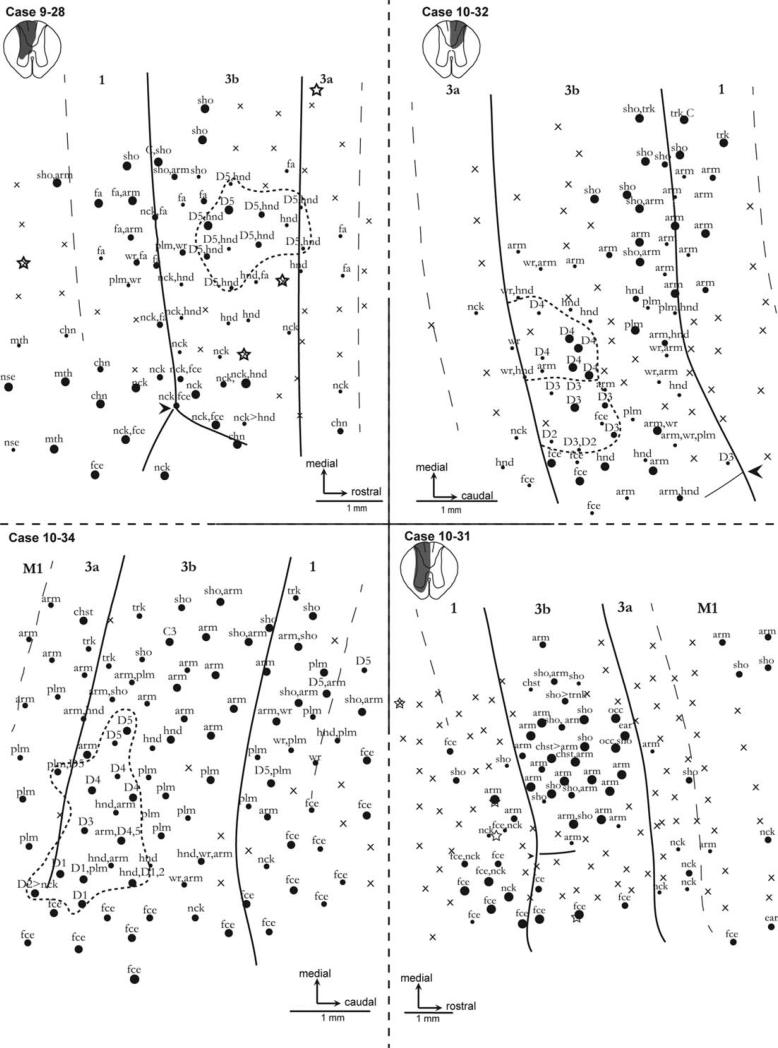
Microelectrode recordings in marmoset 9-28 revealed that this cortex was largely responsive to somatosensory stimuli, but the representation was abnormal. The locations of recording sites in contralateral somatosensory cortex and the recording results are shown in Figure 4. Whereas neurons at most recording sites throughout the explored regions of area 3b were responsive to light touch and taps on the skin, neurons at several recording sites were not. Such unresponsive sites are extremely unusual in normal monkeys, but are common after extensive sensory deprivation (Bowes et al., 2012; Chen et al., 2012; Darian-Smith and Brown, 2000; Jain et al., 1997, 2008; Merzenich et al., 1983a,b, 1984). Normally, the digits are represented from 1 to 5 in a lateromedial sequence in the rostral half of area 3b (Carlson et al., 1986), but that was not the case in marmoset 9-28. Whereas several more medial sites were activated by touch on digit 5 as well as other parts of the hand in this case, neurons at none of the sites were responsive to touch on any of the other digits. Instead, sites over much of the lateral part of the hand–forearm region were activated by touch on the neck, palm, or dorsal hand, whereas more medial sites were activated by the hand and parts of the forearm. Often, sites were activated by more than one body part. All these features are abnormal. Similar abnormal representations as well as unresponsive sites were found in area 3a and area 1, whereas normal face responses were found for sites in lateral 3b, area 1 (possibly including area 2 or S2), and 3a. Thus, the reactivated cortex was abnormal in failing to represent digits 1–4, having large receptive fields involving several body parts, and having neck and forearm representations in portions of area 3b that normally represent the digits and hand.
Figure 4.
Microelectrode recording results from the hand portions of areas 3b, 3a, and 1 of marmoset monkeys 9-28, 10-32, 10-34, and 10-31 after a lesion of the contralateral dorsal columns. Strong multiunit responses are marked by large dots, and smaller dots indicate weaker responses. Unresponsive sites are marked with an X, and stars indicate where small microlesions were placed so results could be related to the cortical architecture later. Note that in case 9-28 a reduced portion of area 3b was responsive to stimulation on the hand, and only digit 5 was represented. Sites in 3b, 3a, and 1 were responsive to parts of the arm. In case 10-32, neurons were activated by stimulation of parts of the arm, whereas some units responded to digits 2–4. In case 10-34, neurons were largely activated by stimulation of parts of the arm. Responses to all digits were found intermingled with sites responsive to stimulation on other parts of the hand and the arm in area 3b. In case 10-31, sites were unresponsive to the digits, whereas sites throughout the hand cortex responded to parts of the arm and chest. Laterally, sites in face cortex responded to face stimulation. Sho, shoulder; Plm, palm; Hnd, hand; Fa, forearm; Wr, wrist; Nck, neck. An arrowhead marks the myelin-light septum that separates the face–hand territories. Scale bar = 1 mm.
Case 10-32
The lesion reconstruction in this case (Fig. 4) depicted a complete severing of the ipsilateral dorsal columns at the C5–6 level. There was some compromise of the dorsal horn. This was consistent with the CTB-immunoreactive labeling in the cuneate nucleus after injections in digits, as no staining was seen ipsilaterally (Fig. 2B). After 5–6 weeks of postlesion recovery, electrophysiological recordings revealed that the hand region of contralateral area 3b was mostly responsive. Considerable reorganization was observed wherein receptive fields for the neck and chin were seen medial to the hand–face border, and cortical territories responsive to stimulation of the dorsal hand were scattered throughout. The palm representation was diminished, and in its place, neurons with receptive fields on the forearm and wrist were found. There was no observed representation of D1 or D5. However, neurons with receptive fields on digits 2, 3, and 4 were recorded. The territory responsive to D2 was much reduced; two electrode penetrations revealed D2-receptive fields; one of these also responded to cutaneous input to D3. Representations of D3 and D4 were observed in their normal territories; however, these were representations of the distal digits only. Limited probing revealed an area 1/2 that was unresponsive lateral to the forearm representation. Medial to the face and hand territories in area 1/2, the recorded receptive fields then progressed normally from arm to shoulder, to the upper trunk with progressively more medial recording sites. Only a few responsive recoding sites were in area 3a, and those with receptive fields on the neck were abnormal.
Case 10-34
The marmoset in this case had a more extensive reactivation of somatosensory cortex after a large, but incomplete dorsal column lesion. When the brainstem of this animal (Fig. 2B) was reacted for CTB immunoreactivity after tracer injections into digits 1, 3, and 5, ipsilateral label was present but sparse. Because the dorsalmost sections of the horizontally sectioned spinal cord were damaged, we were unable to fully reconstruct the lesion, but the sparse CTB label in the cuneate nucleus indicates that the C5–6 spinal cord lesion was incomplete and had left some fibers intact.
Recordings from the contralateral somatosensory cortex (Fig. 4) revealed representations of most of the digits. Our map of area 3a revealed a greater representation of the palm than expected. This was in contrast to very minimal digit representation: specifically, only one penetration revealed a D2-receptive field, and this was shared with the neck. Medial to these unusual receptive fields was a normal progression from the mid-upper arm to trunk. In area 3b, each digit was represented, although the only D2-receptive field observed was shared with the hand. Only one receptive field was observed for D3. Other perturbations included ectopic representation of the forearm; forearm receptive fields were seen as lateral as the D1 representation and within usually digit-exclusive D3–D4 territories. Recordings from area 1 revealed that this representation did not mirror that of area 3b. Instead, face receptive fields were seen in territories 2 mm medial to that seen in area 3b. These territories are usually responsive to the digits. The only digit represented here was D5, which was found only in ectopic locations. A normal progression of the upper arm, shoulder, and trunk representations was observed in cortex medial to the hand and face territories in area 3b and 1.
Case 10-31
Case 10-31 (Fig. 4) had a complete severing of unilateral dorsal column fibers at the C5–6 level. The ipsilateral dorsal and ventral horns were compromised as well. In accordance with the size and location of the lesion, the cuneate nuclei (Fig. 2B) showed dense CTB-immunoreactive staining on the contralateral side, but no staining ipsilaterally.
After 5–6 weeks of recovery, the derived somatosensory map (Fig. 4) indicated that a large-scale reorganization had taken place. As expected, area 3a was mostly unresponsive to tactile stimulation. Recording sites with misplaced receptive fields were observed, however, and these were largely on the neck. The part of area 3b normally responsive to the hand included a number of sites with neurons that had receptive fields on the upper arm, shoulder, and even the chest. Sites with these receptive fields were seen in close proximity to the hand–face border, where D1 is normally represented. Area 3b was highly responsive, with receptive fields from sites that could be rated “good” to “excellent” on a responsiveness scale. No recording sites in area 3b had receptive fields on the digits. This reorganization of area 3b was partially reflected in area 1, where receptive fields on the shoulder and upper arm were obtained at more lateral recording sites than in normal marmosets. Recording sites with receptive fields on the neck were also recorded. There was no observable representation of the hand or digits in area 1 or area 3a. A few sites in motor cortex responded to the arm or shoulder, but not the hand.
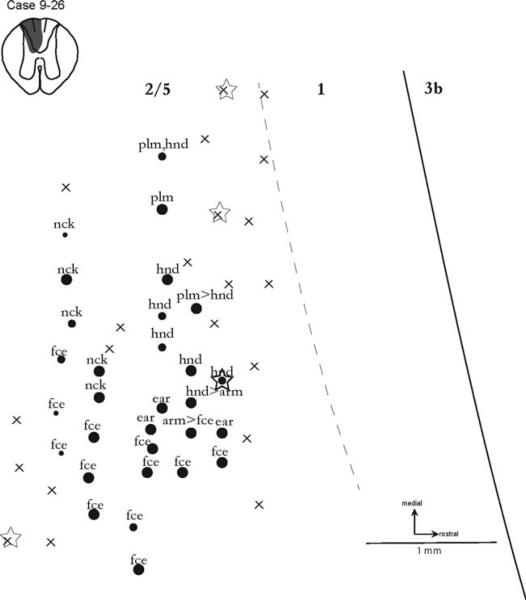
Reorganization of area 2–5
An area 2 and its boundary with area 5 have not been well defined in marmosets. Here we define the region immediately caudal to area 1 as the 2–5 region. As results from partial mapping of the 2–5 region revealed some responsiveness after dorsal column lesion (Fig. 4), recordings were focused on this region in one case (9-26). The spinal cord reconstructed in this case (Fig. 5) indicated that the lesion was almost complete, with minimal sparing of the ventralmost dorsal column fibers. Correspondingly, when the brainstem was visualized (Fig. 2B) after CTB tracing and probed for CTB immunoreactivity, little staining in the cuneate nucleus was evident ipsilateral to the lesion. This was in contrast to the dense staining of the cuneate nucleus on the contralateral side.
Figure 5.
Recording results from the region of area 2 plus area 5 6–7 weeks after a nearly complete lesion of the contralateral dorsal columns in case 9-26. Although the somatotopy of this region of cortex has not been described for marmosets, responses from the hand and palm were obtained, but none from the digits. Laterally, sites responsive to the face occurred. Conventions as in Figure 4.
The somatosensory map (Fig. 5) derived in case 9-26 reflected the likely reorganization of cortex in the traditional location of area 2. These recordings were recorded via low-threshold cutaneous stimulation, were made just over 1 mm caudal to area 3b (Fig. 6), were observed over a 1½-mm rostrocaudal expanse, and were labeled “excellent” on a 5-point “very weak to excellent” responsiveness scale. There appears to be no previous evidence that the 2–5 region in marmosets is responsive to tactile stimulation. Here we describe, for the first time, the topographic organization of robustly responsive cortex immediately caudal to area 1 in the common marmoset after spinal cord injury.
Figure 6.
The locations of recording sites (squares) in areas 2 and 5 relative to myelin-dense area 3b in marmoset 9-26. The recording results are shown in Figure 5. A myelin-sparse septum in lateral 3b (horizontal line) marks the normal location of the hand/face border. Note that recordings are clearly caudal to area 1, and in the expected locations of areas 2 and 5. Conventions as in Figure 3. Scale bar = 1 mm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The region of explored cortex was immediately caudal to the hand representation territories of areas 3b and 1 (Fig. 6). Because area 2 responses to tactile stimuli would be expected to be evoked from parts of the hand (Pons et al., 1985), it was not surprising that a number of sites in the area 2 region did respond to touch on the hand. However, responses specific to touch on the digits were not obtained, and this result is similar to the lack of digit responses in area 3b and 1 after complete, contralateral dorsal column lesion. Other possible abnormalities in the 2–5 region were the sites activated by the neck and face, as the sites were much more medial than expected. Thus, results from case 9-26 indicate that the 2–5 region is at least sometimes responsive to tactile stimulation, and the somatotopy of the region may be altered by long-standing sensory loss.
Cases with extensive forelimb afferent sparing
Results from cases with extensive sparing of forelimb afferents in the dorsal columns had normal or nearly normal cortical representations of the hand. Case 7-86 received a lesion lateral to the dorsal columns at the C3–4 level with no damage to the dorsal columns. Recordings from 33 electrode penetrations revealed no abnormalities in the gross somatotopy, and all the digits were represented in the normal later-omedial sequence in the hand territory of area 3b. Case 7-53 had a restricted C3–4 midline lesion that affected the gracilis fasciculi only. CTB tracing of digit afferents produced dense labeling both ipsi- and contralaterally. Recordings from 70 penetrations in the hand territory revealed that the somatosensory cortex was mostly responsive, and that digits 1–5 were represented in a normal pattern and in normal locations. Case 7-76 was a partial lesion of the cuneate fasciculus at the C3–4 level. The somatotopy of area 3b was disordered such that the digits were all represented but not in the typical lateral-to-medial progression. Case 8-06 was an extensive lesion at the C8 level, and disrupted most of the cuneate fasciculi at that level. As a result of the preservation of input from the hand after a lesion at this level, the topography was largely intact, except where cortical territories normally responsive to touch from digit 4 became responsive to digits 1, 3, 4, and 5.
DISCUSSION
Our major results come from five marmosets. In each of these cases, the lesion of the cuneate fasciculus at the C5–6 level was complete or extensive. CTB was used to label afferents from the digits that terminate in the cuneate nucleus of both sides of the brainstem, and cortical area 3b and/or adjoining cortex was mapped extensively with microelectrodes. In one marmoset, area 2–5 was specifically explored. In four other marmosets, lesions were partial, at a different spinal cord level, or outside the cuneate fasciculus. In the cases with complete or extensive lesions of the cuneate fasciculus, the regions of the normal hand representation in contralateral somatosensory cortex were extensively reactivated by inputs from the hand and forearm, but the somatotopy was abnormal and reliably excluded some digits. In cases in which the contralateral hand region of cortex was not deprived or only nominally deprived, the contralateral somatosensory cortex appeared grossly normal. These results in comparison with previous results after dorsal column lesions in monkeys are discussed below.
The reactivation of area 3b and adjoining areas of cortex
After complete lesions of the cuneate fasciculus at a high cervical level and 6–7 weeks of recovery, our present results indicate that the deprived regions of areas 3b, 1, and 3a of the common marmoset are extensively reactivated, but in abnormal somatotopic patterns. Similarly, after extensive loss of somatosensory inputs from the hand and forearm in other monkeys, the deprived zone of contralateral somatosensory cortex typically becomes almost completely responsive to inputs from the hand, if hand afferents remained, or other parts of the body (Chen et al., 2012; Darian-Smith and Brown, 2000; Florence and Kaas, 1995; Jain et al., 1997, 2008; Merzenich et al., 1983a,b; Pons et al., 1991; Qi et al., 2011; Tandon et al., 2009). Immediately after a lesion that produces deprived zones of cortex, these zones are largely unresponsive to sensory stimuli (Darian-Smith and Brown, 2000; Jain et al., 1997; Merzenich et al., 1983b), but this cortex gradually becomes responsive to tactile stimuli over days to months of recovery, depending on the extent and source of sensory loss. Thus, the loss of input from the section of a nerve serving the glabrous hand is followed by a rapid reactivation of the deprived cortex by other parts of the hand over days to weeks of recovery, whereas an extensive loss of inputs from the hand or arm is followed by a reactivation of contralateral hand–arm cortex by inputs from the face only after a period of 6–8 months (Jain et al., 1997).
After lesions of primary sensory afferents in the dorsal columns of the spinal cord, the reactivation of somatosensory cortex has been largely attributed to incomplete lesions and the preservation of some of the afferents from the hand in the cuneate fasciculus (e.g., Jain et al., 2000), because at least some reactivation from parts of the hand has been commonly reported. The preserved axons were thought to sprout and form new synapses in the cuneate nucleus, where they became more powerful and activated populations of neurons that relayed to the contralateral thalamus and then to cortex. In fact, the microinjection of chondroitinase ABC at the level of the cuneate nucleus to promote sprouting of surviving inputs from digit 1 after spinal cord lesions did result in a greater reactivation of contralateral somatosensory cortex by inputs from digit 1 (Bowes et al., 2012). Thus, surviving dorsal column afferents can be an important part of the recovery process. Other studies (Chen et al., 2012; Qi et al., 2011) also indicate that other pathways can be important, because extensive and nearly complete lesions of the cuneate fasciculus in untreated squirrel monkeys were also followed by considerable reactivation of contralateral somatosensory cortex by inputs from the hand.
In the present study, we demonstrated that extensive reactivation of contralateral somatosensory cortex is possible even after complete lesions of the cuneate fasciculus at levels removing all axon branches from the hand. Some of the reactivation came from parts of the neck, but some came from parts of the hand and adjoining arm. This leads us to consider other possible sources of sensory input. The second-order sensory neurons in the dorsal horn of the spinal cord seem to be a likely source, as these neurons receive the same information from low-threshold mechanoreceptors of the hand as do cuneate nucleus neurons, and some neurons of the dorsal horn project ipsilaterally to the cuneate nucleus (Bennett et al., 1983; Rustioni et al., 1979). These and other neurons may project to the cuneate nucleus via the dorsolateral fasciculus (Enevoldson and Gordon, 1989a,b) or other pathways and join the cuneate fasciculus above the cervical C5–6 level. However, many of the second-order neurons thought to project to the cuneate nucleus are thought to be excited by noxious stimuli or both noxious and tactile stimuli (Bennett et al., 1983), and the roles of these inputs remain uncertain. Other evidence suggests that dorsal horn inputs to the cuneate nucleus largely mediate tonic inhibitory control (Dykes and Craig, 1998). In any case, second-order neurons may largely provide subthreshold activation in intact monkeys, as dorsal column lesions have the immediate impact of inactivating the relevant parts of the cuneate nucleus (Xu and Wall, 1997). However, second-order inputs could be potentiated by the same mechanisms that potentiate surviving dorsal column inputs, leading to a reactivation of cuneate nucleus neurons and target thalamic and cortical neurons.
Another important finding from our three cases with complete lesions is that no cortical recording sites were activated by touching or lightly tapping the digits in two of three cases, and only digit 5 inputs activated sites in the third case. Inputs from digit 5 travel most medially in the cuneate fasciculus (Florence et al., 1989), where they could be favored to survive a lesion of the cuneate fasciculus, but our lesion in case 9-28 with the cortical activation by digit 5 extended well into the gracile fasciculus (Fig. 1A). Although uncertainties remain, there does appear to be a greater dependence on monosynaptic cuneate fasciculus inputs to the cuneate nucleus for the important tactile functions of the fingers.
As for our other relevant finding, a large but incomplete lesion of the cuneate fasciculus had a much more normal representation of the hand in contralateral somatosensory cortex area 3b. This adds to previous evidence that the reactivation patterns in cortex are more normal when more of the cuneate fasciculus inputs are preserved (Jain et al., 1997; Qi et al., 2011).
In other cases, neurons in area 1 and area 3a, and even motor cortex were responsive to tactile stimuli after recovery from cuneate fasciculus lesions, and these responses were likely dependent on activity relayed from area 3b (Garraghty et al., 1990a,b), although reactivation patterns in areas 3b, 3a, and 1 differed. Previous evidence for the reactivation of areas 3a and 1 after dorsal column lesions exists for other primates (Bowes et al., 2012; Jain et al., 2008; Qi et al., 2011).
In another case with an almost complete lesion of the cuneate fasciculus (Fig. 5), neurons likely in area 2–5 caudal to the border of area 1 were activated by the hand, whereas more lateral neurons in area 2 responded to touch on the face. Previously, there has been little information about the effects of dorsal column lesions on the responsiveness of neurons in area 2. Neurons caudal to area 1 in the region of area 2 have not been previously reported to be responsive to somatosensory stimuli in anesthetized marmosets (Carlson et al., 1986; Krubitzer and Kaas, 1990), and the weak responsiveness of neurons in the area 2 region of other small New World monkeys, together with somatotopic features, has led to the proposal that these primates may not have an area 2, or have a combined area 2–5 (Padberg et al., 2005). The present results indicate that cortex in this region is responsive to somatosensory stimuli as in other primates (Padberg et al., 2005, 2007; Pons and Kaas, 1985; Seelke et al., 2012), and can be reactivated after almost complete lesions of the cuneate fasciculus. Our limited results suggest that marmosets, and likely all New World monkeys, do have an area 2 with an orderly somatotopic map, and this representation can be activated after recovery from complete dorsal column lesions.
ACKNOWLEDGEMENTS
We thank Laura Trice for her technical assistance.
ROLE OF AUTHORS
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: CB, MB, JK. Acquisition of data: CB, MB, CC. Analysis and interpretation of data: CB, JK. Drafting of the manuscript: CB, JK. Review of the manuscript: CB, CC, MB, JK.
Grant sponsor: National Institutes of Health; Grant number: NS 16446.
Footnotes
CONFLICT OF INTEREST STATEMENT
No conflict of interest exists for any of the authors.
LITERATURE CITED
- Bennett GJ, Seltzer Z, Lu GW, Nishikawa N, Dubner R. The cells of origin of the dorsal column postsynaptic projection in the lumbosacral enlargements of cats and monkeys. Somatosens Res. 1983;1:131–149. doi: 10.3109/07367228309144545. [DOI] [PubMed] [Google Scholar]
- Bowes C, Massey JM, Burish M, Cerkevich CM, Kaas JH. Chondroitinase ABC promotes selective reactivation of somatosensory cortex in squirrel monkeys after a cervical dorsal column lesion. Proc Natl Acad Sci U S A. 2012;109:2595–2600. doi: 10.1073/pnas.1121604109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M, Huerta MF, Cusick CG, Kaas JH. Studies on the evolution of multiple somatosensory representations in primates: the organization of anterior parietal cortex in the New World Callitrichid, Saguinus. J Comp Neurol. 1986;246:409–426. doi: 10.1002/cne.902460309. [DOI] [PubMed] [Google Scholar]
- Chen LM, Qi HX, Kaas JH. Dynamic reorganization of digit representations in somatosensory cortex of nonhuman primates after spinal cord injury. J Neurosci. 2012;32:14649–14663. doi: 10.1523/JNEUROSCI.1841-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darian-Smith C, Brown S. Functional changes at periphery and cortex following dorsal root lesions in adult monkeys. Nat Neurosci. 2000;3:476–481. doi: 10.1038/74852. [DOI] [PubMed] [Google Scholar]
- Darian-Smith C, Ciferri M. Cuneate nucleus reorganization following cervical dorsal rhizotomy in the macaque monkey: its role in the recovery of manual dexterity. J Comp Neurol. 2006;498:552–565. doi: 10.1002/cne.21088. [DOI] [PubMed] [Google Scholar]
- Dykes RW, Craig AD. Control of size and excitability of mechanosensory receptive fields in dorsal column nuclei by homolateral dorsal horn neurons. J Neurophysiol. 1998;80:120–129. doi: 10.1152/jn.1998.80.1.120. [DOI] [PubMed] [Google Scholar]
- Enevoldson TP, Gordon G. Postsynaptic dorsal column neurons in the cat: a study with retrograde transport of horseradish peroxidase. Exp Brain Res. 1989a;75:611–620. doi: 10.1007/BF00249912. [DOI] [PubMed] [Google Scholar]
- Enevoldson TP, Gordon G. Spinocervical neurons and dorsal horn neurons projecting to the dorsal column nuclei through the dorsolateral fascicle: a retrograde HRP study in the cat. Exp Brain Res. 1989b;75:621–630. doi: 10.1007/BF00249913. [DOI] [PubMed] [Google Scholar]
- Florence SL, Kaas JH. Large-scale reorganization at multiple levels of the somatosensory pathway follows therapeutic amputation of the hand in monkeys. J neurosci. 1995;15:8083–8095. doi: 10.1523/JNEUROSCI.15-12-08083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florence SL, Wall JT, Kaas JH. The somatotopic pattern of afferent projections from the digits to the spinal cord and cuneate nucleus in macaque monkeys. Brain Res. 1988;452:388–392. doi: 10.1016/0006-8993(88)90045-5. [DOI] [PubMed] [Google Scholar]
- Florence SL, Wall JT, Kaas JH. Somatotopic organization of inputs from the hand to the spinal gray and cuneate nucleus of monkeys with observations on the cuneate nucleus of humans. J Comp Neurol. 1989;286:48–70. doi: 10.1002/cne.902860104. [DOI] [PubMed] [Google Scholar]
- Florence SL, Wall JT, Kaas JH. Central projections from the skin of the hand in squirrel monkeys. J Comp Neurol. 1991;311:563–578. doi: 10.1002/cne.903110410. [DOI] [PubMed] [Google Scholar]
- Garraghty PE, Florence SL, Kaas JH. Ablations of areas 3a and 3b of monkey somatosensory cortex abolish cutaneous responsivity in area 1. Brain Res. 1990a;528:165–169. doi: 10.1016/0006-8993(90)90213-u. [DOI] [PubMed] [Google Scholar]
- Garraghty PE, Pons TP, Kaas JH. Ablations of areas 3b (SI proper) and 3a of somatosensory cortex in marmosets deactivate the second and parietal ventral somatosensory areas. Somatosens Motor Res. 1990b;7:125–135. doi: 10.3109/08990229009144703. [DOI] [PubMed] [Google Scholar]
- Huffman KJ, Krubitzer L. Area 3a: topographic organization and cortical connections in marmoset monkeys. Cereb Cortex. 2001;11:849–867. doi: 10.1093/cercor/11.9.849. [DOI] [PubMed] [Google Scholar]
- Jain N, Catania KC, Kaas JH. Deactivation and reactivation of somatosensory cortex after dorsal spinal cord injury. Nature. 1997;386:495–498. doi: 10.1038/386495a0. [DOI] [PubMed] [Google Scholar]
- Jain N, Florence SL, Kaas JH. Reorganization of somatosensory cortex after nerve and spinal cord injury. News Physiol Sci. 1998;13:143–149. doi: 10.1152/physiologyonline.1998.13.3.143. [DOI] [PubMed] [Google Scholar]
- Jain N, Florence S, Qi H-X, Kaas J. Growth of new brainstem connections in adult monkeys with massive sensory loss. Proc Natl Acas Sci USA. 2000;97:5546–5550. doi: 10.1073/pnas.090572597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N, Qi HX, Collins CE, Kaas JH. Large-scale reorganization in the somatosensory cortex and thalamus after sensory loss in macaque monkeys. J Neurosci. 2008;28:11042–11060. doi: 10.1523/JNEUROSCI.2334-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Qi HX, Burish MJ, Gharbawie OA, Onifer SM, Massey JM. Cortical and subcortical plasticity in the brains of humans, primates, and rats after damage to sensory afferents in the dorsal columns of the spinal cord. Exp Neurol. 2008;209:407–416. doi: 10.1016/j.expneurol.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer LA, Kaas JH. The organization and connections of somatosensory cortex in marmosets. J Neurosci. 1990;10:952–974. doi: 10.1523/JNEUROSCI.10-03-00952.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Wall J, Nelson RJ, Sur M, Felleman D. Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience. 1983a;8:33–55. doi: 10.1016/0306-4522(83)90024-6. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Wall JT, Sur M, Nelson RJ, Felleman DJ. Progression of change following median nerve section in the cortical representation of the hand in areas 3b and 1 in adult owl and squirrel monkeys. Neuroscience. 1983b;10:639–665. doi: 10.1016/0306-4522(83)90208-7. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Nelson RJ, Stryker MP, Cynader MS, Schoppmann A, Zook JM. Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol. 1984;224:591–605. doi: 10.1002/cne.902240408. [DOI] [PubMed] [Google Scholar]
- Padberg J, Disbrow E, Krubitzer L. The organization and connections of anterior and posterior parietal cortex in titi monkeys: do New World monkeys have an area 2? Cereb Cortex. 2005;15:1938–1963. doi: 10.1093/cercor/bhi071. [DOI] [PubMed] [Google Scholar]
- Padberg J, Franca JG, Cooke DF, Soares JG, Rosa MG, Fiorani M, Jr, Gattass R, Krubitzer L. Parallel evolution of cortical areas involved in skilled hand use. J Neurosci. 2007;27:10106–10115. doi: 10.1523/JNEUROSCI.2632-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons TP, Kaas JH. Connections of area 2 of somatosensory cortex with the anterior pulvinar and subdivisions of the ventroposterior complex in macaque monkeys. J Comp Neurol. 1985;240:16–36. doi: 10.1002/cne.902400103. [DOI] [PubMed] [Google Scholar]
- Pons TP, Garraghty PE, Cusick CG, Kaas JH. The somatotopic organization of area 2 in macaque monkeys. J Comp Neurol. 1985;241:445–466. doi: 10.1002/cne.902410405. [DOI] [PubMed] [Google Scholar]
- Pons TP, Garraghty PE, Ommaya AK, Kaas JH, Taub E, Mishkin M. Massive cortical reorganization after sensory deafferentation in adult macaques. Science. 1991;252:1857–1860. doi: 10.1126/science.1843843. [DOI] [PubMed] [Google Scholar]
- Qi HX, Chen LM, Kaas JH. Reorganization of somatosensory cortical areas 3b and 1 after unilateral section of dorsal columns of the spinal cord in squirrel monkeys. J Neurosci. 2011;31:13662–13675. doi: 10.1523/JNEUROSCI.2366-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustioni A, Hayes NL, O'Neill S. Dorsal column nuclei and ascending spinal afferents in macaques. Brain. 1979;102:95–125. doi: 10.1093/brain/102.1.95. [DOI] [PubMed] [Google Scholar]
- Seelke AM, Padberg JJ, Disbrow E, Purnell SM, Recanzone G, Krubitzer L. Topographic maps within Brodmann's area 5 of macaque monkeys. Cereb Cortex. 2012;22:1834–1850. doi: 10.1093/cercor/bhr257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon S, Kambi N, Lazar L, Mohammed H, Jain N. Large-scale expansion of the face representation in somatosensory areas of the lateral sulcus after spinal cord injuries in monkeys. J Neurosci. 2009;29:12009–12019. doi: 10.1523/JNEUROSCI.2118-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis WD, Coggeshall RE. Sensory mechanisms of the spinal cord: Vol2, Ascending sensory tracts and their descending control. Kluwer Academic/Plenum; New York: 2004. [Google Scholar]
- Xu J, Wall JT. Rapid changes in brainstem maps of adult primates after peripheral injury. Brain Res. 1997;774:211–215. doi: 10.1016/s0006-8993(97)81706-4. [DOI] [PubMed] [Google Scholar]