Abstract
Dynamic changes in cytoplasmic calcium concentration dictate the immunological fate and functions of lymphocytes. During the past few years important details have been revealed about the mechanism of store-operated calcium entry in lymphocytes, including the molecular identity of calcium-release activated (CRAC) channels and the ER calcium sensor (STIM1) responsible for CRAC channel activation following calcium depletion of stores. However, details of the potential fine regulation of CRAC channel activation that may be imposed on lymphocytes following physiologic stimulation within an inflammatory environment have not been fully addressed. In this review, we discuss several underexplored aspects of store-operated (CRAC-mediated) and store-independent calcium signaling in B lymphocytes. First, we discuss the potential novel requirement for antigen-receptor linked pathways in initiating CRAC channel activation. Second, we will discuss results suggesting that coupling between stores and CRAC channels may be regulated, allowing for graded activation in response to partial depletion of ER stores. Third, we will discuss mechanisms that sustain the duration of calcium entry via CRAC channels. Finally, we discuss distinct calcium permeant non-selective cation channels (NSCCs) that are activated by innate stimuli in B cells, potential means by which these innate calcium signaling pathways and CRAC channels crossregulate one another and the mechanistic basis and physiologic consequences of innate calcium signaling.
Keywords: Calcium, CRAC channels, TRP channels, innate signaling
Overview
Calcium (Ca2+) is a multifunctional second messenger that regulates varied and diverse aspects of lymphocyte differentiation and function (1). The mechanisms of antigen-receptor induced calcium signaling have been widely studied. Following the recent identification of Orai and STIM, the molecular components of store-operated calcium entry, efforts to understand the mechanism by which calcium release from endoplasmic reticulum (ER) stores regulates CRAC channel activation (i.e. calcium entry) following antigen receptor engagement have accelerated. A number of excellent reviews have detailed these mechanisms (2–5); consequently, this review focuses on three under-explored aspects of CRAC channel activation in lymphocytes as well as innate mechanisms of calcium entry that occur independently of CRAC channels. First, several studies challenge the dogma that depletion of Ca2+ from ER stores is both necessary and sufficient for CRAC channel activation (6,7). Accordingly, we will discuss the potential role of antigen-receptor linked kinases in CRAC channel activation. Second, while store-depletion-induced CRAC channel activation has generally been considered an “all or none” event, most studies are carried out under conditions of maximal calcium depletion of ER stores. As it is not yet clear if these responses fully reflect those induced by physiological stimuli in vivo, we will discuss results suggesting that coupling between stores and CRAC channels is regulated, allowing for graded CRAC channel activation in response to partial depletion of ER stores. Third, we will consider the fact that the duration of calcium signaling required for transcription factor activation likely exceeds the duration of proximal BCR induced signals that mobilize calcium. Therefore we will discuss mechanisms that sustain the duration of calcium entry via CRAC channels. Finally, it is becoming increasingly evident that CRAC channels are not solely responsible for calcium signaling in lymphocytes. Specifically, distinct calcium permeant non-selective cation channels are activated by innate stimuli in B cells. As these channels have been shown to have a significant functional impact on B cells, we will assess the mechanistic basis and physiologic consequences of such signaling.
Regulation of cytoplasmic calcium levels lymphocytes
Receptor-induced alterations in intracellular calcium levels regulate most aspects of lymphocyte differentiation and function (1). For example, Ca2+ plays a critical role in the regulation of lymphocyte cell shape and polarity (8,9), localization of antigen receptors with coreceptors and proximal signal transduction effectors (10,11), transcription factor activation/translocation (12–15), and gene expression (1,16). Consequently, pathways that dynamically regulate free intracellular Ca2+ concentration levels play a primary and fundamental role in immune regulation.
Calcium levels are dynamically regulated in lymphocytes by 1) intracellular receptor/channels, including IP3 receptors, responsible for Ca2+ release from internal stores, 2) plasma membrane channels, including Calcium Release Activated Calcium (CRAC) channels, responsible for Ca2+ entry 3) the resting membrane potential (membrane potential, [Vm]), set by voltage-gated and calcium-activated K+ channels (17,18), which provides the electrical driving force for Ca2+ entry, 4) energy dependent transporters (19), 5) non-selective cation channels (NSCCs) (20–23), 6) the Sarco-Endoplasmic Reticulum ATPase (SERCA pump) and Plasma Membrane Ca2+ ATPase (PMCA pump), which return Ca2+ to intracellular stores and extrude it from the cell, respectively (24), and 7) buffering by mitochondria (25–28). Moreover, changes in intracellular calcium concentration further regulates calcium dynamics by potentiating or attenuating CRAC channel (17,26,29–35) and IP3R activity (36). The integrated effect of all of these elements acting in concert ultimately shapes the calcium signals that specify the distinct functional response of B cells (13,15,37,38).
Proximal mechanisms of BCR-induced Ca2+ signaling in B cells
Signaling through the BCR complex is mediated by immunoglobulin-associated proteins Igα and Igβ, which have ITAM (immunoreceptor tyrosine based activation motif) signaling domains within their large cytoplasmic tails (39) (Figure 1). Following BCR engagement, Src family (Lyn) kinase-mediated phosphorylation of tyrosines within the Igα and Igβ ITAMs facilitates recruitment and activation of Syk kinase (40) to the signaling complex. The subsequent Syk-mediated phosphorylation of adapter proteins, which lack catalytic or enzymatic activity, facilitates protein-protein and -lipid interactions between members of the signaling cascade (reviewed in (41,42). An important adaptor in BCR signaling is SLP-65 (or BLNK, (43)) and phosphorylation of SLP-65 tyrosines facilitates its association with Btk and PLCγ2, thereby promoting the activation of these two signaling molecules (44). One of the primary consequences of these complex interactions is the activation of PLCγ–2, which hydrolyzes inositol (4,5)-bisphosphate (PIP2) into diacylglycerol (DAG) and inositol (1,4,5) trisphosphate (IP3). IP3 binds to and activates channels (IP3 receptors [IP3R]) on the ER membrane (for review see (45), causing Ca2+ to be released from ER stores. Following IP3R/channels activation ((46), for review see (36,47), the resulting depletion of ER Ca2+, (not the increase in cytoplasmic [Ca2+]) is a central and prerequisite event in the activation of plasma membrane “store-operated” CRAC channels and Ca2+ entry (2,48,49). Thus, IP3 is the primary physiological trigger for store-operated activation of CRAC channels, a critical event in the response of B cells to antigen stimulation.
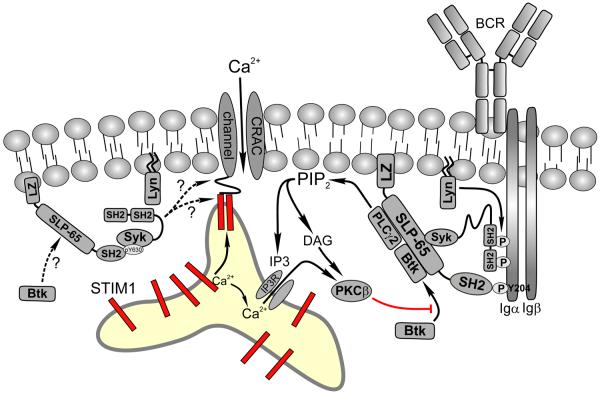
Figure 1. Mechanism of B cell antigen receptor (BCR) mediated calcium mobilization in lymphocytes.
BCR engagement by antigen binding activates the Src kinase Lyn, which phosphorylates immunoreceptor tyrosine activation motifs (ITAMs) on the Igα and Igβ chains of the BCR complex. Syk then binds to phosphorylated ITAMs via its tandem SH2 domains resulting in its activation and subsequent phosphorylation of both Y204 of Igα and the adaptor protein SLP-65. SLP-65 is anchored in the plasma membrane via its N-terminal leucine zipper (LZ) motif and is further stabilized through SH2 domain mediated interactions with pY204 on Igα, bringing it into the proximity of activated Syk. Following further phosphorylation by Syk, phosphorylated SLP-65 functions as a scaffold upon which additional proteins such as PLCγ-2 and Btk bind to form a complex that initiates calcium signaling. Activated PLCγ-2 hydrolyzes membrane PIP2 into diacylglycerol (DAG) and IP3, which binds to and activates IP3 receptors, which are channels through which calcium leaves the ER. When ER Ca2+ levels drop below the Kd of STIM1, the intraluminal N-terminus of STIM1 undergoes a conformational change that facilitates dimerization and these dimers migrate to regions of plasma-ER membrane apposition (termed junctional ER). Direct interactions between the C-terminus of STIM1 and both the N- and C-terminus of Orai1 tetramers triggers CRAC channel activation. CRAC channel activation also appears to be modulated by many of the molecules found in the proximal signaling complex. For example, membrane localized Btk positively regulates the magnitude of calcium entry through CRAC channels following store depletion, an effect that is blocked by PKCβ dependent phosphorylation of Btk. Moreover, recent studies demonstrate that although depletion of ER calcium stores is necessary for CRAC channel activation, it may not be sufficient. In particular, these studies implicate SLP-65, Syk, and Lyn in distal (post store depletion) steps of CRAC channel activation. Recent data also suggest that SLP-65 and Syk regulate sustained calcium entry even after BCR signaling diminishes and IP3 levels drop, perhaps via a novel complex in which Syk remains localized to the plasma membrane via interactions between a phosphorylated C-terminal (Y630) tyrosine that interacts with the SH2 domain of SLP-65 (left side of model). It is currently unknown whether PLCγ-2 and Btk are part of this complex and how they may regulate distal steps of CRAC channel activation.
Molecular mechanism of store-operated calcium entry in lymphocytes
While CRAC channel activation and regulation have been studied extensively in lymphocytes (50–53), the gene which encodes the channel pore (Orai or CRACM, (54–56)) and the proteins that sense calcium levels within the ER (Stromal Interaction molecule (STIM) proteins (57,58)) and relay this information to CRAC channels were identified only recently. CRAC channels are composed of tetramers of Orai subunits (59) with cytoplasmic N- and C-terminal tails. Mutagenesis studies of Orai have identified several residues which dictate ion selectivity, conductance, and sensitivity to divalent cations, establishing Orai as the essential pore forming subunit of CRAC channels (60–62). Of the three known Orai isoforms, Orai1 is the biologically significant variant that encodes CRAC channels expressed in lymphocytes. The critical importance of Orai1 for lymphoid function is evident from a study of two individuals, born to consanguineous parents, who presented with profound immune deficiency due to a point mutation in the protein (54).
STIM1 is a ubiquitously expressed Type I ER transmembrane protein whose N-terminus resides within the ER lumen (57,58). This luminal domain includes calcium-binding EF hand and sterile-α motif (SAM) domains (63). Estimates of the calcium affinity of the STIM1 EF hand domain are in the range of 200–600uM, which is comparable to estimates of the free calcium concentration in the ER (64). Studies of the atomic structure of the EF hand and SAM regions have also revealed an additional “hidden” EF hand, which together with the previously identified domains form a stable intramolecular association when stores are replete with calcium (63,65), generating a folded “inactive” conformation in unstimulated cells.
Following receptor engagement, IP3-mediated depletion of ER calcium stores triggers destabilization and unfolding of the EF hand-SAM structure of STIM1, leading to its oligomerization and subsequent aggregation (59,66–69). Microscopically visible STIM1 puncta relocalize to subdomains of junctional ER, (58,70–75) and move into juxtaposition with oligomers of Orai1 at the plasma membrane (66,69,76). The kinetics of STIM1 puncta formation is relatively slow and half maximal localization in Jurkat T cells in response to store depletion with EGTA occurs over a period of about 30 seconds (71). Importantly, CRAC currents develop with kinetics that are similar to STIM1 aggregation, but with a delay of approximately 20 seconds (67), consistent with a role for STIM1 in activating CRAC channels. Indeed, it was recently shown that direct interactions between C-terminal domains in STIM1 and both the N- and C-terminus of Orai1 trigger channel activation (77,78). Due to this requirement, Ca2+ influx through CRAC channels in lymphocytes is not widely dispersed across the cell plasma membrane, but appears to be limited to CRAC channels juxtaposed to STIM1 puncta. The clustering of STIM1 and Orai in lymphocytes around the “immune synapse” (66) suggests a role for local calcium increases in regulating downstream antigen receptor-induced signaling events. Conversely, as CRAC channels co-cluster with the antigen receptor, signaling effectors activated by the antigen receptor may function in the direct regulation of CRAC channel activation (see Figure 1). In fact, the activity of other calcium permeant channels, including TRPC family members, are known to be regulated by direct interactions with PLCγ isoforms via a split PH domain (79–81); however, whether similar interactions regulate CRAC channel activity is currently unknown.
The mechanisms which regulate STIM1 aggregation and repositioning and its subsequent colocalization with Orai are yet to be defined. A polybasic domain near the STIM1 C–terminal tail targets STIM1 to ER-plasma membrane junctions independently of the CAD/SOAR domain required for activation of channels (77), indicating that these two processes may be independently regulated. Targeting to the ER-plasma membrane junction may reflect an interaction of the polybasic domain with negatively charged phospholipids that preferentially localize to the antigen receptor signaling complex. Remarkably, given the incredibly precise choreography required to colocalize STIM1 and Orai 1, a clear role for the cytoskeleton in the process of antigen receptor mediated calcium signaling has not yet been identified. In fact, it appears that the cytoskeleton is not essential for STIM1/Orai1 relocalization, but instead may primarily be involved in regulating the size and spacing of STIM1/Orai1 puncta (74), thereby fine tuning membrane targeting of STIM1 and Orai1.
Is depletion of ER Ca2+ stores all it's CRACed up to be?
Although current dogma suggests that ER calcium store depletion by physiological stimuli is necessary and sufficient for CRAC channel activation, results from several laboratories suggest that critical aspects of this mechanism require further exploration. First, while depletion of ER calcium stores is necessary for channel activation, it is becoming increasingly apparent that store depletion may not be sufficient to do so. It is yet unknown whether CRAC channel activation and calcium entry are an irrevocable and inevitable consequence of calcium store depletion. Second, the mechanisms that sustain calcium entry over a period of time that exceeds the duration of proximal BCR signaling and IP3 generation are unresolved. Data that suggest potential mechanisms for these issues are presented below.
Distal regulation of CRAC channel activity by proximal BCR effectors
While it is well accepted that BCR-linked kinases Lyn and Syk, and the adaptor SLP-65 play an important proximal role in initiating BCR-induced calcium signaling by virtue of their role in promoting IP3 generation (see above), several studies suggest that these effectors also regulate coupling between calcium stores and CRAC channel activation in B cells. Specifically, such conclusions can be drawn from experiments in which Syk and SLP-65 deficient DT40 B cells were stimulated with IP3 or thapsigargin to directly deplete calcium from stores, thereby circumventing the critical proximal role for these effectors in the generation IP3 required for BCR-induced store-mediated CRAC activation. Notably Syk and SLP-65 deficient DT40 B cells exhibit defective calcium entry in response to store depletion by these agonists. Electrophysiology studies confirm these findings by further demonstrating defective CRAC channel activation in Lyn−/− Syk−/− DT40 B cells, and in SLP-65 deficient cells stimulated with thapsigargin or IP3 (7), and similar results were obtained in WT B cells treated with Lyn and Syk inhibitors (B.F. unpublished data). As CRAC channel activation was not disrupted in Lyn−/− DT40 B cells, Lyn and Syk appear to have distinct roles in coupling; however, it is likely that the pathways that they utilize converge on SLP-65, since Lyn−/− Syk−/− DT40 B cells and SLP-65 deficient cells share a similar phenotype.
If activated Lyn and Syk are indeed required for the initiation of CRAC channel activity, it is not readily apparent how store agonists such as IP3 or thapsigargin would ever induce calcium influx. In this regard, it is possible that the low levels of Lyn and Syk activity observed in resting B cells as a result of tonic BCR signaling (82,83) may be sufficient to initiate CRAC activation. Alternatively, Syk may be activated by calcium released from stores in response to IP3 or thapsigargin (84). Whether thapsigargin-induced calcium release from stores activates Syk sufficiently to support coupling between stores and CRAC channels, and whether primary mammalian B cells exhibit a similar requirement for Syk, Lyn, and SLP-65 in the initiation of CRAC channel activation remains to be determined.
The data outlined above clearly challenge the concept that store depletion alone is both necessary and sufficient for activating CRAC channels. In further support of this idea, we have recently shown that a protein associated with the actin cytoskeleton, namely WAVE2, plays a critical role in CRAC channel activation. We have determined that WAVE2 regulates TCR-mediated calcium entry, but not calcium release from stores (85). Regulation of calcium entry by WAVE2 is not dependent upon its interactions with actin, but rather is mediated by the N-terminal WHD domain of WAVE2 which interacts with a complex of proteins including Rac1, Hem1, PIR121, and Abi1/2 (B.F., unpublished data). Although the precise mechanism by which WAVE2 regulates coupling of store depletion to CRAC channel activation is the focus of ongoing efforts, these studies demonstrate WAVE2, a protein with no intrinsic enzymatic activity, regulates the coupling between calcium store depletion, CRAC channel activation, and calcium influx.
Another molecule that appears to be involved in the direct regulation of Ca2+ influx is Btk, a B cell kinase that binds to SLP-65 and regulates PLCγ-2 activation and IP3 generation (86). In B cells, Btk membrane localization is negatively regulated by PKCβ, which is itself activated by PLCγ-2-mediated increases in DAG and Ca2+ (10). In PKCβ deficient B cells (10) and in primary B cells treated with PKC inhibitors. BCR-induced Ca2+ signals are dramatically enhanced due to an augmentation of CRAC currents and Ca2+ influx via CRAC channels (B.F., unpublished data). These data suggest that localization of Btk to the plasma membrane potentiates CRAC channel activity. It is unclear if Btk directly regulates either CRAC channel activity or the coupling between intracellular Ca2+ stores and CRAC channels; however, this Btk-mediated effect is distinct from direct effects reported for PKC on CRAC channel inactivation (87–89). These data together demonstrate the flexibility that exists in the extent of CRAC channel activation following depletion of ER stores and indicates that, at least in B cells, CRAC channel activity can be regulated by mechanisms that are independent of depletion of ER calcium stores.
The ER calcium threshold for CRAC channel activation
Studies assessing CRAC channel activation typically utilize reagents such as the SERCA inhibitors (thapsigargin and cylcopiazonic acid (CPA)), ionophores (ionomycin), calcium chelators (EGTA and BAPTA), or antibodies that cross link ligand receptors to trigger maximal depletion of calcium from ER stores. Using these approaches, Orai1 activation appears to be tightly coupled to and maximally activated in an “all or none” fashion by store depletion. In Jurkat T cells STIM1 activation and CRAC channel activation exhibits a similar non-linear relationship to steady state ER calcium levels following progressive depletion with the SERCA inhibitor CPA (67). Similarly, data obtained in mast cells revealed that the threshold for calcium depletion of stores and activation of Icrac by IP3 was also non-linear, with maximal CRAC channel activation occurring over a very narrow IP3 concentration range (90). However, the non-linearity of CRAC channel activation by IP3 may reflect rapid IP3 metabolism, as a relatively non-metabolizable IP3 analog induced dose-dependent activation of CRAC channels (91).
This highly non-linear dependence of STIM1 redistribution on [Ca2+]ER is indicative of a cooperative mechanism of STIM1 activation, and the practical consequence is that STIM1 redistribution and CRAC channel activation are activated in a relatively binary fashion by [Ca2+]ER. This is somewhat surprising in that it would seem to limit the possibilities for regulation of CRAC channel activation by ER calcium; however, these results do not, preclude the possibility that modulation of channel activation occurs at a step distal to STIM1 activation. Thus additional molecules, in addition to those described above for B cells, such as the microtubule cytoskeleton ((92,93) positioned at contact sites between STIM1 and Orai1 (94) may regulate channel activation and calcium entry and play a role in generating dynamic changes in calcium required for triggering distinct fates of cells. Moreover, recent studies have identified the domain (CAD/SOAR/OASF) within the cytoplasmic domain of STIM1 that is interacts with and activates of Orai (77,78,95). Data suggest that the CAD domain within STIM1 may not be fully accessible to Orai and that the STIM1 C-terminal tertiary structure may also regulate the gain of coupling between stores and the channel. Currently we have no indication how this apparent conformation might be regulated in B cells, but it is possible that BCR effectors could be involved. Moreover, given that Orai1 and STIM1 expression levels influence the amplitude of whole cell CRAC currents (96), it is possible that, under physiological conditions, the amount of STIM1 that is available for interaction with Orai could also dictate the amplitude of the calcium entry signal.
Mechanisms of sustained CRAC channel activation and calcium entry
Although we have learned much about the mechanistic basis of CRAC channel activation in lymphocytes, including steps involved in calcium-dependent activation of STIM1 and the requirement for direct interactions with Orai, important aspects of the picture are still incomplete. For example, within minutes of B cell activation, the antigen receptor internalizes (97,98), Syk, Lyn and PLCγ-2 activities decrease, and IP3 is degraded and stores refill ((23), B.F., unpublished data). Nonetheless, elevations in calcium persist and are necessary to activate, among other things, transcription factors such as NFAT (14,15). Given the transient nature of proximal BCR signaling, including the rapid degradation of IP3, one wonders how cytoplasmic calcium elevations are sustained. If the primary factor dictating the duration of CRAC channel activation is the calcium content of stores (see above), what is the mechanisms of sustained calcium entry via CRAC channels when calcium stores are refilled and STIM1 presumably resets. In order to fully address these questions, it will be necessary to precisely resolve the relationship between ER Ca2+ levels and the disassembly or dissipation of STIM oligomers, and the mechanism and consequence of STIM1 disassociation from Orai1 on channel activity and sustained calcium entry
It is possible that mechanisms in addition to or distinct from ER calcium concentration independently regulate the disengagement of STIM1 and Orai. Given recent results implicating Lyn, Syk, SLP-65, and WAVE2 in the distal regulation of CRAC channel activation, the question remains as to whether these or additional molecules interact with Orai or STIM to regulate sustained channel activity independently of the calcium content of stores. Although SLP-65 is a substrate for Syk, neither Syk kinase activity nor the Syk tandem SH2 domains that anchor Syk to the Igα and Igβ ITAMS, are required for SLP-65/Syk regulation of distal calcium signaling (99), suggesting that Syk couples SLP-65 to calcium entry in B cells via a kinase-independent mechanism. In fact, following BCR internalization, the pY630 within the Syk kinase domain mediates an association with the SH2 domain of membrane anchored SLP-65, and this interaction appears to be required for sustained calcium signaling (6) (Figure 1). Thus, Lyn, Syk, SLP-65 and possibly WAVE2 may form a signaling complex distinct from that involved in proximal signaling that regulates sustained CRAC channel activity
Innate mechanisms of calcium entry in B lymphocytes
A critical feature of adaptive immune responses is the ability of a small subpopulation of lymphocytes with receptors specific for a particular antigen to proliferate and produce antibodies and immuno-regulatory cytokines in response to that antigen, thereby orchestrating an antigen-specific immune response. However, B cells also can be activated in a polyclonal antigen receptor-independent manner in vivo and in vitro following stimulation with either highly mitogenic pathogen-associated molecular patterns (PAMPs) that bind to pattern recognition receptors (PRRs) or other innate nonmitogenic stimuli to elicit robust proinflammatory responses. Given that dynamic changes in intracellular calcium are necessary and sometimes sufficient to trigger a range of proinflammatory responses by B cells, we examined the mechanisms of calcium signaling initiated by innate stimuli in primary B lymphocytes.
While it is established that sustained calcium signals triggered by antigen receptor engagement result from calcium entry through CRAC channels, our laboratory has show that, in addition to CRAC channels, several distinct calcium permeant transient receptor potential (TRP) channel family members are expressed in B cells. Of the three major mammalian TRP-like gene families identified (for reviews see (100–104)), primary B lymphocytes express mRNA for TPRC1, TRPC3, TRPC6, TRPV2, TPRV4, TRPM1, TRPM5, and TRPM7 (22). Most TRP family members are permeable to both Na+ and Ca2+ and are therefore termed non-selective cation channels (NSCCs). TRP family members are not components of CRAC channels, although recent work suggests that STIM may interact with TRP family members in some cells. This latter finding may explain previous results implicating TRPC1 (105) in BCR-induced store-operated Ca2+ entry and TRPC3 in TCR-dependent CRAC-mediated Ca2+ entry (106). The fact that certain heterologously expressed TRP fragments modulate CRAC channel activity, may be due to competition for STIM proteins that limits their availability for interaction with and full activation of CRAC channels (107–110).
Activation of TRPV4 like channels by mechanical and osmotic stimuli
Calcium signals direct diverse functions of lymphocytes; therefore, distinct mechanisms must exist to generate fate specific signals. Our efforts to understand the mechanisms of signaling by innate proinflammatory stimuli initially focused on those associated with cell motility and adhesion during lymphocyte migration from the microvasculature into inflamed tissues. We examined the response of B cells to fluid shear and osmotic forces, which elicited robust calcium signals in them (22). The primary channel responsible for mechanically-induced calcium signals was found to have biophysical and pharmacological properties similar to TRPV4 (111). Our efforts to understand how mechanical stimuli activate TRPV4-like channels in B cells revealed unexpectedly diverse and complex mechanisms of calcium signaling involving non-selective cation channels, each of which is activated by a different arachidonic acid (AA) derived product (Figure 2). AA is the major polyunsaturated fatty acid component of the plasma membrane and is a precursor of numerous inflammatory mediators (eicosanoids) including prostaglandins, leukotrienes, oxilipids generated by cytochrome P450 (CYP450) enzymes, and lipoxygenase. In primary B cells, three distinct metabolites of AA (the CYP450 epoxygenase product 5,6-epoxyeicosatrienoic acid (5,6-EET), the cytochrome P450 hydroxylase product 20-HETE, and the 5-lipoxygenase product 5-HPETE) induce Ca2+ entry (23) via distinct calcium permeant store-independent NSCCs (21).
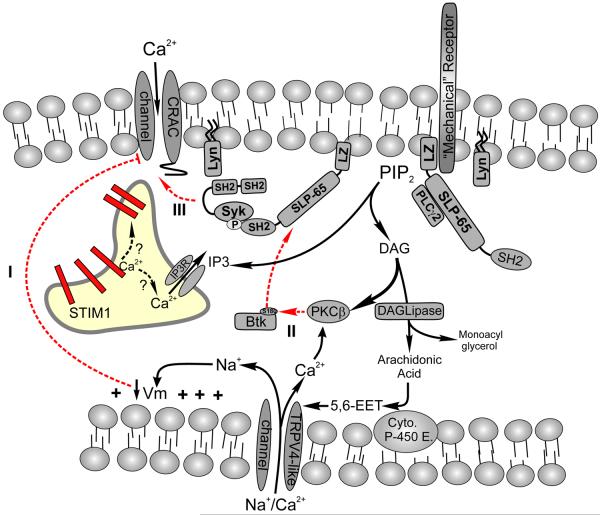
Figure 2. Mechanical regulation of calcium signaling in B lymphocytes.
Shear and osmotic forces mobilize calcium in B lymphocytes via release from intracellular stores and entry across the plasma membrane. The “mechanical receptor” that triggers the calcium signaling cascade in B cells has not been identified yet subsequent steps have been defined. Mechanical and osmotic stress activate PLCγ-2 and the resulting calcium signal is critically dependent on both PLCγ-2 activity and on the expression of the adaptor SLP-65 to which PLCγ-2 anchors. In contrast to BCR-mediated signals, calcium entry in response to mechanical/osmotic stress occurs via a store-independent non-selective cation channel (NSCC) whose pharmacological and biophysical properties most closely resemble those of the transient receptor potential (TRP) channel TRPV4. TRPV4 like channels in B cells are activated by Arachidonic cleaved from DAG which is further metabolized by cytochrome P450 epoxygenase to form 5,6-epoxyeicosatrienoic acid (5,6-EET); a direct channel agonist. Interestingly, although PLCγ-2 is activated and high levels of IP3 are generated by hypotonic stimulation of B cells, CRAC channels do not appear to play any role in the resulting calcium elevations. Several mechanisms could account for the apparent inability to activate CRAC channel mediated calcium entry. Calcium permeation of CRAC channels is highly dependent upon a hyperpolarized membrane potential (Vm). Calcium and sodium permeation of TRPV4-like channels in B cells produces membrane depolarization, which could attenuate Ca2+ permeation of activated CRAC channels (1). Alternatively, because mechanical stimuli produce much higher levels of IP3 than BCR engagement, and therefore DAG, this may activate PKCβ which inhibits Btk membrane localization (2) and this may regulate distal steps in CRAC channel activation following store depletion by IP3 (see text for a more detailed explanation). The mechanism by which Btk potentiates CRAC channel activity is not known.
An intriguing feature of hypotonicity-induced calcium signaling in B cells is its reliance on phospholipase C (PLCγ-2) mediated hydrolysis of PIP2. While hypotonic stress induces significant PLCγ-2-mediated IP3 accumulation and calcium release from stores (23), these stores do regulate hypotonicity-induced NSCC activation, as neither thapsigargin nor IP3-mediated calcium store depletion activates NSCCs in B cells (22). By contrast, the alternative product of PLCγ-2-mediated PIP2 hydrolysis, namely DAG, is the upstream mediator of hypotonicity-induced NSCC activation in B cells. Specifically we found that mechanical and osmotic stimulation triggers DAG lipase-mediated cleavage of DAG and release of AA (23). Notably, although hypotonicity generates AA, calcium entry occurs is mediated by its product 5,6-EET, which directly activates TRPV4-like channels.
While BCR engagement also triggers AA production in mature murine B cells, only mechanical stimuli activates TRPV4-like channels. At first glance, these observations are seemingly paradoxical; if BCR engagement generates DAG, then why aren't NSCCs activated in BCR-stimulated B cells? Although the mechanistic basis for this apparent anomaly is unresolved, it is possible that crucial DAG metabolites such as AA that are generated in response to BCR engagement do not reach stimulatory levels in proximity to these channels. Given that hypotonicity elicits significantly higher steady state IP3 levels than does BCR engagement (23), it is possible that lower DAG levels (equimolar to IP3) generated by the BCR represent insufficient substrate to produce stimulatory levels of AA and 5,6-EET for NSCC activation.
Finally, it is also somewhat unexpected that hypotonicity promotes IP3-mediated calcium release from ER stores, yet store-operated CRAC channels contribute relatively little, if at all, to sustained hypotonicity-induced calcium signals (23). Specifically, the NSCC inhibitor ruthenium red fully suppresses hypotonicity-induced and 5,6-EET-induced calcium signaling to the same extent, yet it has no effect on BCR induced calcium entry. These results indicate that hypotonicity-induced calcium entry is predominantly mediated by TRPV4 like NSCCs. Given that hypotonicity elicits IP3-mediated store release but does not trigger measurable CRAC channel mediated calcium entry, these results suggest that hypotonicity-activated mechanisms suppress CRAC channel activation and/or calcium entry. To do this, hypotonicity may inhibit coupling between store depletion and CRAC channel activation. It is possible that requisite signals, such as Syk or Lyn activation, that are necessary for post-store coupling (see above) are either not induced or are directly inhibited by hypotonicity-induced events. It is also possible that DAG-dependent PKC activation may potentiate CRAC channel inactivation (87). Alternatively, negative regulation of CRAC channels by hypotonicity may result from (DAG- and calcium-induced) PKCβ-mediated Btk phosphorylation, blocking its localization to the BCR signaling complex, and thereby attenuating sustained calcium entry via CRAC channels ((10,86), B.F. unpublished data).
Finally, it is possible that hypotonicity-induced suppression of CRAC channel function is an indirect consequence of NSCC mediated plasma membrane depolarization (21). Work from a number of laboratories demonstrates that a hyperpolarized plasma membrane is required for maximal Ca2+ permeation of CRAC channels (17,33,112,113). Because NSCCs are Na+ as well as Ca2+ permeant and because they have a relatively large conductance (compared with CRAC channels), cation (calcium and sodium) entry via TRP channels depolarizes the plasma membrane and thereby dissipates the electrical driving force for calcium entry through CRAC channels (21). Calcium entry through CRAC channels exhibits a steep dependence on the plasma membrane electrical potential and even modest changes in Vm can fully attenuate calcium entry through open channels (112).
Activation of TRPC3 by pathogen derived CpG DNA
Our recent efforts to understand mechanisms of calcium signaling in B cells have focused upon BCR-independent pathogen signaling pathways. While it is well established that a range of pathogen-associated molecular patterns (PAMPs) are polyclonal B cell mitogens, if and how these molecules mobilize calcium and the role of calcium in these inflammatory responses had not been defined. We focused on unmethylated CpG DNA, the ligand of Toll-like receptor-9 (TLR9), because we found that it triggered a robust calcium signal in primary B cells. We went on to show that CpG-induced calcium influx occurs via store-independent calcium permeant TRPC3 NSCC channels. Notably, we found that TRPC3 activation by CpG does not involve TLR9, but rather utilizes the scavenger receptor B1 (SR-B1), providing the first evidence that CpG stimulation of B cells occurs through multiple receptor systems (114) and that these functionally distinct receptors also mobilize calcium via a distinct mechanism than the BCR (Figure 3). Scavenger receptors are a diverse family of surface molecules that recognize a broad range of endogenous and pathogen-derived molecules (115,116). They are emerging as an important class of activating or co-activating receptors, which like TLR initiate and modulate immune responses via calcium dependent mechanisms.
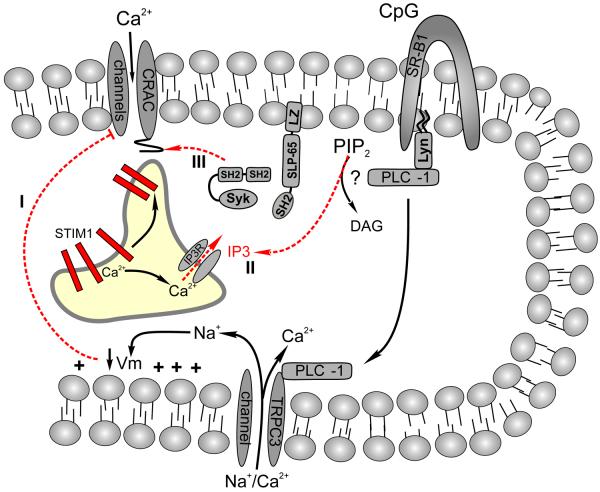
Figure 3. Scavenger receptor mediated calcium signaling in B cells.
Unmethylated CpG DNA triggers a robust calcium signal in primary B cells via the Scavenger Receptor-B1. The mechanism of CpG induced calcium entry is distinct from that elicited by the BCR and by mechanical stimulation in several critical respects. First, PLCγ-1 and not PLCγ-2 is required for SR-B1 mediate calcium mobilization. Second, SR-B1 mediated calcium entry occurs via TRPC3 channels. Finally, although CpG activation SR-B1 triggers calcium release from stores, TRPC3 activation is store independent and; furthermore, PLCγ-1 appears to activate TRPC3 via a direct physical interactions between these two proteins and is not regulated by calcium release from stores. Interestingly, CpG does not activate CRAC channels in spite of its ability to trigger calcium release from ER. This suppression of CRAC channel mediated calcium entry could reflect (1) inhibition of calcium permeation of CRAC channels by TRPC3 mediated membrane depolarization, (2) insufficient IP3 production or depletion of calcium from stores to mobilize STIM1, or (3) sufficient calcium depletion and STIM1 mobilization but an inability to activate Syk, Lyn, or SLP-65 as required for positive regulation of distal coupling between stores and CRAC channels (see text).
Among the intriguing findings to emerge from our studies of SR-B1 signaling in B cells is that CpG triggers calcium release from stores yet it does not elicit detectable CRAC currents. This apparent uncoupling of store depletion from CRAC channel activation lends support to the idea promoted in this review that store depletion may be necessary but not sufficient for CRAC channel activation. A clue into this apparent block in CRAC channel activation was the finding that SR-B1 activation of TRPC3 requires PLCγ-1. This is in contrast with BCR-induced CRAC channel activation, which requires the generation of IP3 by PLCγ-2. Interestingly TRPC3 activation by PLCγ1 appears to be independent of its phosphorylation status or enzyme activity and rather involves direct physical interactions between PLCγ-1 and TRPC3. In fact, CpG does not induce detectable phosphorylation of either PLCγ-1 or PLCγ-2.
One can imagine several explanations for this apparent uncoupling by CpG of store depletion from CRAC channel activation. First, the inability of CpG to activate CRAC channels could reflect insufficient calcium store depletion to trigger STIM1 oligomerization and relocalization to junctional ER domains (67,74). Indeed, recent studies suggest a Kd for Ca2+ regulation of STIM1 activation in the 200–600 μM range (64). Although we found that BCR engagement and CpG both induce Ca2+ release from stores, the amplitude of CpG induced transients were sometimes smaller. Moreover, as our assessment of Ca2+ release was measured by changes in cytoplasmic calcium levels, any differential regulation of calcium store depletion by CpG versus BCR stimulation could also be masked by concurrent regulation of SERCA and PMCA pump activity or cellular buffering. One could also imagine that calcium is released by distinct mechanisms or from stores not in proximity to CRAC channels given the atypical requirement for PLCγ1 in CpG responses and our inability to detect phosphorylation of either PLCγ isoforms. Due to the requirement for STIM1 migration into physical proximity with Orai1 in the plasma membrane, location could be a critical factor in the ability of stores to activate these channels. Accordingly, if IP3 is produced by PLCγ1 or a PLC isoform other than PLCγ-2, it may accumulate in a spatially restricted manner such that CpG activates functionally (or geographically) distinct calcium stores that are not in proximity with CRAC channels (90,117). Given recent work that indicates STIM1 and Orai1 can cluster in a highly polarized manner at the distal pole of migrating T cells, it seems likely that the targeting/localization of key molecules is critical to the cells response (76). It is also possible that TRPC3 activation indirectly regulates calcium permeation of CRAC channels.
As we demonstrated for mechanically activated TRPV4-like channels, sodium and calcium entry via TRPC3 may also depolarize the plasma membrane and dissipate the electrical driving force for calcium entry via CRAC channels.
Finally, the inability of CpG to activate CRAC channels may reflect its inability to activate distal steps involved in coupling store depletion to channel activation. As recent work suggests that Lyn, Syk, and SLP-65 regulate CRAC channel activation by mechanisms that are distinct from their proximal role in PLCγ-2 activation and IP3 generation (6,7,99,118), the inability of SR-B1 to activate Syk (or -Btk and -PLCγ-2) may account for this block in CRAC channel activation.
In summary, we have identified multiple NSCCs in primary B cells that are activated by innate stimuli including endogenous or exogenous AA-derived inflammatory mediators and unmethylated CpG DNA. Although innate and BCR-coupled signaling pathways overlap in many aspects, they are remarkably distinct in their mechanisms of calcium entry. Calcium entry via the BCR is mediated exclusively by CRAC channels; whereas, calcium entry triggered by mechanical and osmotic stimuli and by CpG appears to be mediated exclusively by store-independent activation of NSCCs. Consequently, the ultimate fate of a B cells will reflect the intricate interplay of calcium signaling events dictated by the integrated effect of all of these pathways.
Function of NSCC channels in B cells
Ca2+ triggers a wide range of lymphocyte fates and functions (1,13,15,22) and our studies have identified novel mechanisms by which proinflammatory stimuli initiate calcium-dependent fates of B cells via NSCC activation. These NSCCs mediate specific responses of B cells to a wide array of stimuli including mechanical and osmotic forces, inflammatory mediators produced by B cells themselves and other leukocytes at sites of inflammation, by vascular endothelium, and by pathogens and operate independently of intracellular Ca2+ stores and CRAC channels linked to the BCR, yet work in concert to coordinate the immune response.
For example, we demonstrated that AA plays a central role in the mobilization of Ca2+ in B cells. In fact, AA derived pro-inflammatory eicosanoids have been shown to regulate cytokine production, nitric oxide, and free radicals involved in the pathogenesis of immune mediated inflammatory diseases (119,120). Eicosanoids are also produced by some prokaryotic and eukaryotic pathogens (121) and induce inflammation and host susceptibility to these pathogens (122). Eicosanoids produced by vascular endothelium increase vascular permeability and promote adhesion and transmigration of leukocytes (123). Those produced by leukocytes and the vascular endothelium have important roles in regulating capillary bed perfusion, the permeability of vascular endothelium, and expression of selectins and integrin ligands which enable macrophages, neutrophils and lymphocytes to escape from the microvasculature and lymphatics and migrate through extravascular spaces (124–127). At the same time, these mediators can activate NSCCs and promote integrin-dependent binding, which plays a critical role during B cell entry into the splenic white pulp or by allowing B cells to linger within the marginal zones adjacent to vascular sinuses (128,129). Thus, eicosanoids produced by immune cells or by pathogens at sites of inflammation may mediate “cross-talk” that promotes and coordinate the inflammatory response.
The physiological mechanism by which integrin activation is dynamically regulated and the role of eicosanoids in the activation process is poorly understood. Studies of T cells have suggested that membrane depolarization increases their binding avidity for extracellular matrix proteins. The resting membrane potential of T lymphocytes is regulated primarily by K+ channel activity. Depolarization induced with K+ channel blockers or by dissipating the K+ gradient activates integrins, which mediate cell adhesion to extracellular matrix proteins ICAM-1 and VCAM-1 (130). In B lymphocytes, plasma membrane depolarization induced by NSCC activation also modulates the avidity of integrins for extracellular matrix proteins (21–23,23,23). Why B cells express multiple eicosanoid sensitive NSCCs with similar functions is perplexing, although it is possible that distinct families of receptors or inflammatory mediators are each linked to different Ca2+ permeant channels. Thus, our findings have broad implications for understanding proximal mechanisms of integrin activation by proinflammatory mediators which regulate lymphocyte trafficking and localization. The ultimate response of a lymphocyte in vivo will; therefore, reflect the effect of these overlapping pathways on the gain or dynamic range of calcium signaling.
Interestingly, we found that the pathogen derived molecule, CpG DNA also induces calcium signals and VCAM-1 adhesion of B cells. Why it is that distinct innate stimuli utilize alternative calcium pereant NSCCs, in this case TRPC3, to activate B cells is a mystery, but in the case of SR-B1, it's inability to activate Syk kinase may be critical factor in terms of how CRAC channel activity is inhibited. CpG activation of TRPC3 was found to limit the TLR9-dependent production of proinflammatory cytokines IL-6, IL-10, and IgM. Ongoing efforts are focused on trying to better define how these diverse pathways negatively regulate CRAC channel mediated signaling, and the physiological basis for this negative regulation. These results together provide a novel perspective on the complexity of calcium signaling within B cells and have far reaching implications with respect to the mechanisms regulating B cell activation, migration, and tolerance to innate stimuli including shear force and pathogen-derived molecules.
Summary and Conclusions
With the recent discovery of key molecular components of store-operated calcium signaling in lymphocytes, enormous progress has been made toward understanding the initiation of calcium signaling in lymphocytes. Despite this progress, it is becoming increasingly apparent that the regulation of intracellular calcium in response to antigen receptor-induced events is more complicated than originally thought and is subject to extensive and overlapping regulation. Moreover, evidence is rapidly mounting that complex biological stimuli, including antigen and innate stimuli found within the inflammatory milieu, cooperate to generate dynamic calcium signals in lymphocytes in vivo. As a wealth of information emerges about the molecular mechanisms of CRAC and TRP channel activation and function in lymphocytes, and as we have begun to appreciate that a range of stimuli mobilize calcium in lymphocytes via distinct and interacting pathways, our attention can begin to focus on fine details of the mechanisms of calcium regulation and how these interplay between these signals regulates the fate of immune cells in vivo.
Reference List
- 1.Feske S, Giltnane J, Dolmetsch R, Staudt LM, Rao A. Gene regulation mediated by calcium signals in T lymphocytes. Nat. Immunol. 2001;2:316–324. doi: 10.1038/86318. [DOI] [PubMed] [Google Scholar]
- 2.Oh-Hora M, Rao A. Calcium signaling in lymphocytes. Curr. Opin. Immunol. 2008;20:250–258. doi: 10.1016/j.coi.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feske S. Calcium signalling in lymphocyte activation and disease. Nat. Rev. Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 4.Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446:284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- 5.Engelke M, Engels N, Dittmann K, Stork B, Wienands J. Ca2+ signaling in antigen receptor-activated B lymphocytes. Immunol. Rev. 2007;218:235–246. doi: 10.1111/j.1600-065X.2007.00539.x. 235-46. [DOI] [PubMed] [Google Scholar]
- 6.Kulathu Y, Hobeika E, Turchinovich G, Reth M. The kinase Syk as an adaptor controlling sustained calcium signalling and B-cell development. EMBO J. 2008;27:1333–1344. doi: 10.1038/emboj.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung SC, Limnander A, Kurosaki T, Weiss A, Korenbrot JI. Coupling Ca2+ store release to Icrac channel activation in B lymphocytes requires the activity of Lyn and Syk kinases. J. Cell Biol. 2007;177:317–328. doi: 10.1083/jcb.200702050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Negulescu PA, Krasieva TB, Khan A, Kerschbaum HH, Cahalan MD. Polarity of T cell shape, motility, and sensitivity to antigen. Immunity. 1996;4:421–430. doi: 10.1016/s1074-7613(00)80409-4. [DOI] [PubMed] [Google Scholar]
- 9.Wei X, Tromberg BJ, Cahalan MD. Mapping the sensitivity of T cells with an optical trap: polarity and minimal number of receptors for Ca2+ signaling. Proc. Natl. Acad. Sci. U. S. A. 1999;96:8471–8476. doi: 10.1073/pnas.96.15.8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang SW, Wahl MI, Chu J, Kitaura J, Kawakami Y, Kato RM, Tabuchi R, Tarakhovsky A, Kawakami T, Turck CW, Witte ON, Rawlings DJ. PKCbeta modulates antigen receptor signaling via regulation of Btk membrane localization. EMBO J. 2001;20:5692–5702. doi: 10.1093/emboj/20.20.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su TT, Guo B, Kawakami Y, Sommer K, Chae K, Humphries LA, Kato RM, Kang S, Patrone L, Wall R, Teitell M, Leitges M, Kawakami T, Rawlings DJ. PKC-beta controls I kappa B kinase lipid raft recruitment and activation in response to BCR signaling. Nat. Immunol. 2002;3:780–786. doi: 10.1038/ni823. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Llopis J, Whitney M, Zlokarnik G, Tsien RY. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature. 1998;392:936–941. doi: 10.1038/31965. [DOI] [PubMed] [Google Scholar]
- 13.Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 14.Healy JI, Dolmetsch RE, Timmerman LA, Cyster JG, Thomas ML, Crabtree GR, Lewis RS, Goodnow CC. Different nuclear signals are activated by the B cell receptor during positive versus negative signaling. Immunity. 1997;6:419–428. doi: 10.1016/s1074-7613(00)80285-x. [DOI] [PubMed] [Google Scholar]
- 15.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 16.Negulescu PA, Shastri N, Cahalan MD. Intracellular calcium dependence of gene expression in single T lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 1994;91:2873–2877. doi: 10.1073/pnas.91.7.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu QH, Fleischmann BK, Hondowicz B, Maier CC, Turka LA, Yui K, Kotlikoff MI, Wells AD, Freedman BD. Modulation of Kv channel expression and function by TCR and costimulatory signals during peripheral CD4+ lymphocyte differentiation. J. Exp. Med. 2002;196:897–909. doi: 10.1084/jem.20020381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hess SD, Oortgiesen M, Cahalan MD. Calcium oscillations in human T and natural killer cells depend upon membrane potential and calcium influx. J. Immunol. 1993;150:2620–2633. [PubMed] [Google Scholar]
- 19.Bautista DM, Hoth M, Lewis RS. Enhancement of calcium signalling dynamics and stability by delayed modulation of the plasma-membrane calcium-ATPase in human T cells. J. Physiol. 2002;541:877–894. doi: 10.1113/jphysiol.2001.016154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freedman BD. Mechanisms of calcium signaling and function in lymphocytes. Critical Reviews in Immunology. 2006;26:97–111. doi: 10.1615/critrevimmunol.v26.i2.10. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Zhu P, Freedman BD. Multiple eicosanoid-activated nonselective cation channels regulate B-lymphocyte adhesion to integrin ligands. Am. J. Physiol Cell Physiol. 2006;290:C873–C882. doi: 10.1152/ajpcell.00229.2005. [DOI] [PubMed] [Google Scholar]
- 22.Liu QH, Liu X, Wen Z, Hondowicz B, King L, Monroe J, Freedman BD. Distinct calcium channels regulate responses of primary B lymphocytes to B cell receptor engagement and mechanical stimuli. J. Immunol. 2005;174:68–79. doi: 10.4049/jimmunol.174.1.68. [DOI] [PubMed] [Google Scholar]
- 23.Zhu P, Liu X, Labelle EF, Freedman BD. Mechanisms of hypotonicity-induced calcium signaling and integrin activation by arachidonic acid-derived inflammatory mediators in B cells. J. Immunol. 2005;175:4981–4989. doi: 10.4049/jimmunol.175.8.4981. [DOI] [PubMed] [Google Scholar]
- 24.Fierro L, Parekh AB. Substantial depletion of the intracellular Ca2+ stores is required for macroscopic activation of the Ca2+ release-activated Ca2+ current in rat basophilic leukaemia cells. J. Physiol. 2000;522(Pt 2):247–257. doi: 10.1111/j.1469-7793.2000.t01-1-00247.x. 247-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoth M, Fanger CM, Lewis RS. Mitochondrial regulation of store-operated calcium signaling in T lymphocytes. J. Cell Biol. 1997;137:633–648. doi: 10.1083/jcb.137.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoth M, Button DC, Lewis RS. Mitochondrial control of calcium-channel gating: a mechanism for sustained signaling and transcriptional activation in T lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 2000;97:10607–10612. doi: 10.1073/pnas.180143997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parekh AB. Mitochondrial regulation of store-operated CRAC channels. Cell Calcium. 2008;44:6–13. doi: 10.1016/j.ceca.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Parekh AB. Mitochondrial regulation of intracellular Ca2+ signaling: more than just simple Ca2+ buffers. News Physiol Sci. 2003;18:252–256. doi: 10.1152/nips.01458.2003. 252-6. [DOI] [PubMed] [Google Scholar]
- 29.Zweifach A, Lewis RS. Calcium-dependent potentiation of store-operated calcium channels in T lymphocytes. J. Gen. Physiol. 1996;107:597–610. doi: 10.1085/jgp.107.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis RS, Dolmetsch RE, Zweifach A. Positive and negative regulation of depletion-activated calcium channels by calcium. Soc. Gen. Physiol Ser. 1996;51:241–254. [PubMed] [Google Scholar]
- 31.Zweifach A, Lewis RS. Slow calcium-dependent inactivation of depletion-activated calcium current. Store-dependent and -independent mechanisms. J. Biol. Chem. 1995;270:14445–14451. doi: 10.1074/jbc.270.24.14445. [DOI] [PubMed] [Google Scholar]
- 32.Zweifach A, Lewis RS. Rapid inactivation of depletion-activated calcium current (ICRAC) due to local calcium feedback. J. Gen. Physiol. 1995;105:209–226. doi: 10.1085/jgp.105.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin SC, Boltz RC, Blake JT, Nguyen M, Talento A, Fischer PA, Springer MS, Sigal NH, Slaughter RS, Garcia ML, Kaczorowski GJ, Koo GC. Voltage-gated potassium channels regulate calcium-dependent pathways involved in human T lymphocyte activation. J. Exp. Med. 1993;177:637–645. doi: 10.1084/jem.177.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gelfand EW, Cheung RK, Grinstein S. Role of membrane potential in the regulation of lectin-induced calcium uptake. J. Cell. Physiol. 1984;121:533–539. doi: 10.1002/jcp.1041210312. [DOI] [PubMed] [Google Scholar]
- 35.Leonard RJ, Garcia ML, Slaughter RS, Reuben JP. Selective blockers of voltage-gated K+ channels depolarize human T lymphocytes: mechanism of the antiproliferative effect of charybdotoxin. Proc. Natl. Acad. Sci. U. S. A. 1992;89:10094–10098. doi: 10.1073/pnas.89.21.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyakawa T, Maeda A, Yamazawa T, Hirose K, Kurosaki T, Iino M. Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO J. 1999;18:1303–1308. doi: 10.1093/emboj/18.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freedman BD, Liu QH, Somersan S, Kotlikoff MI, Punt JA. Receptor avidity and costimulation specify the intracellular Ca2+ signaling pattern in CD4+CD8+ thymocytes. J. Exp. Med. 1999;190:943–952. doi: 10.1084/jem.190.7.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dolmetsch RE, Lewis RS. Signaling between intracellular Ca2+ stores and depletion-activated Ca2+ channels generates [Ca2+]i oscillations in T lymphocytes. J. Gen. Physiol. 1994;103:365–388. doi: 10.1085/jgp.103.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cambier JC. Antigen and Fc receptor signaling. The awesome power of the immunoreceptor tyrosine-based activation motif (ITAM) J. Immunol. 1995;155:3281–3285. [PubMed] [Google Scholar]
- 40.Justement LB. Signal transduction via the B-cell antigen receptor: the role of protein tyrosine kinases and protein tyrosine phosphatases. Curr. Top. Microbiol. Immunol. 2000;245:1–51. doi: 10.1007/978-3-642-57066-7_1. [DOI] [PubMed] [Google Scholar]
- 41.Leo A, Schraven B. Adapters in lymphocyte signalling. Curr. Opin. Immunol. 2001;13:307–316. doi: 10.1016/s0952-7915(00)00220-x. [DOI] [PubMed] [Google Scholar]
- 42.Myung PS, Boerthe NJ, Koretzky GA. Adapter proteins in lymphocyte antigen-receptor signaling. Curr. Opin. Immunol. 2000;12:256–266. doi: 10.1016/s0952-7915(00)00085-6. [DOI] [PubMed] [Google Scholar]
- 43.Fu C, Turck CW, Kurosaki T, Chan AC. BLNK: a central linker protein in B cell activation. Immunity. 1998;9:93–103. doi: 10.1016/s1074-7613(00)80591-9. [DOI] [PubMed] [Google Scholar]
- 44.Chiu CW, Dalton M, Ishiai M, Kurosaki T, Chan AC. BLNK: molecular scaffolding through 'cis'-mediated organization of signaling proteins. EMBO J. 2002;21:6461–6472. doi: 10.1093/emboj/cdf658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 46.Sugawara H, Kurosaki M, Takata M, Kurosaki T. Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B-cell antigen receptor. EMBO J. 1997;16:3078–3088. doi: 10.1093/emboj/16.11.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hagar RE, Burgstahler AD, Nathanson MH, Ehrlich BE. Type III InsP3 receptor channel stays open in the presence of increased calcium. Nature. 1998;396:81–84. doi: 10.1038/23954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu MM, Luik RM, Lewis RS. Some assembly required: Constructing the elementary units of store-operated Ca2+ entry. Cell Calcium. 2007;42:163–172. doi: 10.1016/j.ceca.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vig M, Kinet JP. Calcium signaling in immune cells. Nat. Immunol. 2009;10:21–27. doi: 10.1038/ni.f.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zweifach A, Lewis RS. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc. Natl. Acad. Sci. U. S. A. 1993;90:6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kerschbaum HH, Cahalan MD. Single-channel recording of a store-operated Ca2+ channel in Jurkat T lymphocytes. Science. 1999;283:836–839. doi: 10.1126/science.283.5403.836. [DOI] [PubMed] [Google Scholar]
- 52.Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J. Physiol. 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. 359-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kerschbaum HH, Cahalan MD. Monovalent permeability, rectification, and ionic block of store- operated calcium channels in Jurkat T lymphocytes. J. Gen. Physiol. 1998;111:521–537. doi: 10.1085/jgp.111.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 55.Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc. Natl. Acad. Sci. U. S. A. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr., Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Penna A, Demuro A, Yeromin AV, Zhang SL, Safrina O, Parker I, Cahalan MD. The CRAC channel consists of a tetramer formed by Stim-induced dimerization of Orai dimers. Nature. 2008 doi: 10.1038/nature07338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 61.Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, Kinet JP, Penner R. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr. Biol. 2006;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zheng L, Stathopulos PB, Li GY, Ikura M. Biophysical characterization of the EF-hand and SAM domain containing Ca2+ sensory region of STIM1 and STIM2. Biochem. Biophys. Res. Commun. 2008;369:240–246. doi: 10.1016/j.bbrc.2007.12.129. [DOI] [PubMed] [Google Scholar]
- 64.Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: An initiation mechanism for capacitive Ca2+ entry. J. Biol. Chem. 2006;281:35855–35862. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- 65.Stathopulos PB, Zheng L, Li GY, Plevin MJ, Ikura M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell. 2008;135:110–122. doi: 10.1016/j.cell.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 66.Lioudyno MI, Kozak JA, Penna A, Safrina O, Zhang SL, Sen D, Roos J, Stauderman KA, Cahalan MD. Orai1 and STIM1 move to the immunological synapse and are up-regulated during T cell activation. Proceedings Of The National Academy Of Sciences Of The United States Of America. 2008;105:2011–2016. doi: 10.1073/pnas.0706122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature. 2008;454:538–542. doi: 10.1038/nature07065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc. Natl. Acad. Sci. U. S. A. 2007;104:9301–9306. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Navarro-Borelly L, Somasundaram A, Yamashita M, Ren D, Miller RJ, Prakriya M. STIM1-ORAI1 interactions and ORAI1 conformational changes revealed by live-cell FRET microscopy. J. Physiol. 2008 doi: 10.1113/jphysiol.2008.162503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. Journal of Cell Biology. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soboloff J, Spassova MA, Hewavitharana T, He LP, Xu W, Johnstone LS, Dziadek MA, Gill DL. STIM2 is an inhibitor of STIM1-mediated store-operated Ca2+ Entry. Curr. Biol. 2006;16:1465–1470. doi: 10.1016/j.cub.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 73.Xu P, Lu J, Li Z, Yu X, Chen L, Xu T. Aggregation of STIM1 underneath the plasma membrane induces clustering of Orai1. Biochem. Biophys. Res. Commun. 2006;350:969–976. doi: 10.1016/j.bbrc.2006.09.134. [DOI] [PubMed] [Google Scholar]
- 74.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. Journal of Cell Biology. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baba Y, Hayashi K, Fujii Y, Mizushima A, Watarai H, Wakamori M, Numaga T, Mori Y, Iino M, Hikida M, Kurosaki T. Coupling of STIM1 to store-operated Ca2+ entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc. Natl. Acad. Sci. U. S. A. 2006;103:16704–16709. doi: 10.1073/pnas.0608358103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barr VA, Bernot KM, Srikanth S, Gwack Y, Balagopalan L, Regan CK, Helman DJ, Sommers CL, Oh-Hora M, Rao A, Samelson LE. Dynamic movement of the calcium sensor STIM1 and the calcium channel Orai1 in activated T-cells: puncta and distal caps. Mol. Biol. Cell. 2008;19:2802–2817. doi: 10.1091/mbc.E08-02-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–890. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat. Cell Biol. 2009;11:337–343. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Patterson RL, van Rossum DB, Nikolaidis N, Gill DL, Snyder SH. Phospholipase C-gamma: diverse roles in receptor-mediated calcium signaling. Trends Biochem. Sci. 2005;30:688–697. doi: 10.1016/j.tibs.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 80.van Rossum DB, Patterson RL, Sharma S, Barrow RK, Kornberg M, Gill DL, Snyder SH. Phospholipase Cgamma1 controls surface expression of TRPC3 through an intermolecular PH domain. Nature. 2005;434:99–104. doi: 10.1038/nature03340. [DOI] [PubMed] [Google Scholar]
- 81.van Rossum DB, Patterson RL, Kiselyov K, Boehning D, Barrow RK, Gill DL, Snyder SH. Agonist-induced Ca2+ entry determined by inositol 1,4,5-trisphosphate recognition. Proc. Natl. Acad. Sci. U. S. A. 2004;101:2323–2327. doi: 10.1073/pnas.0308565100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Monroe JG. ITAM-mediated tonic signalling through pre-BCR and BCR complexes. Nat. Rev. Immunol. 2006;6:283–294. doi: 10.1038/nri1808. [DOI] [PubMed] [Google Scholar]
- 83.Monroe JG. Ligand-independent tonic signaling in B-cell receptor function. Curr. Opin. Immunol. 2004;16:288–295. doi: 10.1016/j.coi.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 84.Ng SW, Di CJ, Singaravelu K, Parekh AB. Sustained activation of the tyrosine kinase Syk by antigen in mast cells requires local Ca2+ influx through Ca2+ release-activated Ca2+ channels. J. Biol. Chem. 2008;283:31348–31355. doi: 10.1074/jbc.M804942200. [DOI] [PubMed] [Google Scholar]
- 85.Nolz JC, Gomez TS, Zhu PM, Li SX, Medeiros RB, Shimizu Y, Burkhardt JK, Freedman BD, Billadeau DD. The WAVE2 complex regulates actin cytoskeletal reorganization and CRAC-mediated calcium entry during T cell activation. Current Biology. 2006;16:24–34. doi: 10.1016/j.cub.2005.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fluckiger AC, Li Z, Kato RM, Wahl MI, Ochs HD, Longnecker R, Kinet JP, Witte ON, Scharenberg AM, Rawlings DJ. Btk/Tec kinases regulate sustained increases in intracellular Ca2+ following B-cell receptor activation. EMBO J. 1998;17:1973–1985. doi: 10.1093/emboj/17.7.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Parekh AB, Penner R. Depletion-activated calcium current is inhibited by protein kinase in RBL-2H3 cells. Proc. Natl. Acad. Sci. U. S. A. 1995;92:7907–7911. doi: 10.1073/pnas.92.17.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dellis O, Gangloff SC, Paulais M, Tondelier D, Rona JP, Brouillard F, Bouteau F, Guenounou M, Teulon J. Inhibition of the calcium release-activated calcium (CRAC) current in Jurkat T cells by the HIV-1 envelope protein gp160. J. Biol. Chem. 2002;277:6044–6050. doi: 10.1074/jbc.M111831200. [DOI] [PubMed] [Google Scholar]
- 89.Hahn J, Jung W, Kim N, Uhm DY, Chung S. Characterization and regulation of rat microglial Ca2+ release-activated Ca2+ (CRAC) channel by protein kinases. Glia. 2000;31:118–124. [PubMed] [Google Scholar]
- 90.Parekh AB, Fleig A, Penner R. The store-operated calcium current ICRAC: nonlinear activation by InsP3 and dissociation from calcium release. Cell. 1997;89:973–980. doi: 10.1016/s0092-8674(00)80282-2. [DOI] [PubMed] [Google Scholar]
- 91.Glitsch MD, Parekh AB. Ca2+ store dynamics determines the pattern of activation of the store-operated Ca2+ current ICRAC in response to InsP3 in rat basophilic leukaemia cells. J. Physiol. 2000;523(Pt 2):283–290. doi: 10.1111/j.1469-7793.2000.t01-2-00283.x. 283-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smyth JT, DeHaven WI, Bird GS, Putney JW., Jr. Role of the microtubule cytoskeleton in the function of the store-operated Ca2+ channel activator STIM1. J. Cell Sci. 2007;120:3762–3771. doi: 10.1242/jcs.015735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grigoriev I, Gouveia SM, d.van V, Demmers J, Smyth JT, Honnappa S, Splinter D, Steinmetz MO, Putney JW, Jr., Hoogenraad CC, Akhmanova A. STIM1 is a MT-plus-end-tracking protein involved in remodeling of the ER. Curr. Biol. 2008;18:177–182. doi: 10.1016/j.cub.2007.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Varnai P, Toth B, Toth DJ, Hunyady L, Balla T. Visualization and manipulation of plasma membrane-endoplasmic reticulum contact sites indicates the presence of additional molecular components within the STIM1-Orai1 Complex. J. Biol. Chem. 2007;282:29678–29690. doi: 10.1074/jbc.M704339200. [DOI] [PubMed] [Google Scholar]
- 95.Muik M, Fahrner M, Derler I, Schindl R, Bergsmann J, Frischauf I, Groschner K, Romanin C. A Cytosolic Homomerization and a Modulatory Domain within STIM1 C Terminus Determine Coupling to ORAI1 Channels. J. Biol. Chem. 2009;284:8421–8426. doi: 10.1074/jbc.C800229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat. Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pure E, Tardelli L. Tyrosine phosphorylation is required for ligand-induced internalization of the antigen receptor on B lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 1992;89:114–117. doi: 10.1073/pnas.89.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stoddart A, Dykstra ML, Brown BK, Song W, Pierce SK, Brodsky FM. Lipid rafts unite signaling cascades with clathrin to regulate BCR internalization. Immunity. 2002;17:451–462. doi: 10.1016/s1074-7613(02)00416-8. [DOI] [PubMed] [Google Scholar]
- 99.Abudula A, Grabbe A, Brechmann M, Polaschegg C, Herrmann N, Goldbeck I, Dittmann K, Wienands J. SLP-65 signal transduction requires Src homology 2 domain-mediated membrane anchoring and a kinase-independent adaptor function of Syk. J. Biol. Chem. 2007;282:29059–29066. doi: 10.1074/jbc.M704043200. [DOI] [PubMed] [Google Scholar]
- 100.Montell C. The TRP superfamily of cation channels. Sci. STKE. 2005;2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- 101.Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu. Rev. Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- 102.Birnbaumer L. The TRPC class of ion channels: a critical review of their roles in slow, sustained increases in intracellular Ca2+ concentrations. Annu. Rev. Pharmacol. Toxicol. 2009;49:395–426. doi: 10.1146/annurev.pharmtox.48.113006.094928. [DOI] [PubMed] [Google Scholar]
- 103.Owsianik G, Talavera K, Voets T, Nilius B. Permeation and Selectivity of TRP Channels. Annu. Rev. Physiol. 2005 doi: 10.1146/annurev.physiol.68.040204.101406. [DOI] [PubMed] [Google Scholar]
- 104.Harteneck C, Plant TD, Schultz G. From worm to man: three subfamilies of TRP channels. Trends Neurosci. 2000;23:159–166. doi: 10.1016/s0166-2236(99)01532-5. [DOI] [PubMed] [Google Scholar]
- 105.Mori Y, Wakamori M, Miyakawa T, Hermosura M, Hara Y, Nishida M, Hirose K, Mizushima A, Kurosaki M, Mori E, Gotoh K, Okada T, Fleig A, Penner R, Iino M, Kurosaki T. Transient receptor potential 1 regulates capacitative Ca2+ entry and Ca2+ release from endoplasmic reticulum in B lymphocytes. J. Exp. Med. 2002;195:673–681. doi: 10.1084/jem.20011758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Philipp S, Strauss B, Hirnet D, Wissenbach U, Mery L, Flockerzi V, Hoth M. TRPC3 mediates T-cell receptor-dependent calcium entry in human T lymphocytes. J. Biol. Chem. 2003 doi: 10.1074/jbc.M304044200. [DOI] [PubMed] [Google Scholar]
- 107.Zeng W, Yuan JP, Kim MS, Choi YJ, Huang GN, Worley PF, Muallem S. STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol. Cell. 2008;32:439–448. doi: 10.1016/j.molcel.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Worley PF, Zeng W, Huang GN, Yuan JP, Kim JY, Lee MG, Muallem S. TRPC channels as STIM1-regulated store-operated channels. Cell Calcium. 2007;42:205–211. doi: 10.1016/j.ceca.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat. Cell Biol. 2007;9:636–645. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat. Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 111.Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat. Cell Biol. 2000;2:695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- 112.Freedman BD, Price MA, Deutsch CJ. Evidence for voltage modulation of IL-2 production in mitogen- stimulated human peripheral blood lymphocytes. J. Immunol. 1992;149:3784–3794. [PubMed] [Google Scholar]
- 113.Chandy KG, DeCoursey TE, Cahalan MD, McLaughlin C, Gupta S. Voltage-gated potassium channels are required for human T lymphocyte activation. J. Exp. Med. 1984;160:369–385. doi: 10.1084/jem.160.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhu P, Liu X, Treml LS, Cancro MP, Freedman BD. Mechanism and regulatory function of CpG signaling via scavenger receptor-B1 in primary B lymphocytes. J.Biol.Chem. 2009 doi: 10.1074/jbc.M109.018580. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111:927–930. doi: 10.1016/s0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- 116.Mukhopadhyay S, Gordon S. The role of scavenger receptors in pathogen recognition and innate immunity. Immunobiology. 2004;209:39–49. doi: 10.1016/j.imbio.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 117.Peinelt C, Beck A, Monteilh-Zoller MK, Penner R, Fleig A. IP3 receptor subtype-dependent activation of store-operated calcium entry through ICRAC. Cell Calcium. 2009 doi: 10.1016/j.ceca.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Takata M, Sabe H, Hata A, Inazu T, Homma Y, Nukada T, Yamamura H, Kurosaki T. Tyrosine kinases Lyn and Syk regulate B cell receptor-coupled Ca2+ mobilization through distinct pathways. EMBO J. 1994;13:1341–1349. doi: 10.1002/j.1460-2075.1994.tb06387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pinto F, Brenner T, Dan P, Krimsky M, Yedgar S. Extracellular phospholipase A2 inhibitors suppress central nervous system inflammation. Glia. 2003;44:275–282. doi: 10.1002/glia.10296. [DOI] [PubMed] [Google Scholar]
- 120.Kalyvas A, David S. Cytosolic phospholipase A2 plays a key role in the pathogenesis of multiple sclerosis-like disease. Neuron. 2004;41:323–335. doi: 10.1016/s0896-6273(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 121.Noverr MC, Erb-Downward JR, Huffnagle GB. Production of eicosanoids and other oxylipins by pathogenic eukaryotic microbes. Clin. Microbiol. Rev. 2003;16:517–533. doi: 10.1128/CMR.16.3.517-533.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Maccarrone M, Bari M, Battista N, Finazzi-Agro A. Endocannabinoid degradation, endotoxic shock and inflammation. Curr. Drug Targets. Inflamm. Allergy. 2002;1:53–63. doi: 10.2174/1568010023344878. [DOI] [PubMed] [Google Scholar]
- 123.Pratt PF, Rosolowsky M, Campbell WB. Effects of epoxyeicosatrienoic acids on polymorphonuclear leukocyte function. Life Sci. 2002;70:2521–2533. doi: 10.1016/s0024-3205(02)01533-3. [DOI] [PubMed] [Google Scholar]
- 124.Fleming I. Cytochrome p450 and vascular homeostasis. Circ. Res. 2001;89:753–762. doi: 10.1161/hh2101.099268. [DOI] [PubMed] [Google Scholar]
- 125.Luster AD, Tager AM. T-cell trafficking in asthma: lipid mediators grease the way. Nat. Rev. Immunol. 2004;4:711–724. doi: 10.1038/nri1438. [DOI] [PubMed] [Google Scholar]
- 126.Oike M, Droogmans G, Nilius B. Mechanosensitive Ca2+ transients in endothelial cells from human umbilical vein. Proc. Natl. Acad. Sci. U. S. A. 1994;91:2940–2944. doi: 10.1073/pnas.91.8.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pribila JT, Quale AC, Mueller KL, Shimizu Y. Integrins and T cell-mediated immunity. Annu. Rev. Immunol. 2004;22:157–180. doi: 10.1146/annurev.immunol.22.012703.104649. 157-80. [DOI] [PubMed] [Google Scholar]
- 128.Lo CG, Lu TT, Cyster JG. Integrin-dependence of lymphocyte entry into the splenic white pulp. J. Exp. Med. 2003;197:353–361. doi: 10.1084/jem.20021569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lu TT, Cyster JG. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science. 2002;297:409–412. doi: 10.1126/science.1071632. [DOI] [PubMed] [Google Scholar]
- 130.Levite M, Cahalon L, Peretz A, Hershkoviz R, Sobko A, Ariel A, Desai R, Attali B, Lider O. Extracellular K+ and opening of voltage-gated potassium channels activate T cell integrin function: physical and functional association between Kv1.3 channels and beta1 integrins. J. Exp. Med. 2000;191:1167–1176. doi: 10.1084/jem.191.7.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]