Although atherosclerosis is a systemic disease, plaque progression and complications occur in a focal, patchy pattern. It remains challenging to predict which segments of a given coronary artery will show accelerated progression of atherosclerosis.
Coronary endothelial dysfunction, characterized by a segmental vasoconstrictive response to the endothelium-dependent vasodilator acetylcholine, is considered the earliest stage of atherosclerosis in patients with angiographically nonobstructive coronary arteries. Therefore, sites with endothelial dysfunction may signal future progression of segmental coronary atherosclerosis (1). Using serial intravascular ultrasound (IVUS) imaging, we tested the hypothesis that coronary segments with endothelial dysfunction are associated with plaque progression in patients with nonobstructive coronary artery disease.
In this prospective study, 22 patients underwent coronary angiography with coronary endothelial function assessment and IVUS for clinical purposes at baseline and at 6 months (all 22 patients were part of the placebo groups of the prospective National Institutes of Health–sponsored studies: the Correlation of Endothelial Function and Early Coronary Artery Disease in Humans trial [NCT00271492] and the Long-Term l-Arginine Supplementation Improves Small-Vessel Coronary Endothelial Function in Humans trial [HL-03180-01]). Coronary artery segments with normal (n = 22) and abnormal (n = 22) endothelial function were analyzed within the same subject and compared with each other after 6 months of follow-up.
Endothelium-dependent and -independent vasoreactivity was assessed as previously described (1, 2). After completing the endothelial function studies, standard IVUS analysis with automated pullback (0.5 mm/s) of the left anterior descending coronary artery was performed using a 20-MHz, 2.9-F Eagle Eye catheter (Volcano Corporation, Rancho Cordova, California).
Comparisons between measurements on segments with versus without endothelial dysfunction were conducted using Wilcoxon signed rank tests, because each patient contributed 1 segment of each kind. Similarly, comparisons between baseline and 6-month follow-up measures were conducted using Wilcoxon signed rank tests. All statistical tests were 2-sided, and a p value <0.05 was considered to be statistically significant. Analyses were conducted using SAS version 9.2 (SAS Institute, Cary, North Carolina) and SPSS version 11.5 (IBM, Armonk, New York).
There was a significant decrease in total and low-density lipoprotein cholesterol, as well as C-reactive protein levels at 6-month follow-up without a significant change in statin use. Lifestyle changes that may have contributed to these changes were not assessed in the current study protocol.
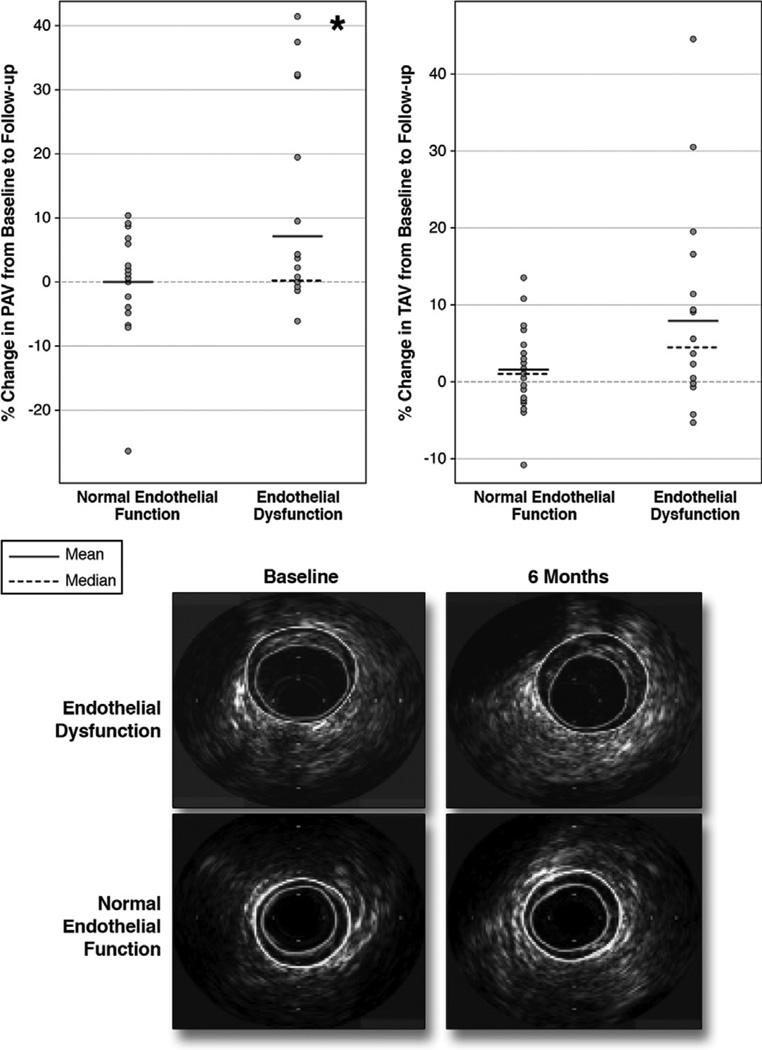
Intraindividual percent change of normalized total atheroma volume (TAV) and percent change of percent atheroma volume (PAV) of the segments with endothelial dysfunction were higher than of those with normal endothelial function, reaching statistical significance only for changes in PAV (Fig. 1). Although there was significant increase in plaque volume in the segments with endothelial dysfunction, lumen size did not change significantly. Although the presence of endothelial dysfunction was associated with coronary plaque progression, there was no significant correlation between the degree of percent change of epicardial segmental coronary arterial diameter with acetylcholine infusion during follow-up and percent change of normalized TAV (r = −0.24, p = 0.40). Likewise, there was no significant correlation between percent change of coronary blood flow with acetylcholine during follow-up and percent change of normalized TAV (r = −0.18, p = 0.52).
Figure 1. Comparison of Intraindividual % Change of Normalized PAV and TAV Between the Segments With Endothelial Dysfunction and Those With Normal Endothelial Function.
The % change of normalized percent atheroma volume (PAV) is shown in the upper left panel and of total atheroma volume (TAV) in the upper right panel. The comparison is between segments with endothelial dysfunction and those with normal endothelial function. Representative examples of intravascular ultrasound (IVUS) findings are shown in the lower panel. Segments with endothelial dysfunction showed greater progression in PAV (*p < 0.05) and TAV (p = 0.065) than segments with normal endothelial function from the same subject. IVUS demonstrates the progression of disease-only segments with endothelial dysfunction (lumen traced in green, external elastic membrane in yellow).
The current study, thus, demonstrates for the first time in patients with mild coronary artery disease using serial volumetric IVUS analysis that, within the same coronary artery, segments with endothelial dysfunction show accelerated plaque progression compared with segments with normal endothelial function.
Our findings expand previous observations and suggest a regional association between coronary endothelial dysfunction and progression of coronary atherosclerosis.
We have previously reported that coronary artery segments with endothelial dysfunction have larger necrotic cores and more features of plaque vulnerability including microcalcification as assessed by virtual histology (VH) (3). In addition, the most-recent data from the PROSPECT (Providing Regional Observations to Study Predictors of Events in the Coronary Tree) study in patients with acute coronary syndromes demonstrated that nonculprit lesions with 70% plaque burden and IVUS-VH–derived thin-capped fibroatheroma are more likely associated with the progression of atherosclerosis and recurrent coronary events (4). Thus, the current study extends these previous observations and demonstrates that coronary artery segments with relatively small plaque burden, but evidence of endothelial dysfunction, show faster plaque progression over a relatively short period of time than segments with similar plaque burden at baseline, but normal endothelial function. Thus, segments with endothelial dysfunction may represent the earliest identifiable site with underlying vascular injury, but abnormal repair prone to accelerated plaque progression and complications.
The mechanism for plaque progression at the segments with endothelial dysfunction may be multifactorial, but the reduced activity of endothelium-dependent vasodilators, particularly nitric oxide activity, an increased activity of vasoconstrictors with mitogenic activity, and altered anti-inflammatory and anticoagulant properties of the endothelium likely contribute to accelerated plaque progression.
The lack of correlation between the severity of endothelial dysfunction and plaque progression suggests that plaque progression is independent of changes in the degree of coronary endothelial reactivity.
In conclusion, atherosclerosis is a diffuse and systemic disease with focal complications. The ability of in vivo identification of coronary artery segments with propensity for plaque vulnerability that are at risk for plaque progression and the development of cardiovascular events may potentially have implications for future site-specific therapy beyond the systemic therapy for patients in early coronary atherosclerosis.
Acknowledgments
This study was supported by the National Institutes of Health (NIH grants HL 92954, HL 03180-01 HL 085307, HL77131, DK73608, and AG 31750). Drs. Gössl and Yoon contributed equally to this work.
REFERENCES
- 1.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 2.Doucette JW, Corl PD, Payne HM, et al. Validation of a Doppler guide wire for intravascular measurement of coronary artery flow velocity. Circulation. 1992;85:1899–1911. doi: 10.1161/01.cir.85.5.1899. [DOI] [PubMed] [Google Scholar]
- 3.Lavi S, McConnell JP, Rihal CS, et al. Local production of lipoprotein-associated phospholipase A2 and lysophosphatidylcholine in the coronary circulation: association with early coronary atherosclerosis and endothelial dysfunction in humans. Circulation. 2007;115:2715–2721. doi: 10.1161/CIRCULATIONAHA.106.671420. [DOI] [PubMed] [Google Scholar]
- 4.Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]