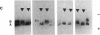Abstract
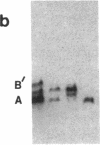
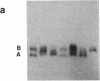
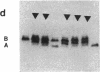
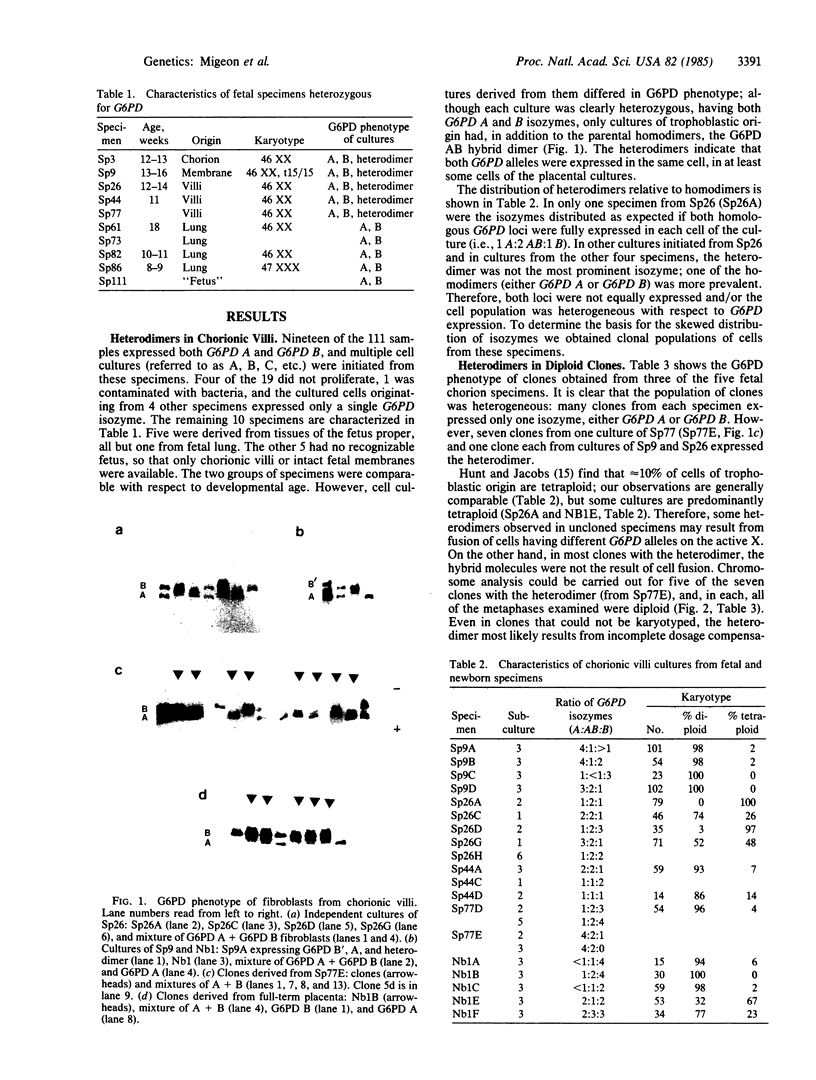
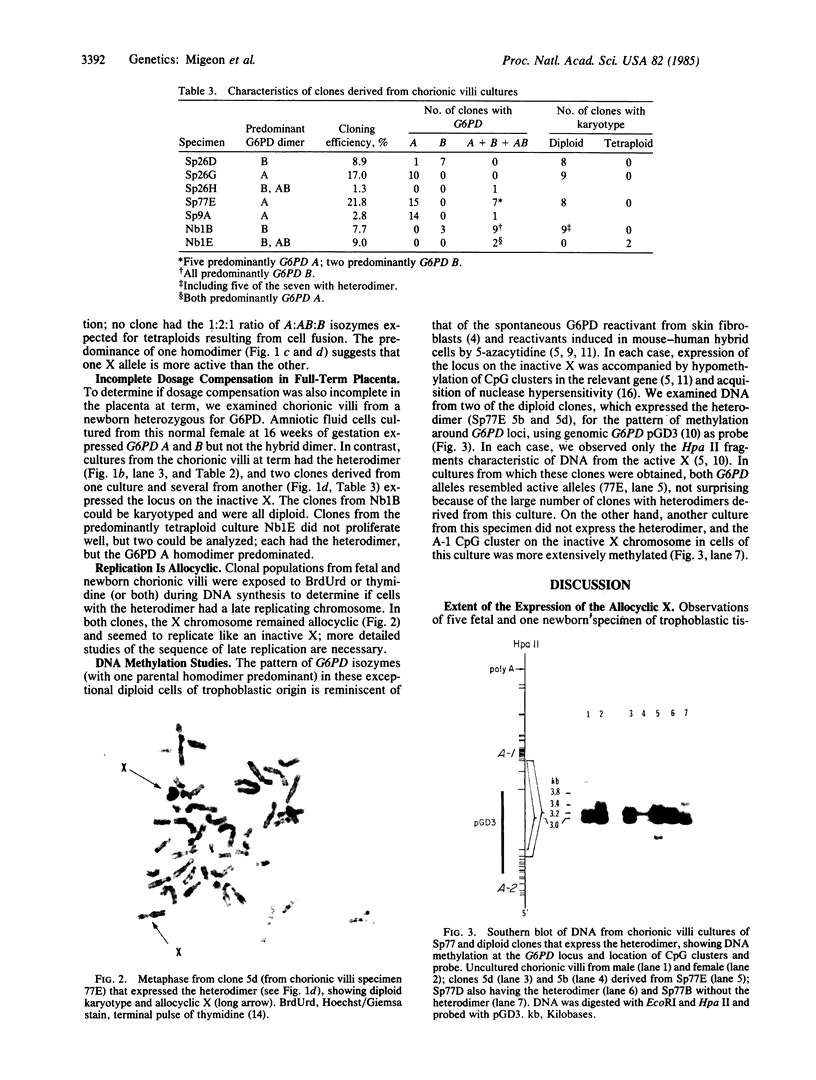
Studies of glucose-6-phosphate dehydrogenase (G6PD) in heterozygous cells from chorionic villi of five fetal and one newborn placenta show that the locus on the allocyclic X is expressed in many cells of this trophectoderm derivative. Heterodimers were present in clonal populations of cells with normal diploid karyotype and a late replicating X chromosome. The expression of the two X chromosomes was unequal, based on ratios of homodimers and heterodimers in clones. Studies of DNA, digested with Hpa II and probed with cloned genomic G6PD sequences, indicate that expression of the locus in chorionic villi is associated with hypomethylation of 3' CpG clusters. These findings suggest that dosage compensation, at least at the G6PD locus, has not been well established or maintained (or both) in placental tissue. Furthermore, the active X chromosome in these human cells of trophoblastic origin can be either the paternal or maternal one; therefore, paternal X inactivation in extraembryonic lineages is not an essential feature of mammalian X dosage compensation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chapman V., Forrester L., Sanford J., Hastie N., Rossant J. Cell lineage-specific undermethylation of mouse repetitive DNA. Nature. 1984 Jan 19;307(5948):284–286. doi: 10.1038/307284a0. [DOI] [PubMed] [Google Scholar]
- Corsaro C. M., Migeon B. R. Gene expression in euploid human hybrid cells: ouabain resistance is codominant. Somatic Cell Genet. 1978 Sep;4(5):531–540. doi: 10.1007/BF01542924. [DOI] [PubMed] [Google Scholar]
- Harper M. I., Fosten M., Monk M. Preferential paternal X inactivation in extraembryonic tissues of early mouse embryos. J Embryol Exp Morphol. 1982 Feb;67:127–135. [PubMed] [Google Scholar]
- Holliday R., Pugh J. E. DNA modification mechanisms and gene activity during development. Science. 1975 Jan 24;187(4173):226–232. [PubMed] [Google Scholar]
- Hunt P. A., Jacobs P. A. In vitro growth and chromosome constitution of placental cells. I. Spontaneous and elective abortions. Cytogenet Cell Genet. 1985;39(1):1–6. doi: 10.1159/000132095. [DOI] [PubMed] [Google Scholar]
- Kratzer P. G., Chapman V. M., Lambert H., Evans R. E., Liskay R. M. Differences in the DNA of the inactive X chromosomes of fetal and extraembryonic tissues of mice. Cell. 1983 May;33(1):37–42. doi: 10.1016/0092-8674(83)90332-x. [DOI] [PubMed] [Google Scholar]
- Lyon M. F. X-chromosome inactivation and developmental patterns in mammals. Biol Rev Camb Philos Soc. 1972 Jan;47(1):1–35. doi: 10.1111/j.1469-185x.1972.tb00969.x. [DOI] [PubMed] [Google Scholar]
- Migeon B. R., Do T. T. In search of non-random X inactivation: studies of fetal membranes heterozygous for glucose-6-phosphate dehydrogenase. Am J Hum Genet. 1979 Sep;31(5):581–585. [PMC free article] [PubMed] [Google Scholar]
- Migeon B. R., Sprenkle J. A., Do T. T. Stability of the "two active X" phenotype in triploid somatic cells. Cell. 1979 Nov;18(3):637–641. doi: 10.1016/0092-8674(79)90118-1. [DOI] [PubMed] [Google Scholar]
- Migeon B. R. Stability of X chromosomal inactivation in human somatic cells. Nature. 1972 Sep 8;239(5367):87–89. doi: 10.1038/239087a0. [DOI] [PubMed] [Google Scholar]
- Migeon B. R., Wolf S. F., Mareni C., Axelman J. Derepression with decreased expression of the G6PD locus on the inactive X chromosome in normal human cells. Cell. 1982 Jun;29(2):595–600. doi: 10.1016/0092-8674(82)90175-1. [DOI] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Shapiro L. J. Reactivation of an inactive human X chromosome: evidence for X inactivation by DNA methylation. Science. 1981 Jan 23;211(4480):393–396. doi: 10.1126/science.6164095. [DOI] [PubMed] [Google Scholar]
- Monk M., Harper M. I. Sequential X chromosome inactivation coupled with cellular differentiation in early mouse embryos. Nature. 1979 Sep 27;281(5729):311–313. doi: 10.1038/281311a0. [DOI] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Sager R., Kitchin R. Selective silencing of eukaryotic DNA. Science. 1975 Aug 8;189(4201):426–433. [PubMed] [Google Scholar]
- Schmidt M., Stolzmann W. M., Baranovskaya L. I. Replication variants of the human inactive X chromosome. I. Variability within lymphocytes of single individuals. Chromosoma. 1982;85(3):405–412. doi: 10.1007/BF00330363. [DOI] [PubMed] [Google Scholar]
- Takagi N., Sasaki M. Preferential inactivation of the paternally derived X chromosome in the extraembryonic membranes of the mouse. Nature. 1975 Aug 21;256(5519):640–642. doi: 10.1038/256640a0. [DOI] [PubMed] [Google Scholar]
- Toniolo D., D'Urso M., Martini G., Persico M., Tufano V., Battistuzzi G., Luzzatto L. Specific methylation pattern at the 3' end of the human housekeeping gene for glucose 6-phosphate dehydrogenase. EMBO J. 1984 Sep;3(9):1987–1995. doi: 10.1002/j.1460-2075.1984.tb02080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S. F., Jolly D. J., Lunnen K. D., Friedmann T., Migeon B. R. Methylation of the hypoxanthine phosphoribosyltransferase locus on the human X chromosome: implications for X-chromosome inactivation. Proc Natl Acad Sci U S A. 1984 May;81(9):2806–2810. doi: 10.1073/pnas.81.9.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S. F., Migeon B. R. Clusters of CpG dinucleotides implicated by nuclease hypersensitivity as control elements of housekeeping genes. Nature. 1985 Apr 4;314(6010):467–469. doi: 10.1038/314467a0. [DOI] [PubMed] [Google Scholar]
- Yen P. H., Patel P., Chinault A. C., Mohandas T., Shapiro L. J. Differential methylation of hypoxanthine phosphoribosyltransferase genes on active and inactive human X chromosomes. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1759–1763. doi: 10.1073/pnas.81.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ploeg L. H., Flavell R. A. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980 Apr;19(4):947–958. doi: 10.1016/0092-8674(80)90086-0. [DOI] [PubMed] [Google Scholar]