Abstract
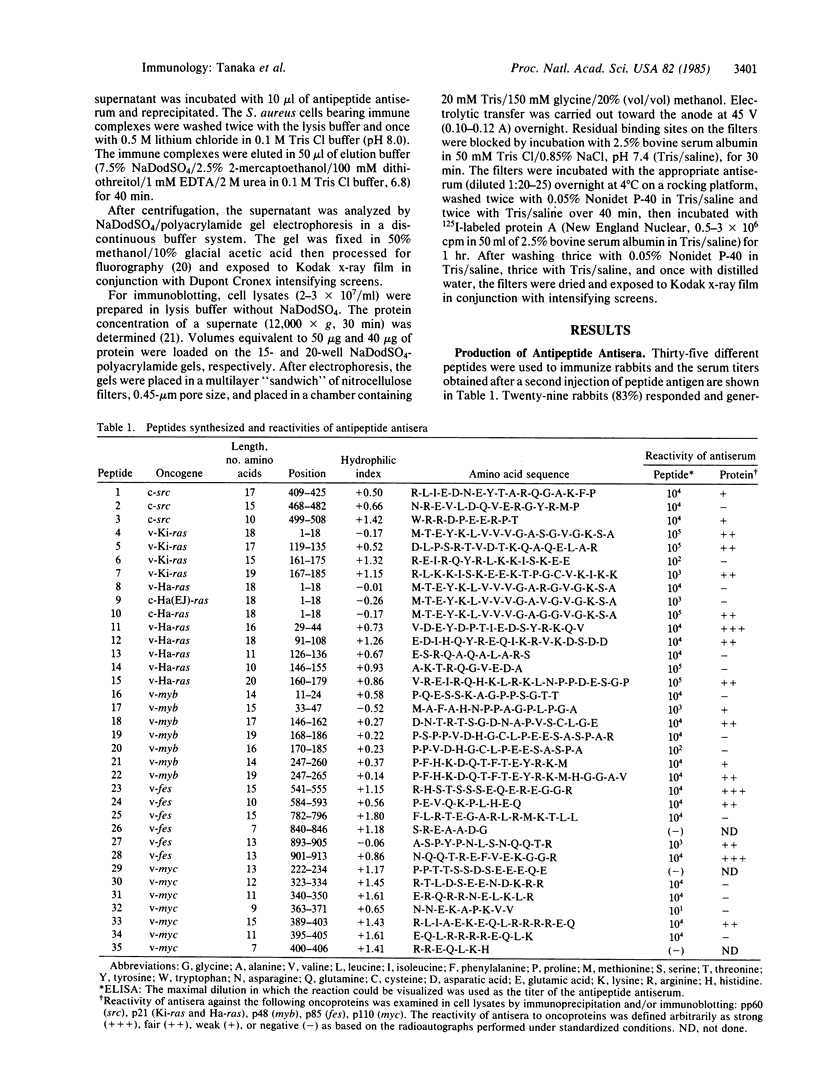
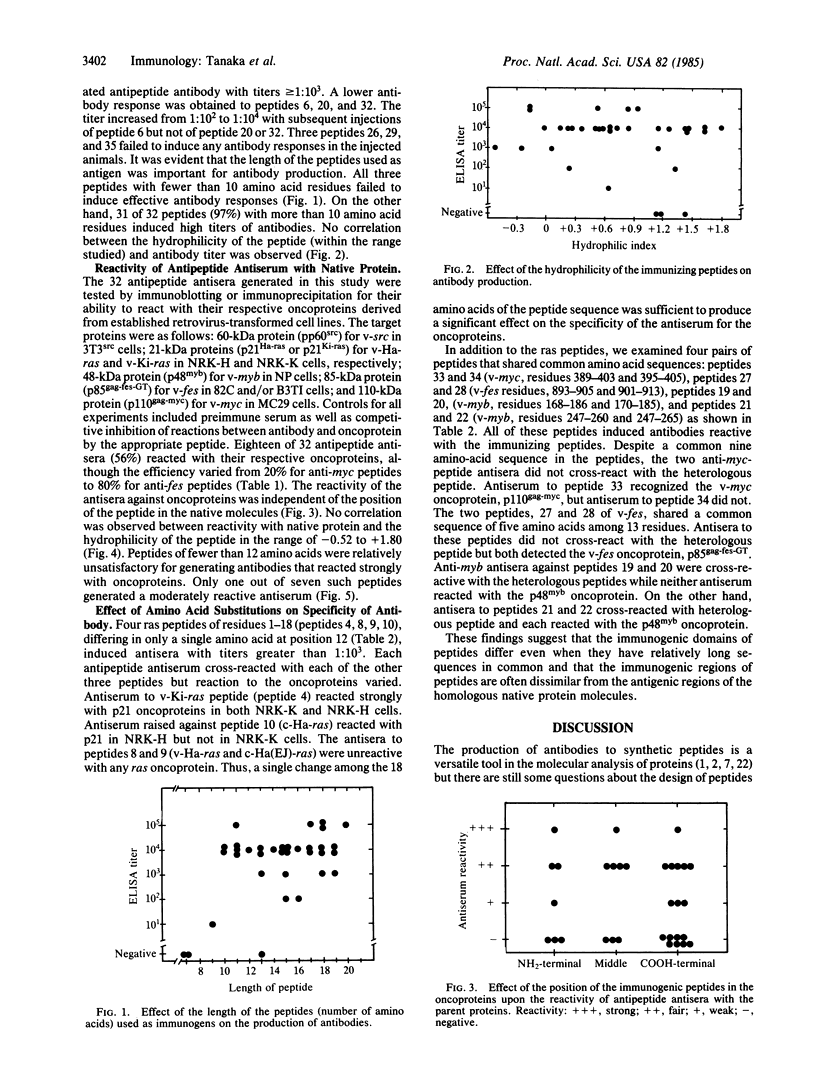
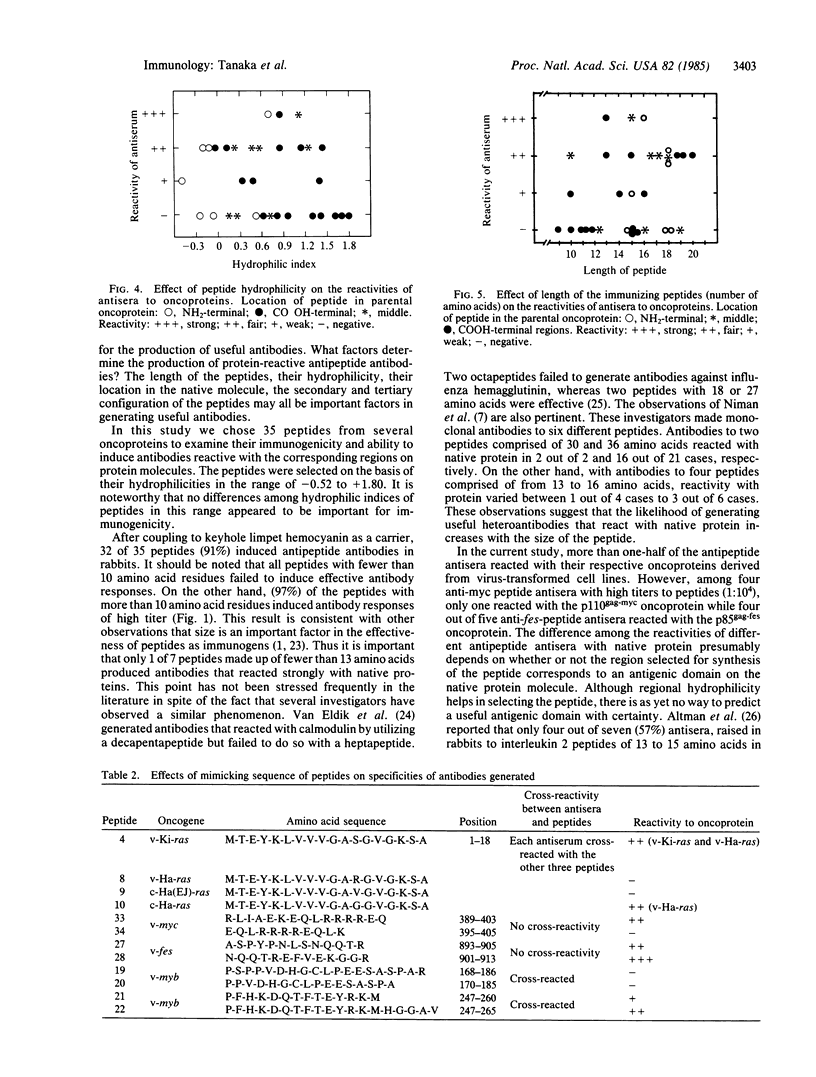
To examine the efficiency of generating protein-reactive antipeptide antibodies, 35 peptides encoded by retroviral or cellular oncogenes were used to immunize rabbits. Thirty-two peptides elicited antipeptide antibodies, of which 56% reacted with their respective oncoproteins. The length of the immunizing peptide was an important factor in generating antibodies reactive with native protein. Similar peptides differing in a single or a few amino acids could elicite antisera of markedly different reactivities.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander H., Johnson D. A., Rosen J., Jerabek L., Green N., Weissman I. L., Lerner R. A. Mimicking the alloantigenicity of proteins with chemically synthesized peptides differing in single amino acids. Nature. 1983 Dec 15;306(5944):697–699. doi: 10.1038/306697a0. [DOI] [PubMed] [Google Scholar]
- Alitalo K., Bishop J. M., Smith D. H., Chen E. Y., Colby W. W., Levinson A. D. Nucleotide sequence to the v-myc oncogene of avian retrovirus MC29. Proc Natl Acad Sci U S A. 1983 Jan;80(1):100–104. doi: 10.1073/pnas.80.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman A., Cardenas J. M., Houghten R. A., Dixon F. J., Theofilopoulos A. N. Antibodies of predetermined specificity against chemically synthesized peptides of human interleukin 2. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2176–2180. doi: 10.1073/pnas.81.7.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron M. H., Baltimore D. Antibodies against the chemically synthesized genome-linked protein of poliovirus react with native virus-specific proteins. Cell. 1982 Feb;28(2):395–404. doi: 10.1016/0092-8674(82)90357-9. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., Lipsick J. S., Reddy E. P., Baluda M. A. Identification of the leukemogenic protein of avian myeloblastosis virus and of its normal cellular homologue. Proc Natl Acad Sci U S A. 1983 May;80(10):2834–2838. doi: 10.1073/pnas.80.10.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cadman E., Bostwick J. R., Eichberg J. Determination of protein by a modified Lowry procedure in the presence of some commonly used detergents. Anal Biochem. 1979 Jul 1;96(1):21–23. doi: 10.1016/0003-2697(79)90548-7. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Cooper G. M. Cellular transforming genes. Science. 1982 Aug 27;217(4562):801–806. doi: 10.1126/science.6285471. [DOI] [PubMed] [Google Scholar]
- Dhar R., Ellis R. W., Shih T. Y., Oroszlan S., Shapiro B., Maizel J., Lowy D., Scolnick E. Nucleotide sequence of the p21 transforming protein of Harvey murine sarcoma virus. Science. 1982 Sep 3;217(4563):934–936. doi: 10.1126/science.6287572. [DOI] [PubMed] [Google Scholar]
- Gentry L. E., Rohrschneider L. R., Casnellie J. E., Krebs E. G. Antibodies to a defined region of pp60src neutralize the tyrosine-specific kinase activity. J Biol Chem. 1983 Sep 25;258(18):11219–11228. [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Hampe A., Laprevotte I., Galibert F., Fedele L. A., Sherr C. J. Nucleotide sequences of feline retroviral oncogenes (v-fes) provide evidence for a family of tyrosine-specific protein kinase genes. Cell. 1982 Oct;30(3):775–785. doi: 10.1016/0092-8674(82)90282-3. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner R. A. Tapping the immunological repertoire to produce antibodies of predetermined specificity. Nature. 1982 Oct 14;299(5884):593–596. doi: 10.1038/299592a0. [DOI] [PubMed] [Google Scholar]
- Niman H. L., Houghten R. A., Walker L. E., Reisfeld R. A., Wilson I. A., Hogle J. M., Lerner R. A. Generation of protein-reactive antibodies by short peptides is an event of high frequency: implications for the structural basis of immune recognition. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4949–4953. doi: 10.1073/pnas.80.16.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkoff J., Lai M. H., Hunter T., Verma I. M. Analysis of transforming gene products from Moloney murine sarcoma virus. Cell. 1981 Nov;27(1 Pt 2):109–119. doi: 10.1016/0092-8674(81)90365-2. [DOI] [PubMed] [Google Scholar]
- Papkoff J., Verma I. M., Hunter T. Detection of a transforming gene product in cells transformed by Moloney murine sarcoma virus. Cell. 1982 Jun;29(2):417–426. doi: 10.1016/0092-8674(82)90158-1. [DOI] [PubMed] [Google Scholar]
- Pincus M. R., van Renswoude J., Harford J. B., Chang E. H., Carty R. P., Klausner R. D. Prediction of the three-dimensional structure of the transforming region of the EJ/T24 human bladder oncogene product and its normal cellular homologue. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5253–5257. doi: 10.1073/pnas.80.17.5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushlow K. E., Lautenberger J. A., Papas T. S., Baluda M. A., Perbal B., Chirikjian J. G., Reddy E. P. Nucleotide sequence of the transforming gene of avian myeloblastosis virus. Science. 1982 Jun 25;216(4553):1421–1423. doi: 10.1126/science.6283631. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Shapira M., Jibson M., Muller G., Arnon R. Immunity and protection against influenza virus by synthetic peptide corresponding to antigenic sites of hemagglutinin. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2461–2465. doi: 10.1073/pnas.81.8.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H. Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell. 1983 Mar;32(3):881–890. doi: 10.1016/0092-8674(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Tamura T., Bauer H., Birr C., Pipkorn R. Antibodies against synthetic peptides as a tool for functional analysis of the transforming protein pp60src. Cell. 1983 Sep;34(2):587–596. doi: 10.1016/0092-8674(83)90391-4. [DOI] [PubMed] [Google Scholar]
- Tsuchida N., Ryder T., Ohtsubo E. Nucleotide sequence of the oncogene encoding the p21 transforming protein of Kirsten murine sarcoma virus. Science. 1982 Sep 3;217(4563):937–939. doi: 10.1126/science.6287573. [DOI] [PubMed] [Google Scholar]
- Van Eldik L. J., Fok K. F., Erickson B. W., Watterson D. M. Engineering of site-directed antisera against vertebrate calmodulin by using synthetic peptide immunogens containing an immunoreactive site. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6775–6779. doi: 10.1073/pnas.80.22.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G., Hutchinson M. A., Hunter T., Eckhart W. Antibodies specific for the polyoma virus middle-size tumor antigen. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4882–4886. doi: 10.1073/pnas.78.8.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]



