Abstract
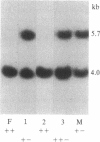
To test the hypothesis that there is a genetic predisposition to nondisjunction and trisomy 21 associated with DNA sequences on chromosome 21, we used DNA polymorphism haplotypes for chromosomes 21 to examine the distribution of different chromosomes 21 in Down syndrome and control families from the same ethnic group. The chromosomes 21 from 20 Greek families with a Down syndrome child and 27 control Greek families have been examined for DNA polymorphism haplotypes by using four common polymorphic sites adjacent to two closely linked single-copy DNA sequences (namely pW228C and pW236B), which map somewhere near the proximal long arm of chromosome 21. Three haplotypes, +, +---, and - with respective frequencies of 43/108, 24/108, and 23/108, account for the majority of chromosomes 21 in the control families. However, haplotype - was found to be much more commonly associated with chromosomes 21 that underwent nondisjunction in the Down syndrome families (frequency of 21/50; X2 for the two distributions is 9.550; P = 0.023; degrees of freedom, 3). The two populations (control and trisomic families) did not differ in the distribution of haplotypes for two DNA polymorphisms on chromosome 17. The data from this initial study suggest that the chromosome 21, which is marked in Greeks with haplotype - for the four above described polymorphic sites, is found more commonly in chromosomes that participate in nondisjunction than in controls. We propose an increased tendency for nondisjunction due to DNA sequences associated with a subset of chromosomes 21 bearing this haplotype.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfi O. S., Chang R., Azen S. P. Evidence for genetic control of nondisjunction in man. Am J Hum Genet. 1980 Jul;32(4):477–483. [PMC free article] [PubMed] [Google Scholar]
- Antonarakis S. E., Boehm C. D., Giardina P. J., Kazazian H. H., Jr Nonrandom association of polymorphic restriction sites in the beta-globin gene cluster. Proc Natl Acad Sci U S A. 1982 Jan;79(1):137–141. doi: 10.1073/pnas.79.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker B. S., Carpenter A. T., Esposito M. S., Esposito R. E., Sandler L. The genetic control of meiosis. Annu Rev Genet. 1976;10:53–134. doi: 10.1146/annurev.ge.10.120176.000413. [DOI] [PubMed] [Google Scholar]
- Davies K. E., Harper K., Bonthron D., Krumlauf R., Polkey A., Pembrey M. E., Williamson R. Use of a chromosome 21 cloned DNA probe for the analysis of non-disjunction in Down syndrome. Hum Genet. 1984;66(1):54–56. doi: 10.1007/BF00275186. [DOI] [PubMed] [Google Scholar]
- Harlap S. A time-series analysis of the incidence of Down's syndrome in West Jerusalem. Am J Epidemiol. 1974 Mar;99(3):210–217. doi: 10.1093/oxfordjournals.aje.a121604. [DOI] [PubMed] [Google Scholar]
- Harlap S. Down's syndrome in West Jerusalem. Am J Epidemiol. 1973 Apr;97(4):225–232. doi: 10.1093/oxfordjournals.aje.a121503. [DOI] [PubMed] [Google Scholar]
- Hassold T. J., Jacobs P. A. Trisomy in man. Annu Rev Genet. 1984;18:69–97. doi: 10.1146/annurev.ge.18.120184.000441. [DOI] [PubMed] [Google Scholar]
- Hassold T., Chiu D., Yamane J. A. Parental origin of autosomal trisomies. Ann Hum Genet. 1984 May;48(Pt 2):129–144. doi: 10.1111/j.1469-1809.1984.tb01008.x. [DOI] [PubMed] [Google Scholar]
- Hook E. B., Harlap S. Differences in maternal age-specific rates of Down syndrome between Jews of European origin and of North African or Asian origin. Teratology. 1979 Oct;20(2):243–248. doi: 10.1002/tera.1420200209. [DOI] [PubMed] [Google Scholar]
- Kunkel L. M., Smith K. D., Boyer S. H., Borgaonkar D. S., Wachtel S. S., Miller O. J., Breg W. R., Jones H. W., Jr, Rary J. M. Analysis of human Y-chromosome-specific reiterated DNA in chromosome variants. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1245–1249. doi: 10.1073/pnas.74.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieman-Hurwitz J., Dafni N., Lavie V., Groner Y. Human cytoplasmic superoxide dismutase cDNA clone: a probe for studying the molecular biology of Down syndrome. Proc Natl Acad Sci U S A. 1982 May;79(9):2808–2811. doi: 10.1073/pnas.79.9.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Kee S. G., Efstratiadis A., Kafatos F. C. Amplification and characterization of a beta-globin gene synthesized in vitro. Cell. 1976 Jun;8(2):163–182. doi: 10.1016/0092-8674(76)90001-5. [DOI] [PubMed] [Google Scholar]
- Martial J. A., Hallewell R. A., Baxter J. D., Goodman H. M. Human growth hormone: complementary DNA cloning and expression in bacteria. Science. 1979 Aug 10;205(4406):602–607. doi: 10.1126/science.377496. [DOI] [PubMed] [Google Scholar]
- Phillips J. A., 3rd, Parks J. S., Hjelle B. L., Herd J. E., Plotnick L. P., Migeon C. J., Seeburg P. H. Genetic analysis of familial isolated growth hormone deficiency type I. J Clin Invest. 1982 Sep;70(3):489–495. doi: 10.1172/JCI110640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A. F., Phillips J. A., 3rd, Migeon B. R. DNA restriction endonuclease analysis for localization of human beta- and delta-globin genes on chromosome 11. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4563–4565. doi: 10.1073/pnas.76.9.4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L., Dafni N., Lieman-Hurwitz J., Groner Y. Nucleotide sequence and expression of human chromosome 21-encoded superoxide dismutase mRNA. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5465–5469. doi: 10.1073/pnas.80.18.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tan Y. H., Tischfield J., Ruddle F. H. The linkage of genes for the human interferon-induced antiviral protein and indophenol oxidase-B traits to chromosome G-21. J Exp Med. 1973 Feb 1;137(2):317–330. doi: 10.1084/jem.137.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]