Abstract
ROCK1 and ROCK2 mediate important processes such as cell migration, invasion and metastasis; making them good targets for the development of antitumor agents. Recently, using a fragment-based approach and X-ray crystallography, we reported on the design and synthesis of novel Rho-kinase inhibitors (RKIs). Here, we selected a pair of RKIs, the closely-related structural analogues RKI-18 (potent; IC50 values of 397 nM (ROCK1) and 349 nM (ROCK2)) and RKI-11 (weak/inactive; IC50 values of 38 µM (ROCK1) and 45 µM (ROCK2), as chemical probes and determined their effects on cytoskeleton organization, signaling, apoptosis, anchorage-dependent and –independent growth, migration and invasion. RKI-18 but not RKI-11 suppresses potently the phosphorylation of the ROCK substrate MLC2 in intact human breast, lung, colon and prostate cancer cells. Furthermore, RKI-18 is highly selective at decreasing the levels of P-MLC2 over those of P-Akt, P-S6 and P-Erk ½. RKI-18 suppresses ROCK-mediated actin fiber formation following stimulation with LPA as well as PAK-mediated lamelipodia and filopodia formation following bradykinin or PDGF stimulation. Furthermore, RKI-18 but not RKI-11 inhibits migration, invasion and anchorage-independent growth of human breast cancer cells. The fact that the active ROCK inhibitor RKI-18 but not the inactive closely related structural analogue RKI-11 is effective at suppressing malignant transformation suggests that inhibition of ROCK with RKI-18 results in preventing migration, invasion and anchorage-independent growth. The potential of this class of RKIs as anti tumor agents warrants further advanced preclinical studies.
Keywords: RKI-18, ROCK1, ROCK2, Invasion, Migration, MLC-2
INTRODUCTION
The Rho associated kinases 1 and 2 (ROCK1 and ROCK2) are Ser/Thr kinases that regulate important cellular processes such as cell morphology, shape, adhesion and migration (1–7). A major mechanism by which ROCKs affect these processes is through the phosphorylation of myosin light chain (MLC), the MLC phosphatase PP1 regulatory subunit MYPT-1 and Lim kinase, all of which regulate actin-myosin contractility. Phosphorylation of MLC activates it to induce cell migration (7, 8) whereas phosphorylation of MYPT-1 inhibits de-phosphorylation of MLC (6). Furthermore, phosphorylation of Lim Kinase activates it to phosphorylate and inactivate cofilin which is known to suppress migration (9). The involvement of ROCKs in malignant transformation has been well studied. For example, ROCKs are over expressed in cancer cells relative to normal cells, and this over expression is associated with metastasis, poor clinical outcome and shorter survival of cancer patients (10, 11). Furthermore, depletion of ROCKs inhibits invasion and metastasis of cancer in vitro and in vivo (10, 12–17). In contrast, forced expression induces migration and invasion (14, 18, 19). Further evidence for the involvement of ROCKs comes from the fact that Rho GTPases such as RhoA and RhoC are the immediate activators of ROCKs and their over expression induces whereas their depletion inhibits migration, invasion and metastasis (20, 21). Furthermore, Rho GTPases have been shown to be overexpressed in a variety of cancer types (22–27), and at least one of these, RhoC, has been suggested as a prognostic biomarker for metastasis in breast, melanoma and pancreatic cancer (21, 26, 27). The overwhelming data supporting the contributions of ROCKs and their affecters Rho GTPases in metastasis prompted us and others to investigate the possibility of identifying ROCK inhibitors as potential anti tumor agents. In this report we describe the ability of novel ROCK inhibitors that we have recently identified (28) to suppress anchorage-independent growth, migration and invasion of cancer cells. We also describe the ability of the ROCK inhibitors to suppress cytoskeletal and cell morphological changes that are associated with migration and invasion.
RESULTS AND DISCUSSION
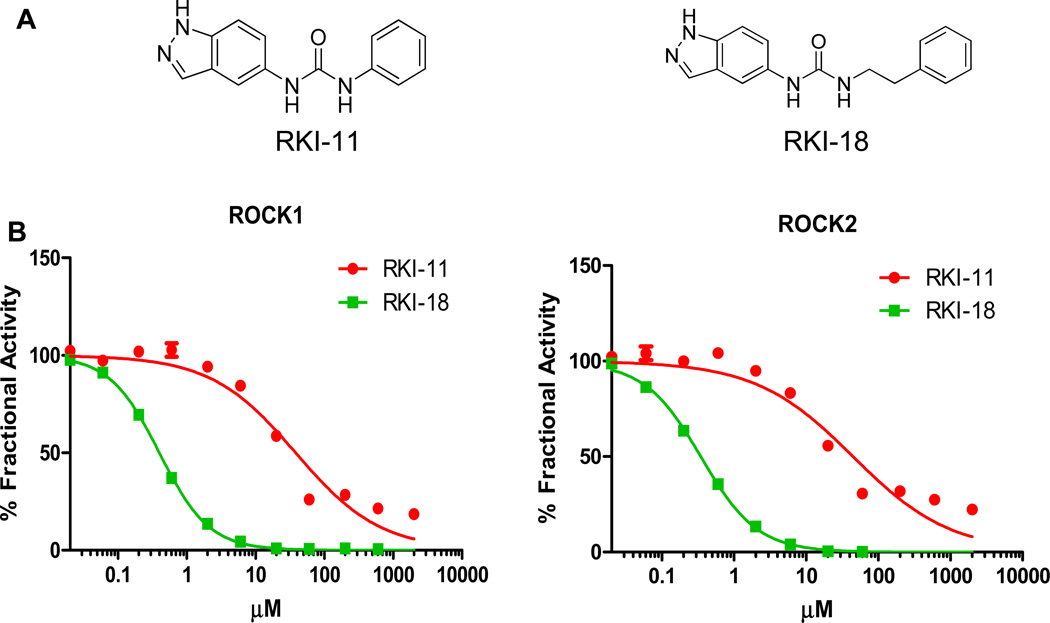
Identification of a pair of closely-related structural analogues RKI-18 (potent) and RKI-11 (weak/inactive) ROCK inhibitors
Our recent chemistry efforts using fragment-based drug design coupled with X-ray crystallography resulted in the identification of potent Rho Kinase Inhibitors (RKIs) (28). In an effort to investigate the effects of these inhibitors on signaling, anchorage-dependent and -independent tumor cell growth, apoptosis, migration and invasion we selected a pair of closely-related analogues, one potent and the other weak/inactive RKI. RKI-18 and RKI-11 are structurally very close indazole urea-based analogues where in RKI-18 the indazole urea and the phenyl group are linked by the two carbon ethylene, whereas in RKI-11 they are attached directly without a linker (Figure 1A). Figure 1B shows that RKI-18 and RKI-11 inhibited ROCK1 with IC50 values of 397 nM and 38 µM. Figure 1B also shows that RKI-18 and RKI-11 inhibited ROCK2 with IC50 values of 349 nM and 45 µM, respectively. Thus, RKI-18 was 96- to 129-fold more potent than RKI-11, providing an ideal pair of potent / weak (inactive) chemical probes for investigating the effects of ROCK inhibition on malignant transformation.
Figure 1.
A. Chemical structures of Rho-kinase Inhibitors RKI-11 and RKI-18. B. In vitro inhibitory activity of RKI-18 and RKI-11 against ROCK 1 and ROCK2 kinase activities.
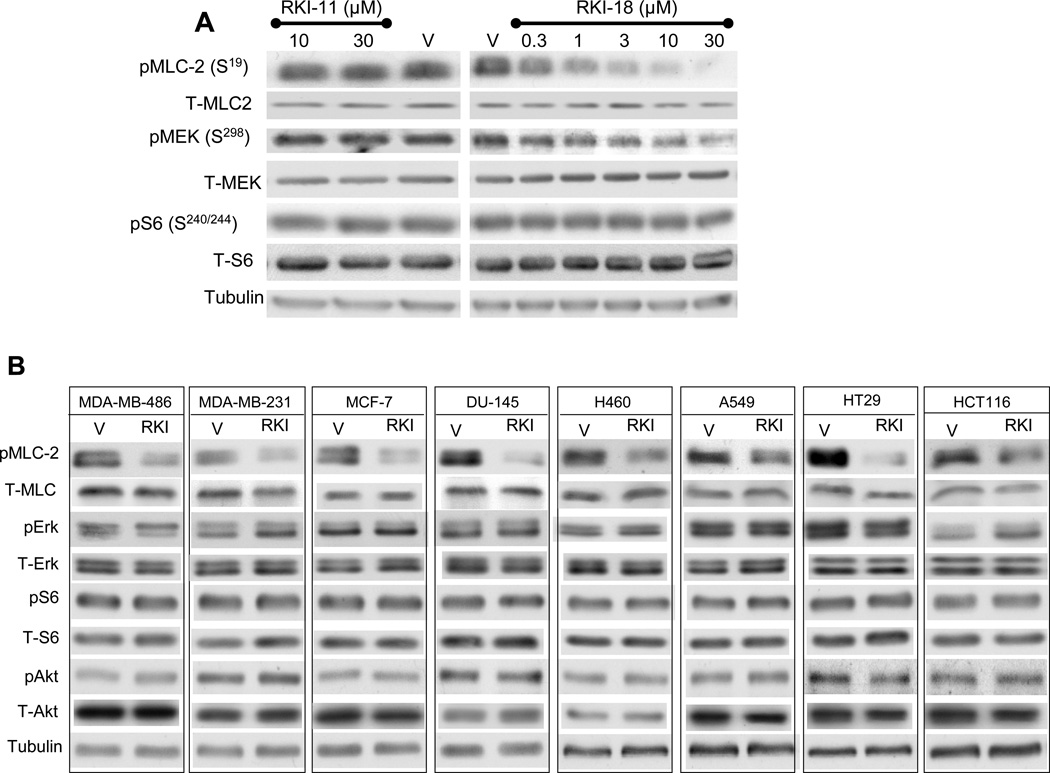
RKI-18 but not RKI-11 inhibits phosphorylation of the ROCK substrate MLC-2 selectively over the phosphorylation of Akt, Erk and S6 kinases in human cancer cells
In order to determine the activity of the ROCK inhibitors in intact human cancer cells, MDA-MB-231 breast cancer cells were treated with various concentrations of RKI-11, RKI-18, or vehicle, and their ability to inhibit the phosphorylation of MLC-2 at ser19, a well-known substrate of ROCK was assessed by western blotting as described under Materials and Methods. Figure 2A shows that RKI-18 suppressed phospho MLC-2 levels at concentration as low as 1 µM whereas RKI-11, consistent with its weak in vitro activity, had no effect at concentrations as high as 30 µM. RKI-18 also inhibited the phosphorylation of MEK at Ser298, a site known to be phosphorylated by p21-activated kinase (PAK) (29) but RKI-18 was more potent towards inhibiting P-MLC2 than P-Mek. In contrast, RKI-18 had little effects on phospho Ser-240/244-S6 (mTORC1 substrate) (Figure 2A). To determine whether RKI-18 can inhibit the phosphorylation of MLC-2 in other human cancer cell lines, we treated 3 breast (MCF-7, MDA-MB-468 and MDA-MB-231), 2 lung (A549 and H460), 2 colon (HT29 and HCT116) and one prostate (DU145) cancer cell lines with either vehicle or RKI-18 and processed the cells for Western blotting as described under Materials and Methods. Figure 2 B shows that in all 8 cell lines RKI-18 inhibited the phosphorylation levels of MLC-2. RKI-18 was highly selective for P-MLC-2 over P-S240/244-S6 (mTORC1 substrate), P-S473-Akt (mTORC2 substrate) and P-Erk ½ (Mek substrate) (Figure 2B). Taken together these results demonstrate that RKI-18 is highly selective for ROCK over other kinases such as mTORC1, mTORC2 and Mek. Finally, consistent with the in vitro kinase activity assay results, RKI-18 was much more potent than RKI-11 in intact cells.
Figure 2. RKI-18 but not RKI-11 inhibits the phosphorylation of ROCK substrate MLC-2 in human cancer cells of different origins.
A. MDA-MB-231 cells were treated with different concentrations of RKI-11or RKI-18 and processed for western immunoblotting as described under Materials and Methods. B. MDA-MB-468, MDA-MB-231, MCF-7, DU-145, H460, A549, HT29 and HCT116 cells were treated with either vehicle (V) or 3 µM RKI-18 (RKI) and processed for western immunoblotting as described under Material and Methods. Data are representative of three (A) and one (B) independent experiments.
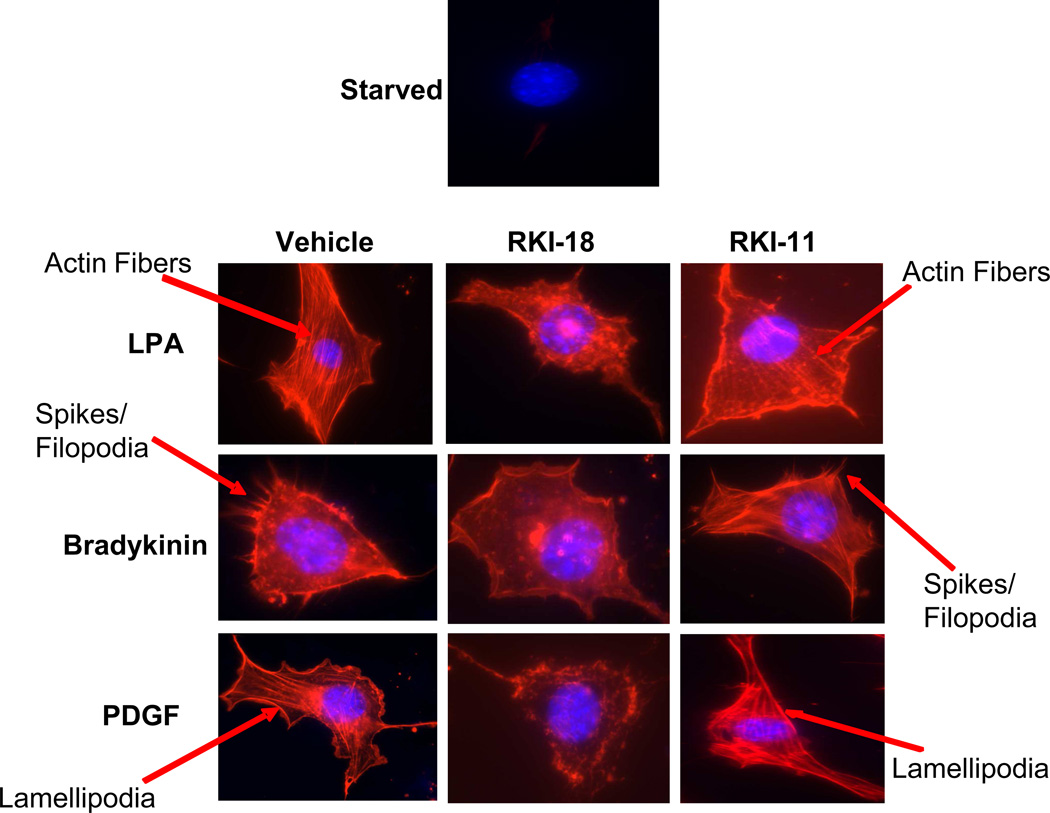
RKI 18 but not RKI-11 inhibits LPA-, Bradykinin- and PDGF-induced stress fiber formation, filopodia and lamelipodia, respectively
Stress fiber formation upon stimulation with LPA (Lysophosphatidic Acid) is a cytoskeleton re-organization process mediated by the activation of RhoA/ROCK pathway whereas PDGF stimulation of lamelipodia formation is mediated by the RAC1/PAK pathway and Bradykinin stimulation of filopodia (spikes) formation is mediated by the CDC42/PAK pathway (30). To determine the effects of our RKIs on these morphological changes, we starved NIH 3T3 cells and treated them with either RKI-11, RKI-18 or vehicle prior to stimulating them with either LPA, PDGF or bradykinin, and then staining the cells with phalloidin as described under Materials and Methods. Figure 3 shows that RKI-18 but not RKI-11 inhibited LPA-induced stress fiber formation, bradykinin-induced filopodia formation and PDGF-induced lamelipodia formation (Figure 3). The ability of RKI-18 to inhibit the formation of actin stress fiber, filopodia and lamelipodia, cytoskeletal changes that are critical to cell motility, suggests that this inhibitor may interfere with the ability of cancer cells to migrate and invade.
Figure 3. RKI-18 but not RKI-11, inhibits the formation of stress fibers, filopodia and lamellipodia upon stimulation with LPA, bradykinin and PDGF respectively.
Starved NIH 3T3 cells were treated with either vehicle or RKIs (10 µM) for 1 hour before treatment with either LPA, bradykinin or PDGF. The cells were then fixed and stained with Texas-red Phalloidin and DAPI as described in Materials and Methods. Data are representative of three independent experiments.
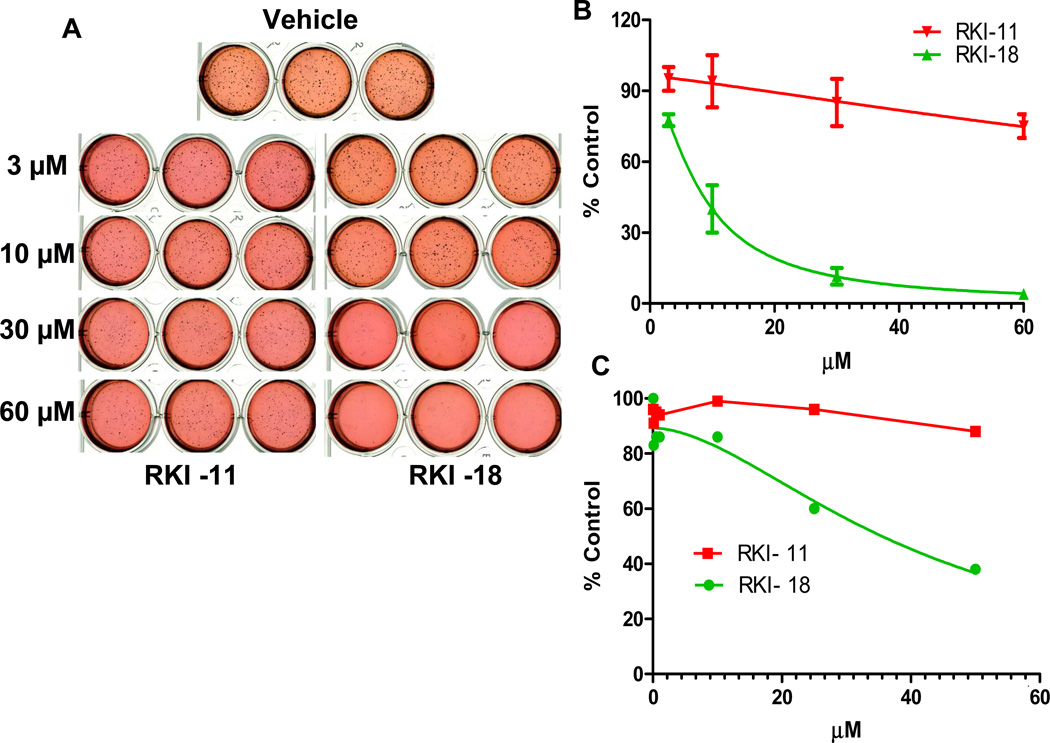
RKI-18 but not RKI-11 inhibits anchorage-independent growth, migration and invasion of human breast cancer cells
The ability of cancer cells to metastasize depends on their ability to grow in an anchorage-independent manner, migrate and invade. We, therefore, investigated the effects of RKI-18 and RKI-11 to affect these cancer hallmarks. To this end, we first determined the ability of these compounds to inhibit anchorage-independent growth in Soft Agar assay as described under Materials and Methods. The results from Figures 4A and 4B demonstrate that RKI-18 inhibited soft agar colony formation of MDA-MB-231 cells with an IC50 of 8 µM whereas the closely related analog and less active ROCK inhibitor RKI-11 had little effect at 60 µM on anchorage-independent growth. To determine the effects of RKI-18 and RKI-11 on anchorage-dependent proliferation and survival, MDA-MB-231 cells were treated with increasing concentrations of the inhibitors and processed for MTT assays as well as Western blotting as described under Materials and Methods. Figure 4C shows that RKI-18 was 4-fold less potent at inhibiting anchorage-dependent proliferation (IC50 = 32 µM) as compared to anchorage-independent growth (IC50 = 8 µM). Furthermore, RKI-18 treatment (up to 30 µM) of these cells did not induce apoptosis as measured by caspase 3 activation and PARP cleavage (data not shown). These results suggest that the ability of RKI-18 to inhibit the anchorage-independent growth of cancer cell on soft agar is not due to inhibition of anchorage-dependent proliferation and/or induction of apoptosis.
Figure 4. RKI-18 but not RKI-11 inhibits anchorage-independent growth of human breast cancer cells.
MDA-MB-231 cells were treated with various concentrations of RKIs (3 µM, 10 µM, 30 µM and 60 µM) and processed for anchorage-independent soft agar growth (A and B) as described under Materials and Methods section (Data show the average of 2 independent experiments). (C) Cells were treated with either vehicle or various concentrations of RKIs (0.05 – 50 µM) for 72 hours and stained with MTT (2mg/ml). Data are representative of 2 independent experiments.
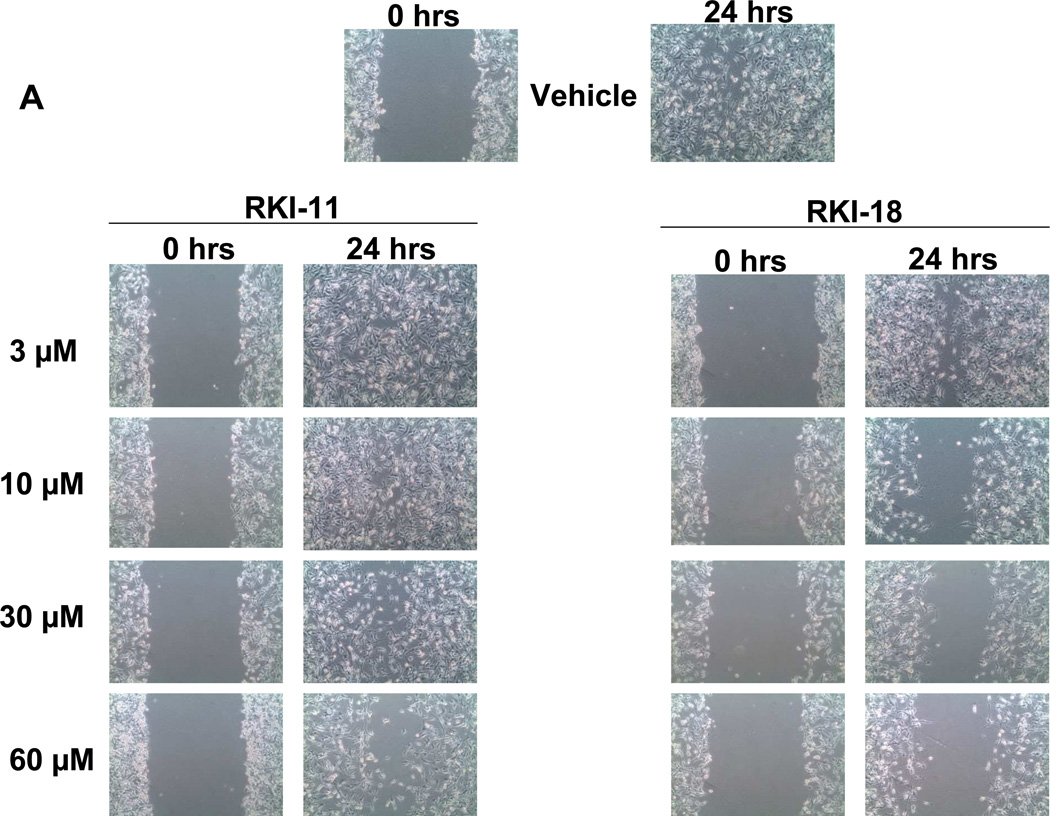
We next determined the ability of these compounds to inhibit migration of breast cancer cells in a wound healing scratch assay as described under Materials and Methods. RKI-18 inhibited the migration of MDA-MB-231 cells in a concentration dependent manner starting at 3 µM (Figure 5A). In contrast, RKI-11 at 60 µM did not inhibit the migration of these cells (Figure 5A). The ability of cancer cells to metastasize depends on their ability to invade; we therefore investigated the ability of RKIs to inhibit invasion as described under Materials and Methods. Figure 5B shows that RKI-18 at 10 µM inhibited the cancer cells from invading through Matrigel by 67% whereas RKI-11 had no effect on cell invasion at 60 µM.
Figure 5. RKI-18 but not RKI-11 inhibits migration and invasion of human breast cancer cells.
A. Cells were plated at 4X105 cells per well, starved for 24 hours, scratched and treated with vehicle or RKIs as described under Materials and Methods section (Data representative of 3 independent experiments). B. MDA-MB-231 cells were treated with vehicle or RKIs and plated at 20,000 cells per insert in Corning Transwell inserts coated with Matrigel and allowed to invade for 48 hours, after which they were analyzed as described under Materials and Methods. Data shows average of 3 independent experiments.
In summary, this manuscript describes the anti-migratory and anti-invasive activities of novel ROCK inhibitors as well as their ability to suppress anchorage-independent tumor cell growth and to inhibit cytoskeletal changes associated with migration and invasion. We chose two pairs of inhibitors consisting of potent and weak/inactive RKIs to be able to demonstrate a correlation between inhibition of the biochemical target and the modulation of the biological phenotypes and cancer hallmarks. RKI-18 and RKI-11 differ by only a two carbon linker, yet RKI-18 is 96- and 129-fold more potent towards ROCK1 and ROCK2, respectively. This was respected in intact breast cancer cells where RKI-18 suppressed the phosphorylation of the ROCK substrate MLC2 whereas RKI-11 had little effect. Importantly, RKI-18 but not RKI-11 inhibited migration, invasion and anchorage-independent growth. The tight correlation between inhibition of ROCK in vitro, inhibition of P-MLC2 levels and suppression of migration, invasion and anchorage-independent tumor cell growth suggest that the ability of RKI-18 to suppress these cancer hallmarks is at least in part due to their ability to inhibit ROCK. RKI-18 inhibited the phosphorylation of MCL2 selectively over the phosphorylation of Akt, Erk 1/2 and S6 suggesting that this inhibitor is not promiscuous. However, RKI-18 also inhibited, although to a lesser extent, the phosphorylation of Ser-298-Mek suggesting that they also inhibit PAK. Consistent with this is the fact that RKI-18 inhibited not only the Rho-ROCK-dependent LPA stimulation of stress fiber formation but also the Rac/PAK-dependent bradykinin stimulation of filopodia formation as well as the CDC42/PAK-mediated PDGF-stimulation of lamellipodia formation. Therefore, the ability of RKI-18 to inhibit migration, invasion and anchorage-independent tumor cell growth may also be mediated at least in part by inhibition of PAK. Inhibition of PAK is desirable since this is a well validated cancer drug discovery target. It is interesting to note here that we have recently reported on another ROCK inhibitor, RKI-1447, with a different pharmacophore than RKI-18 and that was highly selective in its effects on cytoskeletal organization (31). Indeed RKI-1447 inhibited LPA- but not Bradykinin- or PDGF-induced cytoskeletal changes. Consistent with this, RKI-1447 did not inhibit the phosphorylation of S298 on Mek (31). Finally, RKI-18 did not induce apoptosis and inhibited anchorage-dependent proliferation at concentrations that are much higher than those needed to inhibit anchorage-independent growth, migration and invasion. This suggests that the ability of RKI-18 to inhibit anchorage-independent growth, migration and invasion is not due to inhibition of proliferation and/or induction of apoptosis. This is consistent with a mechanism involving inhibition of ROCKs, kinases known to be involved in migration and invasion and not proliferation and survival (6, 32).
MATERIALS AND METHODS
ROCK1 and ROCK2 kinase assays
The assay conditions and quantification procedures were exactly the same as described previously by us (28).
Western Blot Analysis
MDA-MB-468, MDA-MB-231, MCF-7, DU-145, H460, A549, HT29 and HCT116 cells (ATCC, Rockville, MD) were plated in 6-well tissue culture plate. The cells were then treated next day with different concentrations of the RKIs for 1 hour. After incubation the cells were harvested, lysed, quantified and blotted for P-MLC2 (Cat# 3671S), Total-MLC2 (Cat# 3672S), P-MEK (S298) (Cat# 9128S), Total-MEK (Cat# 9122S), P-S6 (Cat# 2155S), Total-S6 (2217S), P-Erk (Cat# 9101L), Total Erk (Cat# 9102L), P-Akt (Cat# 9271L), Total Akt (Cat# 9272L) (Cell Signaling, Danvers, MA) and Tubulin (Cat# 5168) (Sigma, St. Louis, MO) antibodies as described by us previously (31).
Cell Morphology Assay
NIH 3T3 cells were plated at 8000 cells per well were plated in 8-chamber slide in serum free media (serum starvation) for 24 hours. After serum starvation the cells were treated with vehicle or the RKIs for 1 hour. After treatment, the cells were stimulated with 10µM LPA, 200ng/mL bradykinin or 30ng/mL PDGF for 30mins. After stimulation, the cells were fixed with 4% Para-formaldehyde, permeabilized using and 0.1% Triton X-100 and stained with Texas-Red Phalloidin (Cat# T7471) (Invitrogen, Eugene, OR) and DAPI (4’,6 diamidino-2-phenylindole: counterstains DNA) (Cat#H-1200; Vector Laboratories, Burlingame, CA). The cells were imaged using Zeiss Upright Fluorescence Microscope.
Soft agar assay
MDA-MB-231 cells were seeded in a 12 well tissue culture plate at 1200 cells per well in regular growth media and 0.3% agar as described by us (33). The cells were treated with different doses of RKI-11and RKI-18, allowed to grow for 4 weeks after which the colonies were stained overnight with 1mg/ml MTT and counted as described by us (33).
MTT assay
MTT assay was performed to determine the effects of RKIs on anchorage-dependent cell proliferation. Briefly, cells were plated in a 96 well tissue culture plate (1200 cells per well) and incubated for 24 hours. After incubation the cells were treated with vehicle or increasing concentrations of RKI-11 or RKI-18 (0.05 -50 µM). After 72 hour incubation, freshly prepared MTT (2mg/ml) was added to each well and incubated for 3 hours. After incubation the plates were read at 540nm.
Wound healing-Migration assay
MDA-MB-231 cells were seeded at 4X105 cells per well in a 6-well plate and allowed to grow overnight at 37 degree C and 5% CO2. The cells were starved for 24 hours and scratched. The cells were then treated with different concentrations of RKIs in regular growth media for 24 hours. Images of the scratch were acquired at 0 hours and 24 hours.
Invasion assay
Invasion assay was performed in Corning Transwell inserts coated with Matrigel. MDA-MB-231 cells were seeded at 3.5X105 and allowed to grow overnight in a 6well-plate. The cells were treated with either vehicle or different doses of RKIs for 24 hours. After treatment, the cells were trypsinized, resuspended and plated in the inserts as described by us previously (31). The bottom chamber contained 20% FBS as the “chemoattractant”. The cells were kept in the incubator for 48 hours. After incubation the cells were stained and membrane was quantified exactly the same way as described by us previously (31).
Acknowledgements
The authors would like to thank Nan Sun (Moffitt Cancer Center) and the following Moffitt Cancer Center core facilities: the Chemical Biology Core, the Analytical Microscopy Core and Comparative Medicine Core facility.
Grant Support: This work was supported by NCI grant U19 CA 067771.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
REFERENCES
- 1.Amano M, Fukata Y, Kaibuchi K. Regulation and functions of Rho-associated kinase. Exp Cell Res. 2000;261(1):44–51. doi: 10.1006/excr.2000.5046. Epub 2000/11/18. [DOI] [PubMed] [Google Scholar]
- 2.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83(4):1325–1358. doi: 10.1152/physrev.00023.2003. Epub 2003/09/25. [DOI] [PubMed] [Google Scholar]
- 3.Zicha D, Dobbie IM, Holt MR, Monypenny J, Soong DY, Gray C, et al. Rapid actin transport during cell protrusion. Science. 2003;300(5616):142–145. doi: 10.1126/science.1082026. Epub 2003/04/05. [DOI] [PubMed] [Google Scholar]
- 4.Redowicz MJ. Rho-associated kinase: involvement in the cytoskeleton regulation. Arch Biochem Biophys. 1999;364(1):122–124. doi: 10.1006/abbi.1999.1112. Epub 1999/03/24. [DOI] [PubMed] [Google Scholar]
- 5.Tang Y, Olufemi L, Wang MT, Nie D. Role of Rho GTPases in breast cancer. Front Biosci. 2008;13:759–776. doi: 10.2741/2718. Epub 2007/11/06. [DOI] [PubMed] [Google Scholar]
- 6.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nature reviews Molecular cell biology. 2003;4(6):446–456. doi: 10.1038/nrm1128. Epub 2003/06/05. [DOI] [PubMed] [Google Scholar]
- 7.Olson MF. Applications for ROCK kinase inhibition. Curr Opin Cell Biol. 2008;20(2):242–248. doi: 10.1016/j.ceb.2008.01.002. Epub 2008/02/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olson MF, Sahai E. The actin cytoskeleton in cancer cell motility. Clin Exp Metastasis. 2009;26(4):273–287. doi: 10.1007/s10585-008-9174-2. Epub 2008/05/24. [DOI] [PubMed] [Google Scholar]
- 9.Scott RW, Olson MF. LIM kinases: function, regulation and association with human disease. J Mol Med (Berl) 2007;85(6):555–568. doi: 10.1007/s00109-007-0165-6. Epub 2007/02/13. [DOI] [PubMed] [Google Scholar]
- 10.Liu S, Goldstein RH, Scepansky EM, Rosenblatt M. Inhibition of rho-associated kinase signaling prevents breast cancer metastasis to human bone. Cancer research. 2009;69(22):8742–8751. doi: 10.1158/0008-5472.CAN-09-1541. Epub 2009/11/06. [DOI] [PubMed] [Google Scholar]
- 11.Lane J, Martin TA, Watkins G, Mansel RE, Jiang WG. The expression and prognostic value of ROCK I and ROCK II and their role in human breast cancer. Int J Oncol. 2008;33(3):585–593. Epub 2008/08/13. [PubMed] [Google Scholar]
- 12.Routhier A, Astuccio M, Lahey D, Monfredo N, Johnson A, Callahan W, et al. Pharmacological inhibition of Rho-kinase signaling with Y-27632 blocks melanoma tumor growth. Oncol Rep. 2010;23(3):861–867. Epub 2010/02/04. [PubMed] [Google Scholar]
- 13.Ying H, Biroc SL, Li WW, Alicke B, Xuan JA, Pagila R, et al. The Rho kinase inhibitor fasudil inhibits tumor progression in human and rat tumor models. Mol Cancer Ther. 2006;5(9):2158–2164. doi: 10.1158/1535-7163.MCT-05-0440. Epub 2006/09/21. [DOI] [PubMed] [Google Scholar]
- 14.Itoh K, Yoshioka K, Akedo H, Uehata M, Ishizaki T, Narumiya S. An essential part for Rho-associated kinase in the transcellular invasion of tumor cells. Nat Med. 1999;5(2):221–225. doi: 10.1038/5587. Epub 1999/02/04. [DOI] [PubMed] [Google Scholar]
- 15.Imamura F, Mukai M, Ayaki M, Akedo H. Y-27632, an inhibitor of rho-associated protein kinase, suppresses tumor cell invasion via regulation of focal adhesion and focal adhesion kinase. Jpn J Cancer Res. 2000;91(8):811–816. doi: 10.1111/j.1349-7006.2000.tb01018.x. Epub 2000/08/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wyckoff JB, Pinner SE, Gschmeissner S, Condeelis JS, Sahai E. ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr Biol. 2006;16(15):1515–1523. doi: 10.1016/j.cub.2006.05.065. Epub 2006/08/08. [DOI] [PubMed] [Google Scholar]
- 17.Yoshioka K, Foletta V, Bernard O, Itoh K. A role for LIM kinase in cancer invasion. Proc Natl Acad Sci U S A. 2003;100(12):7247–7252. doi: 10.1073/pnas.1232344100. Epub 2003/06/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourguignon LY, Zhu H, Shao L, Zhu D, Chen YW. Rho-kinase (ROK) promotes CD44v(3,8-10)-ankyrin interaction and tumor cell migration in metastatic breast cancer cells. Cell Motil Cytoskeleton. 1999;43(4):269–287. doi: 10.1002/(SICI)1097-0169(1999)43:4<269::AID-CM1>3.0.CO;2-5. Epub 1999/07/28. [DOI] [PubMed] [Google Scholar]
- 19.Li B, Zhao WD, Tan ZM, Fang WG, Zhu L, Chen YH. Involvement of Rho/ROCK signalling in small cell lung cancer migration through human brain microvascular endothelial cells. FEBS Lett. 2006;580(17):4252–4260. doi: 10.1016/j.febslet.2006.06.056. Epub 2006/07/11. [DOI] [PubMed] [Google Scholar]
- 20.Yoshioka K, Nakamori S, Itoh K. Overexpression of small GTP-binding protein RhoA promotes invasion of tumor cells. Cancer research. 1999;59(8):2004–2010. Epub 1999/04/23. [PubMed] [Google Scholar]
- 21.Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406(6795):532–535. doi: 10.1038/35020106. Epub 2000/08/22. [DOI] [PubMed] [Google Scholar]
- 22.Ridley AJ. Rho proteins and cancer. Breast Cancer Res Treat. 2004;84(1):13–19. doi: 10.1023/B:BREA.0000018423.47497.c6. Epub 2004/03/05. [DOI] [PubMed] [Google Scholar]
- 23.Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2(2):133–142. doi: 10.1038/nrc725. Epub 2003/03/15. [DOI] [PubMed] [Google Scholar]
- 24.Fritz G, Just I, Kaina B. Rho GTPases are over-expressed in human tumors. Int J Cancer. 1999;81(5):682–687. doi: 10.1002/(sici)1097-0215(19990531)81:5<682::aid-ijc2>3.0.co;2-b. Epub 1999/05/18. [DOI] [PubMed] [Google Scholar]
- 25.Burbelo P, Wellstein A, Pestell RG. Altered Rho GTPase signaling pathways in breast cancer cells. Breast Cancer Res Treat. 2004;84(1):43–48. doi: 10.1023/B:BREA.0000018422.02237.f9. Epub 2004/03/05. [DOI] [PubMed] [Google Scholar]
- 26.Suwa H, Ohshio G, Imamura T, Watanabe G, Arii S, Imamura M, et al. Overexpression of the rhoC gene correlates with progression of ductal adenocarcinoma of the pancreas. Br J Cancer. 1998;77(1):147–152. doi: 10.1038/bjc.1998.23. Epub 1998/02/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleer CG, van Golen KL, Zhang Y, Wu ZF, Rubin MA, Merajver SD. Characterization of RhoC expression in benign and malignant breast disease: a potential new marker for small breast carcinomas with metastatic ability. Am J Pathol. 2002;160(2):579–584. doi: 10.1016/S0002-9440(10)64877-8. Epub 2002/02/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li R, Martin MP, Liu Y, Wang B, Patel RA, Zhu JY, et al. Fragment-based and structure-guided discovery and optimization of Rho kinase inhibitors. Journal of medicinal chemistry. 2012;55(5):2474–2478. doi: 10.1021/jm201289r. Epub 2012/01/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slack-Davis JK, Eblen ST, Zecevic M, Boerner SA, Tarcsafalvi A, Diaz HB, et al. PAK1 phosphorylation of MEK1 regulates fibronectin-stimulated MAPK activation. The Journal of cell biology. 2003;162(2):281–291. doi: 10.1083/jcb.200212141. Epub 2003/07/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bishop AL, Hall A. Rho GTPases and their effector proteins. The Biochemical journal. 2000;348(Pt 2):241–255. Epub 2000/05/19. [PMC free article] [PubMed] [Google Scholar]
- 31.Patel RA, Forinash KD, Pireddu R, Sun Y, Sun N, Martin MP, et al. RKI-1447 is a potent inhibitor of the Rho-associated ROCK kinases with anti-invasive and anti-tumor activities in breast cancer. Cancer research. 2012 doi: 10.1158/0008-5472.CAN-12-0954. Epub 2012/08/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vigil D, Kim TY, Plachco A, Garton AJ, Castaldo L, Pachter JA, et al. ROCK1 and ROCK2 are Required for Non-Small Cell Lung Cancer Anchorage-Independent Growth and Invasion. Cancer research. 2012 doi: 10.1158/0008-5472.CAN-11-2373. Epub 2012/09/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balasis ME, Forinash KD, Chen YA, Fulp WJ, Coppola D, Hamilton AD, et al. Combination of farnesyltransferase and Akt inhibitors is synergistic in breast cancer cells and causes significant breast tumor regression in ErbB2 transgenic mice. Clin Cancer Res. 2011;17(9):2852–2862. doi: 10.1158/1078-0432.CCR-10-2544. Epub 2011/05/04. [DOI] [PMC free article] [PubMed] [Google Scholar]