Abstract
Purpose: We report on the clinical process, quality assurance, and geometric and dosimetric results of the first clinical implementation of electromagnetic transponder-guided MLC tracking which occurred on 28 November 2013 at the Northern Sydney Cancer Centre.
Methods: An electromagnetic transponder-based positioning system (Calypso) was modified to send the target position output to in-house-developed MLC tracking code, which adjusts the leaf positions to optimally align the treatment beam with the real-time target position. Clinical process and quality assurance procedures were developed and performed. The first clinical implementation of electromagnetic transponder-guided MLC tracking was for a prostate cancer patient being treated with dual-arc VMAT (RapidArc). For the first fraction of the first patient treatment of electromagnetic transponder-guided MLC tracking we recorded the in-room time and transponder positions, and performed dose reconstruction to estimate the delivered dose and also the dose received had MLC tracking not been used.
Results: The total in-room time was 21 min with 2 min of beam delivery. No additional time was needed for MLC tracking and there were no beam holds. The average prostate position from the initial setup was 1.2 mm, mostly an anterior shift. Dose reconstruction analysis of the delivered dose with MLC tracking showed similar isodose and target dose volume histograms to the planned treatment and a 4.6% increase in the fractional rectal V60. Dose reconstruction without motion compensation showed a 30% increase in the fractional rectal V60 from that planned, even for the small motion.
Conclusions: The real-time beam-target correction method, electromagnetic transponder-guided MLC tracking, has been translated to the clinic. This achievement represents a milestone in improving geometric and dosimetric accuracy, and by inference treatment outcomes, in cancer radiotherapy.
Keywords: real-time adaptation, beam-tumor targeting, MLC tracking, clinical translation
INTRODUCTION
The clinical implementation of electromagnetic transponder-guided MLC tracking represents an important step in a long pathway from the bench to the bedside. The first experimental study of MLC tracking was published in 2001 relying on preprogrammed one-dimensional (1D) motion compensation and manual synchronization.1 The integration with real-time feedback on a Millennium MLC (Varian, Palo Alto, CA) via an optical position monitoring system (RPM, Varian) to correct for 1D motion was described in 2006,2 with 3D motion compensation demonstrated in 2008.3 Experimental implementation of MLC tracking on a Siemens MLC was demonstrated in 2010,4 and on an Elekta MLC in 2012.5 The closest to clinical human implementation prior to the current work has been the irradiation of pigs using a stereotactic radiotherapy protocol with conformal fields, where the real-time position was provided by autosegmentation of implanted bronchial stents in electronic portal images.6
To be used clinically, MLC tracking requires a real-time tumor position monitoring system. The advent of the Calypso (Varian) internal real-time position monitoring system using electromagnetic transponders represented a pathway to the clinical translation of real-time MLC tracking. The investigation of the integration of electromagnetic transponder-guided MLC tracking on a Varian MLC has spanned several domains including geometric accuracy,7 dosimetric fidelity,8 quality assurance,9 compatibility with volumetric modulated arc therapy (VMAT) (Ref. 10) and the ability to correct for in-plane rotation.11 Note that the current study only corrects for real-time tumor translation and not real-time rotation. Electromagnetic transponder-guided MLC tracking was also demonstrated on a Siemens MLC where sub-mm tracking accuracy and substantial dosimetric improvements were observed.12
The clinical driver for implementing MLC tracking is the improved geometric accuracy, leading to corresponding improvements in the delivered dose to the patient and ultimately patient outcomes with higher tumor control with lower side effects and improved quality of life.
In this letter, we report on the clinical process, quality assurance, and geometric and dosimetric results of the first clinical implementation of electromagnetic transponder-guided MLC tracking.
METHOD
The ethics, governance, legal, and regulatory processes were completed prior to the initiation of the clinical trial.
Software and algorithm
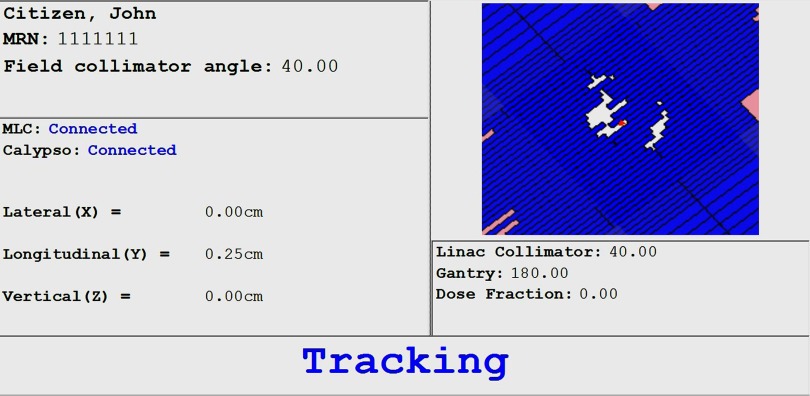
The MLC tracking code used for the study is noncommercial user-written research code built on the code used for previous nonclinical studies. In addition to performance improvements and various bug fixes, the user interface was rewritten for a streamlined clinical process. The user interface, developed in conjunction with radiation therapist input, is shown in Fig. 1. The key information is displayed clearly, with either “Tracking” or “Beam hold” displayed to alert the therapist as to the current status and to check that the Linac is also following the same instruction.
Figure 1.
The user interface developed with input from the radiation therapists.
The algorithm used to determine the optimal leaf positions takes the leaf positions, f, from the treatment plan by interpolating the VMAT control points based on the measured gantry angle. The Calypso position signal, T, is then collapsed onto the treatment beam view, to scale f to f′ based on magnification along the beam view, and then translated in two dimensions. The ideal motion compensated aperture would be g = f′°T. However, due to the finite width of the leaves, an optimization procedure occurs that balances overexposure and underexposure to determine the best approximation to g. More details can be found in Ruan et al.13 A preset number of adjacent leaves are left next to open apertures (two were used in this case) and other leaves not participating at the current time are moved under the jaws, as described in Sawant et al.3
As prostate motion is slow with respect to the system response time and does not have a significant periodic component, no motion prediction algorithm was used.
Quality assurance
The system quality assurance (QA) procedures followed the processes developed through a failure mode and effects analysis (FMEA) performed by Sawant et al.9 The key features included testing the coordinate system, latency, beam-holds in the case of anomalous conditions and dosimetric tests.
Patient-specific quality assurance procedures included checklists and cross checking of all additional process steps, independent monitor unit calculation, portal dosimetry analysis of patient plan delivered using MLC tracking software with zero motion trajectory file, and dose reconstruction of the QA delivery with zero motion trajectory on planning CT dataset.
Clinical process
The treatment plan was developed with dual-arc volumetric modulated arc therapy (RapidArc, Varian) at 6 MV beam energy to deliver 80 Gy to 95% of the planning target volume in 2 Gy fractions.
The pretreatment clinical processes for electromagnetic transponder-guided MLC tracking included several steps necessary for a research implementation of MLC tracking that would presumably be automated in a commercial application. These included opening the jaws by 8 mm to allow for prostate motion tracking (>8 mm of motion will cause a beam hold) and opening an MLC leaf pair outside of the jaw field to set the carriage positions. From the dicom plan files an MLC text file used by the MLC tracking software was extracted for each arc.
The Calypso system displays the target rotation prior to treatment which can be used to adjust the patient's pose on the treatment couch. For the current clinical protocol if any residual rotation value exceeds 10° a cone beam CT scan will be acquired to assess target coverage, and if necessary the patient will be replanned.
The MLC tracking software (Fig. 1) was installed on a separate computer within the Linac local area network. Preparing the software for clinical use required two mouse clicks, starting the application and selecting the patient field. After treatment, the system was restored to normal clinical operation with a one word command.
To estimate the clinical benefit of MLC tracking, post-treatment motion including dose reconstruction was performed using the method of Poulsen et al.14 The MLC tracking dose was calculated using the Calypso-measured motion and the MLC positions from the log files. The nontracking dose was calculated using the Calypso-measured motion and the planned MLC positions. Both the MLC tracking and no motion correction dose distributions were compared with that from the treatment plan.
RESULTS
Quality assurance
All of the system QA tests of Sawant et al.9 for MLC tracking were in tolerance. The MLC field correctly tracked the target motion in all three directions, with varying gantry and collimator values. The latency was measured as 230 ± 20 ms. The beam hold test for anomalous conditions passed. The end-to-end dosimetric results with MLC tracking demonstrated almost identical results to no motion compensation in the absence of motion, and showed consistently and significantly higher 3 mm/3% γ-pass results than no motion compensation in the presence of considerable patient-derived prostate motion.
Patient treatment
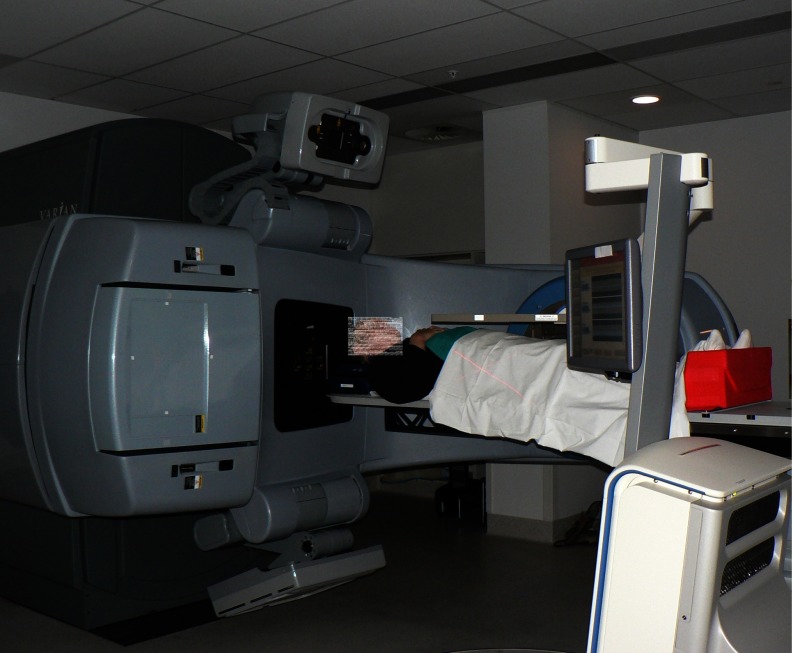
The setup of the first patient treated with MLC tracking is shown in Fig. 2. The patient was initially aligned using the in-room Calypso signal, followed by a cone beam CT scan. The total time from entering to exiting the treatment room for fraction 1 of patient 1 was 21 min. Much of this time was spent reviewing the pretreatment cone beam CT scans for anatomic verification and review of patient compliance with the bladder and rectal filling protocols.15 The treatment itself took 2 min with no beam holds or additional time for MLC tracking.
Figure 2.
Patient setup on the day of the first MLC tracking treatment.
Patient treatment measurements
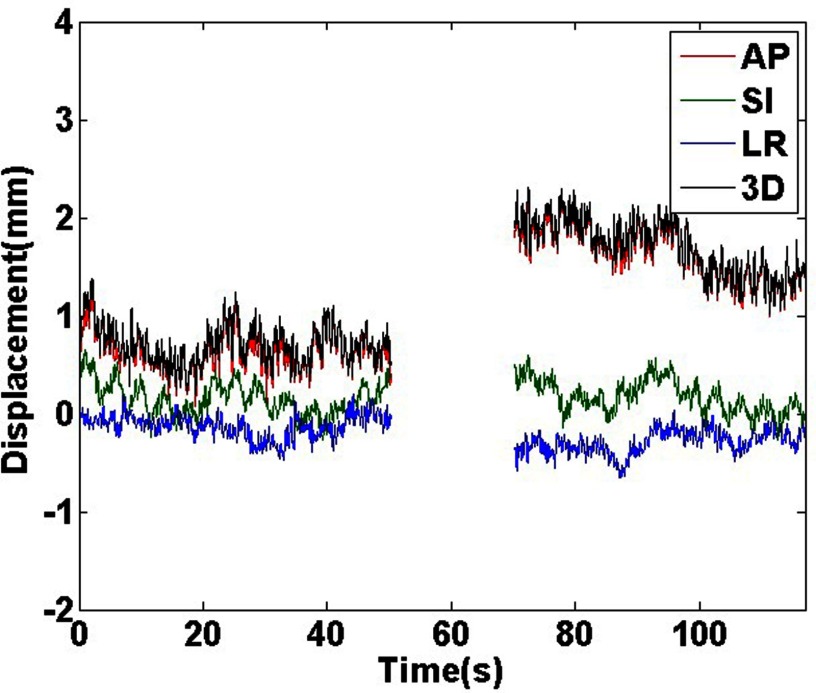
The Calypso measured geometric results for fraction 1 of patient 1 are shown in Fig. 3. Motion data are only shown while the beam is on (a two-arc VMAT treatment). There was a small (∼1 mm) anterior shift between the two arcs. The average prostate position from the initial setup was 1.2 mm.
Figure 3.
Calypso measured motion traces for fraction 1 of patient 1. Motion is only shown when the treatment beam was on during the two arcs. Anterior, superior, and left shifts are in the positive direction.
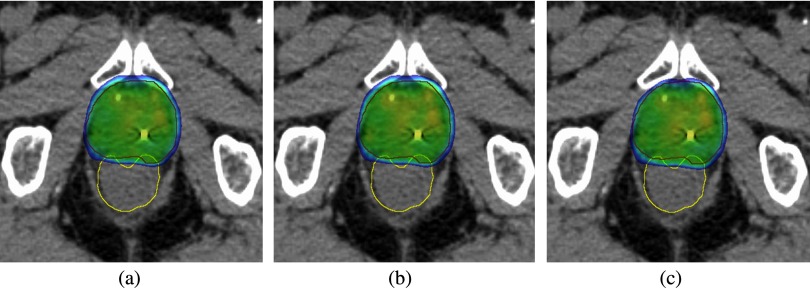
The dose reconstruction results for fraction 1 of patient 1 are shown in Fig. 4 (isodose curves) and Fig. 5 [dose volume histograms (DVHs)]. In Fig. 4, when comparing the MLC tracking isodose curves with the no motion correction isodose curves there are three differences: (1) the MLC tracking isodose curves more closely match the planned isodose curves, (2) the PTV is better centered within the isodose curves, and (3) the amount of dose extending into the rectum is smaller. It should also be noted that the patient had SpaceOAR (Augmenix, Waltham, MA) to further move the rectum from the prostate to decrease the rectal dose. The rectal anatomy at treatment may differ from the plan CT in Fig. 4, however, independently of these differences the rectal dose will still be lower if anterior patient shifts are accounted for with MLC tracking.
Figure 4.
(a) Planned, (b) delivered with MLC tracking, and (c) simulated no-motion correction isodose distributions for fraction 1 of patient 1. Dose levels >95% are shown.
Figure 5.
Planned, delivered with MLC tracking and simulated no-motion correction isodose volume histograms for fraction 1 of patient 1. In 40 fractions, 80 Gy is prescribed to 95% of the PTV.
The DVHs in Fig. 5 show small improvements with tracking over no motion compensation in the PTV and CTV DVHs. For MLC tracking, the PTV D95 is the same as the planned dose. With no motion correction, the PTV D95 is 1% cooler than the planned dose. The most obvious DVH difference in Fig. 5 is the increase in the rectal DVH without motion compensation. For rectal toxicity, the volume of rectum receiving >60 Gy (V60) is consistently associated with the risk of Grade >2 rectal toxicity or rectal bleeding.16 Despite the relatively small motion of 1.2 mm on average, the fractional V60, i.e., the dose which would have been delivered had a similar shift been observed throughout the treatment without motion compensation, was increased by 30% from the planned dose. With MLC tracking, the fractional V60 was increased by 4.6%.
DISCUSSION
This paper reports on the first clinical implementation of MLC tracking. MLC tracking represents the third application of real-time adaptation through beam-tumor targeting. The CyberKnife Synchrony system was commercially available in 2004 (Accuray, Sunnyvale, CA) using a robot to target the Linac at the tumor. The Mitsubishi MHI-TM2000/Vero was first used clinically for lung tumor tracking with a gimbaled Linac in 2011.17 There are three differentiating features of the real-time adaptation methods used in the current study to these prior clinical innovations:
The first is the use of direct tumor position measurement via electromagnetic tracking rather than internal–external correlation model building. Note that for prostate treatments, the Cyberknife system acquires images at a user-requested frequency, typically 15–60 s, followed by a robotic correct for motion, which is not real-time.
The second is in the broad applicability of MLC tracking, given that the vast majority of linear accelerators purchased today have multileaf collimators which can in principle be used for MLC tracking.
The third is that the MLC, unlike using only the Linac, can be extended to correct for deformation observed in real-time which positions this technology well for future innovations such as real-time MRI and ultrasound guidance.
Another broadly available technology yet to be clinically implemented for real-time adaptation is the treatment couch, for which there has been considerable research and development, e.g., Refs. 18 and 19 and subsequent papers. The community awaits the clinical translation of this technology which could be used as the sole adaptation method or in conjunction with MLC tracking and other degrees of freedom that could be modified on a modern linear accelerator, such as the gantry and collimator angles to improve beam-tumor targeting.
The initial clinical application of MLC tracking was for conventionally fractionated prostate cancer patients treated with VMAT. A future direction for MLC tracking is to apply the technology to stereotactic body radiotherapy prostate cancer patients, where an ASTRO Emerging Technology Report states “A precise ability to localize the target tumor is essential to fully benefit from SBRT techniques.”20 Additional future directions include applying MLC tracking to other tumor sites, particularly those in the thorax and abdomen, where intrafraction motion is considerably larger than that of the prostate. As MLC tracking for the current study only corrected for tumor translation, longer term directions include accounting for higher order tumor motion, such as rotation11 and deformation.21
CONCLUSION
The first clinical implementation of electromagnetic transponder-guided MLC tracking has been performed. Dosimetric analysis demonstrates improved rectal dose coverage with MLC tracking compared to no motion correction, even for the modest 1.2 mm average shift observed.
ACKNOWLEDGMENTS
This study was supported by an NHMRC Australia Fellowship, US NIH R01 93626 and Varian Medical Systems. From Varian, Herbert Cattell, Scott Johnson, and Andrea Morgan have contributed significantly to the clinical realization of MLC tracking over the years. Amit Sawant, Dan Ruan, and others have made critical contributions to the program. Professor Radhe Mohan and Professor Jeffrey F. Williamson are gratefully thanked for their early nurturing of this project and assisting in securing ongoing funding. The authors thank the patient enrolled in the study and the many contributing staff from the Northern Sydney Cancer Centre. The authors also thank Chen-Yu Huang for help with the Hexamotion, Jude Dineley for Fig. 2 photograph and Julie Baz for improving the clarity of this paper.
References
- Keall P. J., Kini V. R., Vedam S. S., and Mohan R., “Motion adaptive x-ray therapy: A feasibility study,” Phys. Med. Biol. 46, 1–10 (2001). 10.1088/0031-9155/46/1/301 [DOI] [PubMed] [Google Scholar]
- Keall P. J., Cattell H., Pokhrel D., Dieterich S., Wong K. H., Murphy M. J., Vedam S. S., Wijesooriya K., and Mohan R., “Geometric accuracy of a real-time target tracking system with dynamic multileaf collimator tracking system,” Int. J. Radiat. Oncol., Biol., Phys. 65, 1579–1584 (2006). 10.1016/j.ijrobp.2006.04.038 [DOI] [PubMed] [Google Scholar]
- Sawant A., Venkat R., Srivastava V., Carlson D., Povzner S., Cattell H., and Keall P., “Management of three-dimensional intrafraction motion through real-time DMLC tracking,” Med. Phys. 35, 2050–2061 (2008). 10.1118/1.2905355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacke M. B., Nill S., Krauss A., and Oelfke U., “Real-time tumor tracking: Automatic compensation of target motion using the Siemens 160 MLC,” Med. Phys. 37, 753–761 (2010). 10.1118/1.3284543 [DOI] [PubMed] [Google Scholar]
- Crijns S. P. M., Raaymakers B. W., and Lagendijk J. J. W., “Proof of concept of MRI-guided tracked radiation delivery: Tracking one-dimensional motion,” Phys. Med. Biol. 57, 7863–7872 (2012). 10.1088/0031-9155/57/23/7863 [DOI] [PubMed] [Google Scholar]
- Poulsen P. R., Carl J., Nielsen J., Nielsen M. S., Thomsen J. B., Jensen H. K., Kjaergaard B., Zepernick P. R., Worm E., Fledelius W., Cho B., Sawant A., Ruan D., and Keall P. J., “Megavoltage image-based dynamic multileaf collimator tracking of a NiTi stent in porcine lungs on a linear accelerator,” Int. J. Radiat. Oncol., Biol., Phys. 82, e321–e327 (2012). 10.1016/j.ijrobp.2011.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawant A., Smith R. L., Venkat R. B., Santanam L., Cho B., Poulsen P., Cattell H., Newell L. J., Parikh P., and Keall P. J., “Toward submillimeter accuracy in the management of intrafraction motion: The integration of real-time internal position monitoring and multileaf collimator target tracking,” Int. J. Radiat. Oncol., Biol., Phys. 74, 575–582 (2009). 10.1016/j.ijrobp.2008.12.057 [DOI] [PubMed] [Google Scholar]
- Smith R. L., Sawant A., Santanam L., Venkat R. B., Newell L. J., Cho B. C., Poulsen P., Catell H., Keall P. J., and Parikh P. J., “Integration of real-time internal electromagnetic position monitoring coupled with dynamic multileaf collimator tracking: An intensity-modulated radiation therapy feasibility study,” Int. J. Radiat. Oncol., Biol., Phys. 74, 868–875 (2009). 10.1016/j.ijrobp.2009.01.031 [DOI] [PubMed] [Google Scholar]
- Sawant A., Dieterich S., Svatos M., and Keall P., “Failure mode and effect analysis-based quality assurance for dynamic MLC tracking systems,” Med. Phys. 37, 6466–6479 (2010). 10.1118/1.3517837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keall P. J., Sawant A., Cho B., Ruan D., Wu J., Poulsen P., Petersen J., Newell L. J., Cattell H., and Korreman S., “Electromagnetic-guided dynamic multileaf collimator tracking enables motion management for intensity-modulated arc therapy,” Int. J. Radiat. Oncol., Biol., Phys. 79, 312–320 (2011). 10.1016/j.ijrobp.2010.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Ruan D., Cho B., Sawant A., Petersen J., Newell L. J., Cattell H., and Keall P. J., “Electromagnetic detection and real-time DMLC adaptation to target rotation during radiotherapy,” Int. J. Radiat. Oncol., Biol., Phys. 82, e545–e553 (2012). 10.1016/j.ijrobp.2011.06.1958 [DOI] [PubMed] [Google Scholar]
- Krauss A., Nill S., Tacke M., and Oelfke U., “Electromagnetic real-time tumor position monitoring and dynamic multileaf collimator tracking using a Siemens 160 MLC: Geometric and dosimetric accuracy of an integrated system,” Int. J. Radiat. Oncol., Biol., Phys. 79, 579–587 (2011). 10.1016/j.ijrobp.2010.03.043 [DOI] [PubMed] [Google Scholar]
- Ruan D. and Keall P., “Dynamic multileaf collimator control for motion adaptive radiotherapy: An optimization approach,” in 2011 IEEE Power Engineering and Automation Conference (PEAM) (IEEE, New York, 2011), Vol. 3, pp. 100–103.
- Poulsen P. R., Schmidt M. L., Keall P., Worm E. S., Fledelius W., and Hoffmann L., “A method of dose reconstruction for moving targets compatible with dynamic treatments,” Med. Phys. 39, 6237–6246 (2012). 10.1118/1.4754297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eade T. N., Guo L., Forde E., Vaux K., Vass J., Hunt P., and Kneebone A., “Image-guided dose-escalated intensity-modulated radiation therapy for prostate cancer: Treating to doses beyond 78 Gy,” BJU Int. 109, 1655–1660 (2012). 10.1111/j.1464-410X.2011.10668.x [DOI] [PubMed] [Google Scholar]
- Michalski J. M., Gay H., Jackson A., Tucker S. L., and Deasy J. O., “Radiation dose-volume effects in radiation-induced rectal injury,” Int. J. Radiat., Oncol., Biol., Phys. 76, S123–S129 (2010). 10.1016/j.ijrobp.2009.03.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka M., Matsuo Y., Sawada A., Ueki N., Miyaba Y., Nakamura M., Yano S., Kaneko S., Mizowaki T., and Kokubo M., “Realization of dynamic tumor tracking irradiation with real-time monitoring in lung tumor patients using a gimbaled X-ray head radiation therapy equipment,” Int. J. Radiat. Oncol., Biol., Phys. 84, S560–S561 (2012). 10.1016/j.ijrobp.2012.07.1493 [DOI] [Google Scholar]
- D’Souza W. D., Naqvi S. A., and Yu C. X., “Real-time intra-fraction-motion tracking using the treatment couch: A feasibility study,” Phys. Med. Biol. 50, 4021 (2005). 10.1088/0031-9155/50/17/007 [DOI] [PubMed] [Google Scholar]
- Wilbert J., Meyer J., Baier K., Guckenberger M., Herrmann C., Heß R., Janka C., Ma L., Mersebach T., Richter A., Roth M., Schilling K., and Flentje M., “Tumor tracking and motion compensation with an adaptive tumor tracking system (ATTS): System description and prototype testing,” Med. Phys. 35, 3911–3921 (2008). 10.1118/1.2964090 [DOI] [PubMed] [Google Scholar]
- Konski A. A., Wallner P. E., Harris E. E. R., R. A.PriceJr., Buyyounouski M., Miller R., Schefter T., Tome W., and Parsai I., “Stereotactic body radiotherapy (SBRT) for primary management of early-stage, low-intermediate risk prostate cancer,” Report of the ASTRO Emerging Technology Committee, 2008. [DOI] [PubMed]
- Ge Y., O’Brien R., and Keall P., “Real-time tumor deformation tracking using dynamic multileaf collimator (DMLC),” Int. J. Radiat. Oncol., Biol., Phys. 84, S83 (2012). 10.1016/j.ijrobp.2012.07.219 [DOI] [Google Scholar]