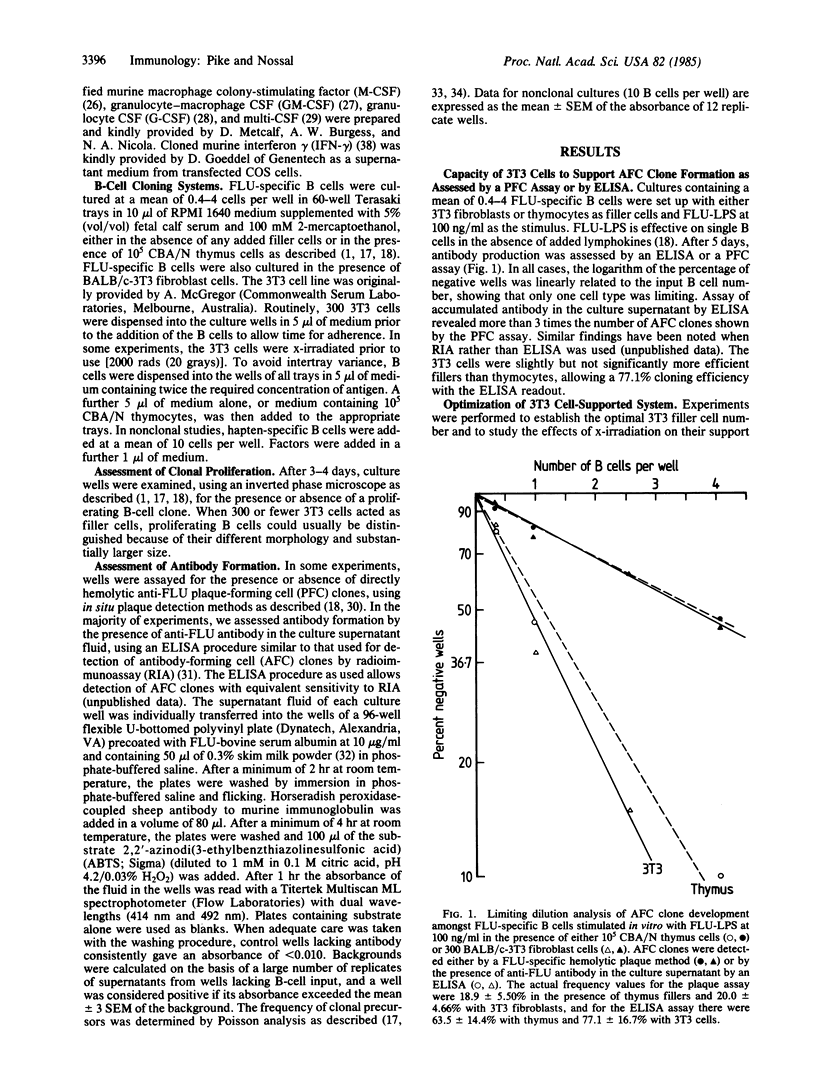
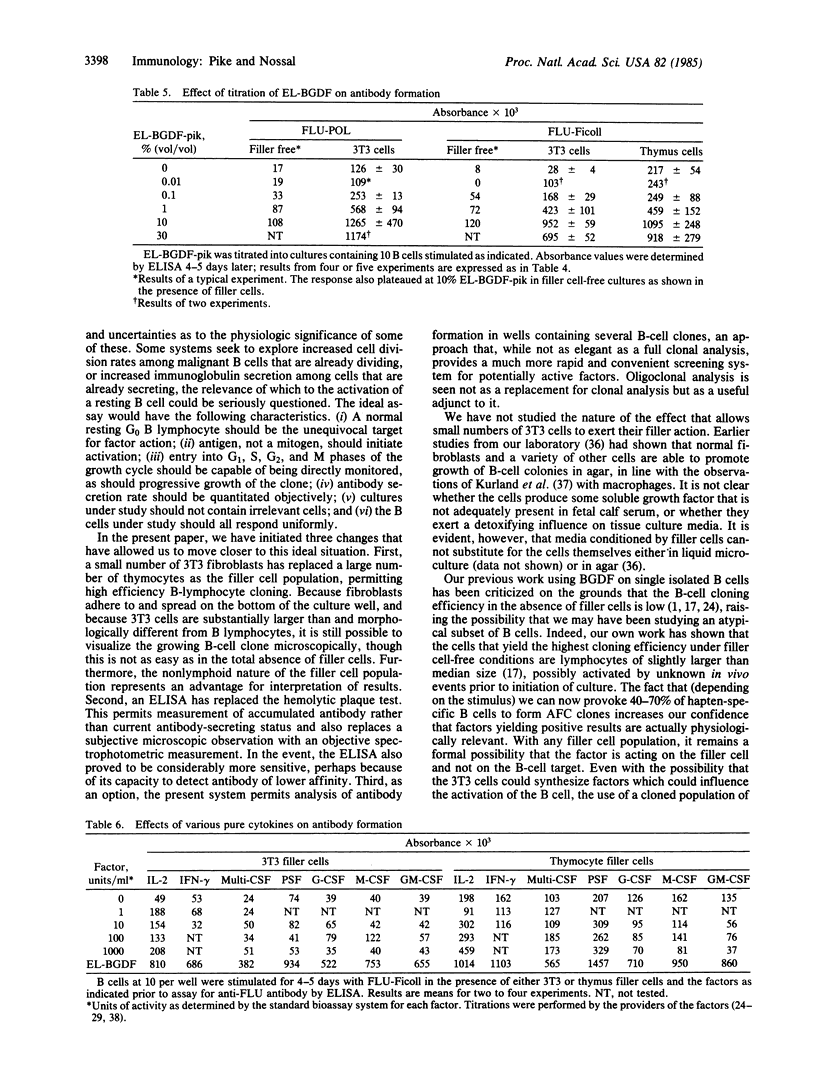
Abstract
Fluorescein (FLU)-specific murine splenic B lymphocytes from nonimmunized adult mice were prepared by the hapten-gelatin fractionation technique and cultured singly or in very small numbers in 10-microliters culture wells. Growth and differentiation to antibody-secreting status were promoted by polymeric FLU-conjugated antigens with or without added T-lymphocyte-derived conditioned media or purified cytokines. In some cultures, 3T3 fibroblasts or CBA/N thymocytes provided a source of filler cells. Anti-FLU antibody formation was detected by a sensitive enzyme-linked immunosorbent assay (ELISA). With an optimal number (around 300) of 3T3 cells per well, up to 77% of the B cells could be induced to produce detectable antibody. The ELISA permitted detection of antibody formation in essentially all wells where B-cell proliferation occurred, and it was more efficient in detecting antibody-forming clones than the hemolytic plaque assay, whether filler cells were present or not. When 10 B cells rather than 1 were included per well, the ELISA, detecting absorbance in standard fashion, provided a useful method for assessment of B-cell growth- and differentiation-promoting factors (BGDF). It was found that 3T3 cells gave less background stimulation than thymus cells, permitting the detection of as little as 1/100th as much BGDF as with thymocytes, thus offering a dynamic range of up to 30 between control absorbance in the absence of factors and the optimal factor level. Use of 3T3 cells also avoids a potential lymphokine cascade. The system has confirmed that interleukin-2 acts as a BGDF, but it has failed to establish an effect of interferon-gamma on B cells. It has also shown the inactivity of a variety of hemopoietic growth factors on B lymphocytes. This system thus promises to be a useful tool in the further analysis of B-lymphocyte activation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Booth R. J., Prestidge R. L., Watson J. D. Constitutive production by the WEHI-3 cell line of B cell growth and differentiation factor that co-purifies with interleukin 1. J Immunol. 1983 Sep;131(3):1289–1293. [PubMed] [Google Scholar]
- Burgess A. W., Camakaris J., Metcalf D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem. 1977 Mar 25;252(6):1998–2003. [PubMed] [Google Scholar]
- Burgess A. W., Metcalf D., Russell S. H., Nicola N. A. Granulocyte/macrophage-, megakaryocyte-, eosinophil- and erythroid-colony-stimulating factors produced by mouse spleen cells. Biochem J. 1980 Feb 1;185(2):301–314. doi: 10.1042/bj1850301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Lewis I., Kent S. B., Schrader J. W. Purification to apparent homogeneity of a factor stimulating the growth of multiple lineages of hemopoietic cells. J Biol Chem. 1984 Jun 25;259(12):7488–7494. [PubMed] [Google Scholar]
- Corbel C., Melchers F. The synergism of accessory cells and of soluble alpha-factors derived from them in the activation of B cells to proliferation. Immunol Rev. 1984 Apr;78:51–74. doi: 10.1111/j.1600-065x.1984.tb00476.x. [DOI] [PubMed] [Google Scholar]
- Farrar J. J., Benjamin W. R., Hilfiker M. L., Howard M., Farrar W. L., Fuller-Farrar J. The biochemistry, biology, and role of interleukin 2 in the induction of cytotoxic T cell and antibody-forming B cell responses. Immunol Rev. 1982;63:129–166. doi: 10.1111/j.1600-065x.1982.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Gearhart P. J., Cebra J. J. Differentiated B lymphocytes. Potential to express particular antibody variable and constant regions depends on site of lymphoid tissue and antigen load. J Exp Med. 1979 Jan 1;149(1):216–227. doi: 10.1084/jem.149.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M. F., Boyd A. W., Nossal G. J. Analysis of true anti-hapten cytotoxic clones in limit dilution microcultures after correction for "anti-self" activity: precursor frequencies, Ly-2 and Thy-1 phenotype, specificity, and statistical methods. J Immunol. 1983 May;130(5):2046–2055. [PubMed] [Google Scholar]
- Gray P. W., Goeddel D. V. Cloning and expression of murine immune interferon cDNA. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5842–5846. doi: 10.1073/pnas.80.19.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., Mizel S. B., Lachman L., Ansel J., Johnson B., Paul W. E. Role of interleukin 1 in anti-immunoglobulin-induced B cell proliferation. J Exp Med. 1983 May 1;157(5):1529–1543. doi: 10.1084/jem.157.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., Nakanishi K., Paul W. E. B cell growth and differentiation factors. Immunol Rev. 1984 Apr;78:185–210. doi: 10.1111/j.1600-065x.1984.tb00482.x. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Henderson L., Klein F., Palaszynski E. Procedures for the purification of interleukin 3 to homogeneity. J Immunol. 1982 Dec;129(6):2431–2436. [PubMed] [Google Scholar]
- Kishimoto T., Yoshizaki K., Kimoto M., Okada M., Kuritani T., Kikutani H., Shimizu K., Nakagawa T., Nakagawa N., Miki Y. B cell growth and differentiation factors and mechanism of B cell activation. Immunol Rev. 1984 Apr;78:97–118. doi: 10.1111/j.1600-065x.1984.tb00478.x. [DOI] [PubMed] [Google Scholar]
- Kurland J. I., Kincade P. W., Moore M. A. Regulation of B-lymphocyte clonal proliferation by stimulatory and inhibitory macrophage-derived factors. J Exp Med. 1977 Nov 1;146(5):1420–1435. doi: 10.1084/jem.146.5.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibson H. J., Gefter M., Zlotnik A., Marrack P., Kappler J. W. Role of gamma-interferon in antibody-producing responses. 1984 Jun 28-Jul 4Nature. 309(5971):799–801. doi: 10.1038/309799a0. [DOI] [PubMed] [Google Scholar]
- Lernhardt W., Corbel C., Wall R., Melchers F. T-cell hybridomas which produce B lymphocyte replication factors only. Nature. 1982 Nov 25;300(5890):355–357. doi: 10.1038/300355a0. [DOI] [PubMed] [Google Scholar]
- Marrack P., Graham S. D., Jr, Kushnir E., Leibson H. J., Roehm N., Kappler J. W. Nonspecific factors in B cell responses. Immunol Rev. 1982;63:33–49. doi: 10.1111/j.1600-065x.1982.tb00410.x. [DOI] [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D., Matsumoto M., Johnson G. R. Purification of a factor inducing differentiation in murine myelomonocytic leukemia cells. Identification as granulocyte colony-stimulating factor. J Biol Chem. 1983 Jul 25;258(14):9017–9023. [PubMed] [Google Scholar]
- Nossal G. J., Pike B. L., Battye F. L. Sequential use of hapten-gelatin fractionation and fluorescence-activated cell sorting in the enrichment of hapten-specific B llymphocytes. Eur J Immunol. 1978 Mar;8(3):151–157. doi: 10.1002/eji.1830080302. [DOI] [PubMed] [Google Scholar]
- Nossal G. J., Pike B. L. Single cell studies on the antibody-forming potential of fractionated, hapten-specific B lymphocytes. Immunology. 1976 Feb;30(2):189–202. [PMC free article] [PubMed] [Google Scholar]
- Pike B. L., Jennings G., Shortman K. A simple semi-automated plaque method for the detection of antibody-forming cell clones in microcultures. J Immunol Methods. 1982;52(1):25–37. doi: 10.1016/0022-1759(82)90346-5. [DOI] [PubMed] [Google Scholar]
- Pike B. L., Nossal G. J. A reappraisal of "T-independent" antigens. I. Effect of lymphokines on the response of single adult hapten-specific B lymphocytes. J Immunol. 1984 Apr;132(4):1687–1695. [PubMed] [Google Scholar]
- Pike B. L., Raubitschek A., Nossal G. J. Human interleukin 2 can promote the growth and differentiation of single hapten-specific B cells in the presence of specific antigen. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7917–7921. doi: 10.1073/pnas.81.24.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike B. L., Vaux D. L., Clark-Lewis I., Schrader J. W., Nossal G. J. Proliferation and differentiation of single hapten-specific B lymphocytes is promoted by T-cell factor(s) distinct from T-cell growth factor. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6350–6354. doi: 10.1073/pnas.79.20.6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike B. L., Vaux D. L., Nossal G. J. Single cell studies on hapten-specific B lymphocytes: differential cloning efficiency of cells of various sizes. J Immunol. 1983 Aug;131(2):554–560. [PubMed] [Google Scholar]
- Puré E., Isakson P. C., Takatsu K., Hamaoka T., Swain S. L., Dutton R. W., Dennert G., Uhr J. W., Vitetta E. S. Induction of B cell differentiation by T cell factors. I. Stimulation of IgM secretion by products of a T cell hybridoma and a T cell line. J Immunol. 1981 Nov;127(5):1953–1958. [PubMed] [Google Scholar]
- Rosenberg S. A., Grimm E. A., McGrogan M., Doyle M., Kawasaki E., Koths K., Mark D. F. Biological activity of recombinant human interleukin-2 produced in Escherichia coli. Science. 1984 Mar 30;223(4643):1412–1414. doi: 10.1126/science.6367046. [DOI] [PubMed] [Google Scholar]
- Schrader J. W., Nossal G. J. Strategies for the analysis of accessory-cell function: the in vitro cloning and characterization of the P cell. Immunol Rev. 1980;53:61–85. doi: 10.1111/j.1600-065x.1980.tb01040.x. [DOI] [PubMed] [Google Scholar]
- Sidman C. L., Marshall J. D., Shultz L. D., Gray P. W., Johnson H. M. Gamma-interferon is one of several direct B cell-maturing lymphokines. 1984 Jun 28-Jul 4Nature. 309(5971):801–804. doi: 10.1038/309801a0. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Heard P. M. Factors regulating macrophage production and growth. Purification and some properties of the colony stimulating factor from medium conditioned by mouse L cells. J Biol Chem. 1977 Jun 25;252(12):4305–4312. [PubMed] [Google Scholar]
- Swain S. L., Wetzel G. D., Soubiran P., Dutton R. W. T cell replacing factors in the B cell response to antigen. Immunol Rev. 1982;63:111–128. doi: 10.1111/j.1600-065x.1982.tb00413.x. [DOI] [PubMed] [Google Scholar]
- Takatsu K., Tanaka K., Tominaga A., Kumahara Y., Hamaoka T. Antigen-induced T cell-replacing factor (TRF). III. Establishment of T cell hybrid clone continuously producing TRF and functional analysis of released TRF. J Immunol. 1980 Dec;125(6):2646–2653. [PubMed] [Google Scholar]
- Vaux D. L., Pike B. L., Nossal G. J. Antibody production by single, hapten-specific B lymphocytes: an antigen-driven cloning system free of filler or accessory cells. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7702–7706. doi: 10.1073/pnas.78.12.7702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A., Lu S. D., Mark D. F. Site-specific mutagenesis of the human interleukin-2 gene: structure-function analysis of the cysteine residues. Science. 1984 Jun 29;224(4656):1431–1433. doi: 10.1126/science.6427925. [DOI] [PubMed] [Google Scholar]
- Wetzel G. D., Kettman J. R. Activation of murine B lymphocytes. III. Stimulation of B lymphocyte clonal growth with lipopolysaccharide and dextran sulfate. J Immunol. 1981 Feb;126(2):723–728. [PubMed] [Google Scholar]



