Abstract
This study examined relations among neuritic and diffuse plaques, neurofibrillary tangles, age, and apolipoprotein E (APOE) in two large samples of neuropathology cases, the Religious Orders Study and the Memory and Aging Project. Cognitive status ranged from normal to demented and AD neuropathology ranged from none to severe. Confirmatory factor analysis identified a best fitting four factor solution to describe interrelationships among plaques and tangles: 1) a global neuritic plaque factor, 2) a global diffuse plaque factor, 3) a factor defined by medial temporal neurofibrillary tangles, and 4) a neocortical tangle factor. Results supported a hypothesis that neuritic plaques mediate the association of age and APOE with neocortical tangles, and similarly mediate the effect of APOE on medial temporal tangles. However, medial temporal tangles were related to age independent of neuritic plaques. These results support a primary amyloid based AD process that accounts for neocortical tangles and makes the largest contribution medial temporal tangles. A second, age related but non-amyloid process likely contributes to medial temporal lobe tangles.
Keywords: Alzheimer’s disease, neuropathology, age, APOE, latent variable modeling
Alzheimer’s disease (AD) has been defined by neuropathological features of neuritic plaques and neurofibrillary tangles since it was first identified in 1906 and these two types of neuropathology continue to constitute the basis for the neuropathological diagnosis of AD (Hyman, 1997; Hyman et al., 2012; Montine et al., 2012). There is an extensive literature on the relationship of these neuropathological features to clinical status (Bennett, Schneider, Wilson, Bienias, & Arnold, 2004; Gomez-Isla, Spires, De Calignon, & Hyman, 2008; Nelson, Braak, & Markesbery, 2009). A widely accepted model of neuropathological and clinical progression of AD posits that neurofibrillary tangles initially appear in the entorhinal cortex and hippocampus and later spread to neocortex. This forms the basis for the widely used Braak and Braak staging of AD pathology (Braak & Braak, 1991; Braak, Braak, & Bohl, 1993; Thal, Griffin, & Braak, 2008) and provides an anatomical explanation for the progression of clinical symptoms. Because the hippocampus is a critical anatomical substrate of episodic memory, the early presence of neurofibrillary tangles in the hippocampus helps to explain the amnesia associated with early AD. Neurofibrillary tangle pathology in neocortical areas in later stages of AD corresponds to broader cognitive impairment affecting non-memory domains and resulting in clinical dementia. Regional and spatial patterns of neuritic plaques do not show the same characteristic pattern as for neurofibrillary tangles (Longstreth et al., 2009; Sonnen et al., 2008; Thal et al., 2008), and specifically, neuritic plaques are likely to be distributed throughout neocortical regions in the earliest stages of plaque development (Thal et al., 2008).
At the level of molecular biology, the “amyloid cascade hypothesis” has emerged as the leading theory to explain the development of AD neuropathology (Hardy & Allsop, 1991; Hardy & Higgins, 1992; Selkoe, 1991). This hypothesis posits that the formation and deposition of amyloid-β peptide (Aβ), the primary constituent of neuritic plaques, is the first step in a sequence of events that ultimately leads to impaired cell function, the formation of neurofibrillary tangles, cell death, cognitive decline, and clinical dementia (Jack et al., 2010). It is supported by the observation that all three genetic mutations associated with early onset AD and the apolipoprotein E polymorphism (APOE) alter the metabolism of amyloid, are associated with development of neuritic plaques composed of Aβ, and manifest tangle formation. Further, preclinical data support a linkage between Aβ processing and tangle formation (Klein, Stine, & Teplow, 2004; Oddo et al., 2006).
A comprehensive explanation of the genesis of AD should incorporate neurobiology, neuropathology, and clinical characteristics. The amyloid cascade hypothesis explains many of the findings related to AD, but is problematic in some respects. Notably, this hypothesis would suggest that presence of neurofibrillary tangles should mirror presence of amyloid plaques (Longstreth et al., 2009). But repeated observations indicate that this is not the case; neurofibrillary tangles are commonly observed in medial temporal lobes in the absence of amyloid plaques (Jellinger & Bancher, 1998; Murray et al., 2011; Nelson, Abner, et al., 2009).
An alternate explanation for the observation of medial temporal neurofibrillary tangles in the absence of amyloid plaques is that medial temporal tangles could result from a second, non-AD process (Nelson, Abner, et al., 2009). We hypothesize that two independent processes may better explain the complex relation of amyloid and tangles to aging, AD, and cognition. Process 1 is prototypical AD and leads to amyloid deposition throughout the neocortex and medial temporal lobes and to widespread tangle formation and impairment in multiple cognitive domains. Process 2 is a different age related process, is associated with tangle formation in the medial temporal lobes, and presumably contributes to episodic memory deficits. It is well known that mixed pathologies commonly contribute to cognitive impairment in old age (Petrovitch et al., 2005; Schneider, Arvanitakis, Bang, & Bennett, 2007; Sonnen et al., 2007; Troncoso et al., 2008). It is also well accepted that other diseases such as fronto-temporal lobe dementia can manifest pathologically with tangles in the absence of amyloid (Dickson, 2009; Nelson, Abner, et al., 2009). One- and two-process explanations of tangle formation generate different predictions about relations of major risk factors such as age and APOE with plaques and tangles. In the one-process formulation, age and APOE relate to tangles via mediating effects of plaques. The two-process account would predict that medial temporal tangles have an additional relationship with age that cannot be explained by plaques.
The goal of this study was to evaluate competing hypotheses from one- and two-process models to account for relationships among age, APOE, and regional measures of the characteristic neuropathological features of AD, neuritic plaques, diffuse plaques, and neurofibrillary tangles. One hypothesis is that these features all are manifestations of a unitary AD process. In this case, neurofibrillary pathology throughout the brain would be related to age and APOE via the mediating effects of neuritic plaques and would not be related to these risk factors after controlling for neuritic plaques. An alternate hypothesis would posit a typical AD process that would explain much of the observed neuropathology, but also an additional process that would provide incremental explanation of neurofibrillary tangles in the medial temporal lobes. This explanation would predict that the relation of age with medial temporal neurofibrillary tangles would fall outside the primary AD pathway and would not be entirely explained by neuritic plaques, while in contrast, APOE effects would be AD related and mediated by neuritic plaques.
This study took advantage of a relatively large neuropathology sample with well-characterized and broadly variable AD neuropathology. We used latent variable modeling to characterize the plaque and tangle neuropathology of AD and test competing hypotheses about how neuropathology indices relate to one another and to age and APOE. Latent variables are statistical representations of conceptual constructs that are not directly measurable. They are empirically derived to explain covariance among observable but imperfect indicators of the constructs of interest, and in so doing, may help to define, measure, and clarify these constructs and how they relate to directly observed external variables.
Methods
Participants
The Religious Orders Study (ROS) and Memory and Aging Project (MAP) are both community-based, prospective cohort studies of risk factors for incident AD and other chronic conditions of aging conducted by the Rush Alzheimer’s Disease Center. Recruitment and inclusion criteria for these studies and subject evaluations have been previously described in detail (R. Wilson, Barnes, & Bennett, 2003; R. S. Wilson, Beckett, et al., 2002; R. S. Wilson, Mendes De Leon, et al., 2002). Briefly, both studies recruit older individuals without known dementia who agree to receive clinical and psychological evaluation each year and to donate their brains for postmortem examination. The annual attrition rate in both cohorts is below 1% among survivors and the autopsy rate exceeds 80% in both cohorts. These studies share similar clinical and neuropathology protocol (Bennett, Schneider, Arvanitakis, et al., 2006). All individuals from these two samples who had available neuropathology data were included in this study; individuals with and without AD pathology were included and individuals with other neuropathologies, like Lewy bodies and vascular brain injury, also were included.
Clinical Methods
Clinical diagnosis of dementia and AD followed NINCD-ADRDA criteria and were implemented in a three-step process as described previously (Bennett, Schneider, Aggarwal, et al., 2006). Mild cognitive impairment (MCI) referred to persons with cognitive impairment based on neuropsychological test performance in the absence of dementia as described previously (Bennett et al., 2002; Boyle, Wilson, Aggarwal, Tang, & Bennett, 2006). These criteria are similar to cognitive impairment no dementia (CIND). Persons without dementia or MCI were characterized as no cognitive impairment (NCI). APOE genotyping was performed by Agencourt Bioscience Corporation as previously described (Buchman et al., 2009).
Table 1 presents a summary of the sample characteristics by clinical diagnosis before death. The analytical sample for the present study consisted of 591 subjects with complete data on most of the neuropathology variables included in the analysis (sample sizes for individual variables ranged from 496 to 591). The sample was predominantly white, non-Hispanic (96%) and was 59% female with a mean age at death of 87 years and an average education level of about 17 years. Approximately 30% of the participants were carriers of at least one APOE ε4 allele. In the last clinical evaluation, about a third of the sample had NCI, 25% had MCI, and the remaining 43% had AD or other form of dementia. The average interval from last psychological evaluation to brain autopsy was 6.5 months (SD = 3.9, range 0-20 months). Overall, sample characteristics were very similar across ROS and MAP.
Table 1.
Sample Characteristics.
| Variable | ROS | MAP | Combined |
|---|---|---|---|
|
| |||
| Total N | 392 | 199 | 591 |
|
| |||
| Age at Death | |||
| Mean (SD, range) | 86.3 (7.0, 66-104) | 88.0 (5.8, 66-102) | 86.9 (6.7, 66-104) |
|
| |||
| Education (years completed) |
|||
| Mean (SD, range) | 18.0 (3.5, 3-30) | 14.7 (2.9, 6-22) | 16.9 (3.7, 3-30) |
|
| |||
| Gender | |||
| N (%) Female | 233 F (59.4%) | 115 F (57.8%) | 348 F (59%) |
| N (%) Male | 159 M (40.6%) | 84 M (42.2%) | 243 M (41%) |
|
| |||
| Race | |||
| N (%) Caucasian | 377 C (96.2%) | 192 C (96.5%) | 569 C (96.3%) |
| N (%) African American | 13 AA (3.6%) | 7 AA (3.5%) | 20 AA (3.4%) |
| N (%) Hispanic | 2 H (0.1%) | 2 H (0.3%) | |
|
| |||
| APOE Status | |||
| N (%) ε4 negative | 275 ε4− (70.9%) | 134 ε4− (69.8%) | 409 ε4− (70.5%) |
| N (%)ε 4 positive | 113 ε4+ (29.1%) | 58 ε4+ (30.2%) | 171 ε4+ (29.5%) |
|
| |||
| Clinical Diagnosis, Last | |||
| Evaluation | |||
| N (%) Normal | 125 N (31.9%) | 66 N (33.2%) | 191 N (32.3%) |
| N (%) MCI | 89 M (22.7%) | 57 M (28.6%) | 146 M (24.7%) |
| N (%) Dementia | 178 D (45.4%) | 76 D (38.2%) | 254 D (43.0%) |
|
| |||
| Neuritic Plaque Counts (mean, range) |
|||
| mid-frontal | 9.7 (0 – 81) | 8.5 (0 – 48) | 9.3 (0 – 81) |
| mid-temporal | 9.7 (0 – 93) | 8.8 (0 – 64) | 9.4 (0 – 93) |
| inferior parietal | 9.6 (0 – 67) | 10.3 (0 – 55) | 9.8 (0 – 67) |
| hippocampal | 4.3 (0 – 46) | 4.4 (0 – 34) | 4.3 (0 – 46) |
| entorhinal | 8.1 (0 – 50) | 9.2 (0 – 58) | 8.4 (0 – 58) |
|
| |||
| Diffuse Plaque Counts (mean, range) |
|||
| mid-frontal | 18.5 (0 – 193) | 17.6 (0 – 121) | 18.4 (0 – 193) |
| mid-temporal | 17.6 (0 – 147) | 14.6 (0 – 89) | 16.6 (0 – 147) |
| inferior parietal | 15.1 (0 – 139) | 15.4 (0 – 108) | 15.2 (0 – 139) |
| hippocampal | 2.1 (0 – 29) | 2.3 (0 – 27) | 2.2 (0 – 29) |
| entorhinal | 7.3 (0 – 69) | 7.9 (0 – 57) | 7.5 (0 – 69) |
|
| |||
| Neurofibrillary Tangle Counts (mean, range) |
|||
| mid-frontal | 1.3 (0 – 32) | 1.3 (0 – 65) | 1.3 (0 – 65) |
| mid-temporal | 4.6 (0 – 66) | 4.6 (0 – 72) | 4.6 (0 – 72) |
| inferior parietal | 1.9 (0 – 48) | 2.0 (0 – 54) | 2.0 (0 – 54) |
| hippocampal | 19.9 (0 – 154) | 24.9 (0 – 134) | 21.8 (0 – 154) |
| entorhinal | 21.0 (0 – 130) | 24.1 (0 – 100) | 22.1 (0 – 130) |
Neuropathology Methods
AD neuropathology indices were obtained from a standard neuropathology protocol as previously described (Bennett, Schneider, Tang, Arnold, & Wilson, 2006; Bennett et al., 2003). AD neuropathology variables of interest in this study included counts in a 1mm2 area of greatest density of neuritic plaques (NP), diffuse plaques (DP), and neurofibrillary tangles (NFT) from five brain regions: hippocampal CA1 sector, entorhinal cortex, midfrontal, middle temporal, and inferior parietal cortices. Due to the skewness of the distributions of these AD neuropathology measures we recoded the values into deciles. The potential loss of information due to the discretization of these measures into deciles was offset by gains in meeting distributional assumptions of latent variable models and improvements in overall model fit. Table 1 shows summary statistics for raw plaque and tangle counts.
Data Analysis
Overview
Latent variable modeling methods were used to identify latent variables to characterize and explain the regional distribution of AD neuropathology ratings (measurement model) and to evaluate relationships among these latent variables, age, and APOE (structural model). Two structural models were used to test study hypotheses about how neurofibrillary tangles relate to age, APOE, and diffuse and neuritic plaques. Multiple group analyses were used to replicate results across the ROS and MAP samples. The measurement model was initially developed using the larger ROS sample, and then was applied to the MAP sample in a multiple group analysis. Replicability of structural relations was similarly tested in a multiple group analysis.
Measurement Model
The first step in model building identified a measurement model that explained the relations among measures of AD neuropathology in the ROS sample. Confirmatory factor analysis (CFA) was used to test alternate a-priori models to explain covariance among the regional ratings of neuritic plaques (NP), diffuse plaques (DP), and neurofibrillary tangles (NFT). Alternate models that were tested included: M-1) a single factor model (global AD) in which all 15 neuropathology indicators defined one AD dimension, M-2) a two factor model (neocortical versus medial temporal) with one factor defined by neocortical NP, DP, and NFT and the second defined by medial temporal NP, DP, and NFT, M-3) a two factor model defined by plaques (DP and NP) and tangles (NFT), M-4) a three factor model with one factor defined by NP, one defined by DP, and one by NFT, M-5) a four factor model with single factors for NP and DP and two factors for NFT (medial temporal and neocortical), M-6) a four factor model with medial temporal and neocortical factors for NP and single factors for DP and NFT, M-7) a similar four factor model with two DP factors and single NP and NFT factors, and M-8) a six factor model with two factors each for NP, DP and NFT (neocortical and medial temporal). Tests of these models estimated the fit of the hypothesized relations in each model to the data; the model that fits best, and fits well, represents the hypothesis with the strongest support from the data.
We then performed a multiple group analysis to evaluate whether the best fitting of the eight measurement models, derived from the ROS sample, was also applicable in the MAP sample. In multiple group CFA, a common model for both groups is specified on an a priori basis, and then group differences in individual parameters can be systematically tested. The best fitting model from the ROS sample was used as the base model and model parameters were constrained to be the same for the ROS and MAP groups. Specifically, the same number of factors accounting for AD neuropathology were used for both groups, factor loadings and thresholds were constrained to be the same, and correlations among factors were constrained to be equal. Factor means and variances were allowed to differ, reflecting the likelihood of real-world sample differences in distributions of AD neuropathology. Fit of this model was compared with fit of a less restrictive model in which loadings, thresholds, and correlations among factors were allowed to differ across groups. In this less restrictive model, the loadings and thresholds for one indicator for each factor were constrained to be the same so that the model would be statistically identifiable.
Structural Model
This step of model development added age and APOE to the best fitting measurement model and examined structural relations among these risk factor variables and the latent variables underlying AD neuropathology ratings. This step was designed to test the hypothesis that the effects of age and APOE on NFT are mediated by NP. Evidence for mediation would be found if: 1) age and APOE have simple bivariate effects on NFT and NP, 2) NP has a direct association with NFT, but 3) age and APOE are not related to NFT independent of NP. These analyses also examined relationships of DP with age, APOE, NP, and NFT.
The primary structural model (S-1) built upon the best fitting measurement model, and evidence of mediation of types 1, 2 or 3 above was obtained as follows. NP, DP, and NFT factors were regressed on age and APOE. In addition, NFT factors were regressed on NP and DP factors. The residual correlation between NP and DP factors was freely estimated. The paths in this model from NP to NFT tested whether there was a direct relation of NFT with NP (mediation evidence type 2). The direct paths from age and APOE to NFT tested whether risk factors were not related to NFT independent of NP (mediation evidence type 3). All parameters in S-1 were simultaneously estimated, including parameters of the measurement model (factor loadings, thresholds, residual variances) and regression coefficients defining structural relationships. Multiple group CFA was used to evaluate whether results were invariant across the ROS and MAP samples. A secondary model (S-2) was used to test whether risk factors had simple bivariate relations with NFT and NP (mediation evidence type 1). This was a re-parameterization of S-1 in which NP, NFT, and DP factors were regressed on age and APOE and residual correlations among these factors were freely estimated. This model differed from S-1 in that regression paths from NP and DP to NFT were not included, and consequently, age and APOE effects on NFT were not adjusted for NP and DP.
Model estimation was performed with Mplus version 6.0 (Muthén & Muthén, 1998-2012). Neuropathology indicators were modeled as categorical variables using a weighted least squares mean and variance adjusted estimator (WLSMV) applied to a mean and covariance data structure. Model fit was evaluated using an overall chi square test supplemented by fit indices: the comparative fit index (CFI; Bentler, 1990), the Tucker-Lewis index (TLI; Tucker & Lewis, 1973), and the root mean square error of approximation (RMSEA; Browne & Cudek, 1993). CFI and TLI values of 0.95 and higher indicate good fit; RMSEA value of .08 and lower indicate acceptable fit. A modified chi-square difference test appropriate for the WLSMV estimator (Muthén & Muthén, 1998-2012) was used to compare fit of nested models and determine if fit significantly improved as a result of freely estimating one or more parameters in a model.
Results
Measurement Model (M-1 to M-8)
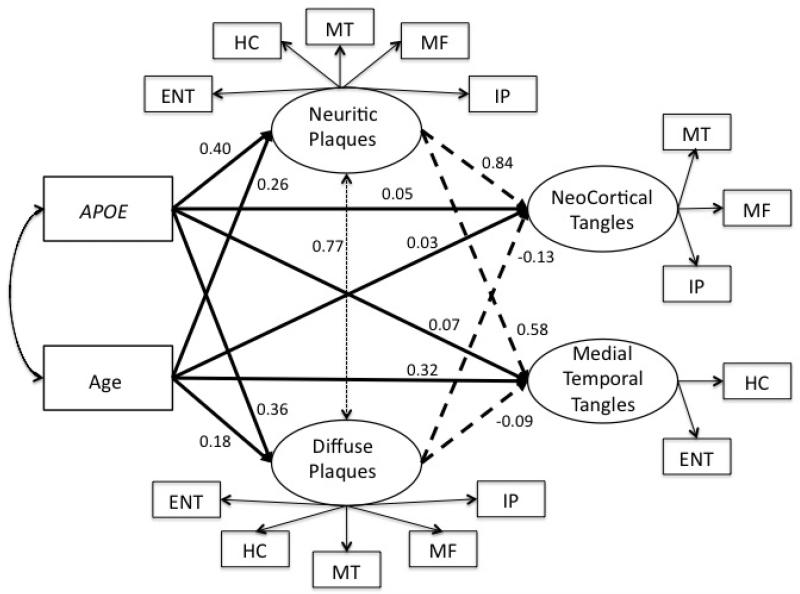
Fit of alternative models for explaining relations among the neuropathology indicators of AD using the ROS sample is summarized in Table 2. Model fit was generally good for the three and four factor models but was better for solutions with more factors. The six-factor solution (M-8) had the best fit but there was technical evidence suggesting that too many factors were being extracted. The four-factor solution with single NP and DP factors and two NFT factors showed the best fit among the remaining alternative models and was selected as the base measurement model for further analyses. This four-factor solution is depicted in Figure 1, specifically the relationships of the latent factors to the observed neuropathology indicators.
Table 2.
Fit Indices for Alternate Models of Dimensions of AD Neuropathology. Results are based on the ROS sample (n=392).
| Model | Overall χ2[df] |
CFI | TLI | RMSEA (90% CI) |
|---|---|---|---|---|
|
| ||||
| 1f – global AD | 1147.2 [90] | 0.938 | 0.928 | 0.173 (0.164-0.182) |
|
| ||||
| 2f – neocortical, medial temporal | 1055.0 [89] | 0.943 J | 0.933 | 0.166 (0.157-0.175) |
|
| ||||
| 2f – plaques, tangles | 684.7 [89] | 0.965 | 0.959 | 0.131 (0.122-0.140) |
|
| ||||
| 3f – NP, DP, NFT | 292.4 [87] | 0.988 | 0.985 | 0.078 (0.068-0.088) |
|
| ||||
| 4f – NP, DP, medial temporal NFT, neocortical NFT |
222.6 [84] | 0.992 | 0.990 | 0.065 (0.055-0.075) |
|
| ||||
| 4f –- DP, NFT, medial temporal NP, neocortical NP |
261.7 [84] | 0.990 | 0.987 | 0.073 (0.063-0.084) |
|
| ||||
| 4f – NP, NFT, medial temporal DP, neocortical DP |
259.0 [84] | 0.990 | 0.987 | 0.073 (0.063-0.083) |
Figure 1.
Analytic model and structural relationships among age, APOE, and latent neuropathology factors. Heavy solid lines show effects of age and APOE on neuropathology factors. Heavy dashed lines show effects on neuritic and diffuse plaques on neurofibrillary tangles. This represents the primary structural model (S-1) in which all of the indicated parameters were simultaneously estimated. Factor loadings were estimated in this model but are not presented. Paths from neuritic and diffuse plaques to neurofibrillary tangle (heavy dashed lines) were dropped in a secondary analysis to evaluate simple effects of age and APOE on neurofibrillary tangles. ENT – entorhinal cortex, HC = hippocampus, MT = mid-temporal cortex, MF = mid-frontal cortex, IP = inferior parietal cortex.
A multiple group CFA was used to test the invariance of the four-factor solution across the MAP and ROS samples. Model fit was not significantly worse for the fully constrained model in which loadings, thresholds, and correlations among factors were constrained to be the same in MAP and ROS in comparison with the less restrictive model where those parameters were allowed to differ across groups (modified chi square difference test χ2[111] =117.9, p=.31). This supported the invariance of results across the two samples, and consequently, the two groups were combined to generate the final measurement model. Standardized factor loadings from this four-factor measurement model applied to the combined ROS-MAP sample are presented in Table 3. All factor loading were 0.75 or higher, and all but two exceeded 0.80, indicating that variance in observed indicators was well explained by the latent factors. Correlations among latent factors ranged from 0.46 to 0.80 (Table 4). These results support the presence of four distinct factors but also show substantial correlation among factors.
Table 3.
Standardized factor loadings for four factor model, combined ROS and MAP sample.
| Latent Factor | Observed Indicator | Loading |
|---|---|---|
|
| ||
| Neuritic Plaques | Mid Frontal NP | 0.89 |
|
| ||
| Mid Temporal NP | 0.93 | |
|
| ||
| Inferior Parietal NP | 0.88 | |
|
| ||
| Hippocampal NP | 0.86 | |
|
| ||
| Entorhinal NP | 0.88 | |
|
| ||
| Neocortical Diffuse Plaques | Mid Frontal DP | 0.93 |
|
| ||
| Mid Temporal DP | 0.89 | |
|
| ||
| Inferior Parietal DP | 0.92 | |
|
| ||
| Hippocampal DP | 0.75 | |
|
| ||
| Entorhinal DP | 0.75 | |
|
| ||
| Neocortical Neurofibrillary Tangles | Mid Frontal NFT | 0.87 |
|
| ||
| Mid Temporal NFT | 0.94 | |
|
| ||
| Inferior Parietal NFT | 0.90 | |
|
| ||
| Medial Temporal Neurofibrillary Tangles | Hippocampal NFT | 0.82 |
|
| ||
| Entorhinal NFT | 0.88 | |
Table 4.
Inter-correlation of factors from four-factor model, combined ROS and MAP samples.
| NP | DP | Neocortical NFT | |
|---|---|---|---|
| DP | 0.80 | ||
|
| |||
| Neocortical NFT | 0.77 | 0.58 | |
|
| |||
| Medial Temporal NFT | 0.61 | 0.46 | 0.70 |
Structural Model
We next added age and APOE to the measurement model and examined structural relations among these risk factor variables and the latent variables, paying particular attention to theoretically important relationships between age, APOE, NP and neocortical and medial temporal NFT. Figure 1 graphically presents the analytic model and structural results. The primary structural model (S-1) simultaneously estimated all paths, including those of the measurement model. First, we evaluated whether the ROS and MAP samples could be combined for this analysis. We used a multiple group analysis to test sample invariance of the structural parameters in S-1, depicted in Figure 1. Model fit did not significantly improve when these regression parameters were allowed to be freely estimated across ROS and MAP groups as compared to being constrained to be equal in the two groups (χ2[14] =10.7, p=.71), therefore, subsequent analyses used the combined ROS-MAP sample.
Model S-1 fit well in the combined sample (χ2[106] = 353.6, CFI = 0.988, TLI = 0.984, RMSEA = 0.063 (95% CI = 0.056 – 0.071). Table 5 shows independent effects of age, APOE, NP, and DP on NFT factors (Multivariate Effect column). These results are also presented in Figure 1. Both neocortical NFT and medial temporal NFT were strongly related to NP (standardized coefficients of 0.84 and 0.58). Medial temporal NFT was related to age in this analysis (0.32), but neocortical NFT was not related to age and neither NFT factor was significantly related to APOE. DP was strongly correlated with NP (standardized coefficient = 0.77), but was not independently related to medial temporal or neocortical NFT.
Table 5.
Structural relationships among age, APOE, neurofibrillary tangle factors, and neuritic and diffuse plaque factors in the combined ROS and MAP samples. Coefficients represent the effects of the independent variables on the dependent variables. Values in the Simple Effect (S-2) columns show simple effects of age and APOE on neuropathology latent variables. Values in the Multivariate Effect (S-1) columns show multivariate effects adjusted for the other effects in the model. Significant effects in bold.
| Simple Effect (S-2) | Multivariate Effect (S-1) | ||||
|---|---|---|---|---|---|
|
| |||||
| Dependent Variable | Independent Variable |
Standardized Regression Coefficient |
p | Standardized Regression Coefficient |
p |
|
| |||||
| NP | Age | 0.26 | 0.001 | ||
|
| |||||
| APOE ε4+ | 0.40 | 0.001 | |||
|
| |||||
| DP | Age | 0.18 | 0.001 | ||
|
| |||||
| APOE ε4+ | 0.36 | 0.001 | |||
|
| |||||
| Neocortical NFT | Age | 0.22 | 0.001 | 0.03 | 0.55 |
|
| |||||
| APOE ε4+ | 0.34 | 0.001 | 0.05 | 0.22 | |
|
| |||||
| DP | −0.13 | 0.12 | |||
|
| |||||
| NP | 0.84 | 0.001 | |||
|
| |||||
| Medial Temporal NFT | Age | 0.45 | 0.001 | 0.32 | 0.001 |
|
| |||||
| APOE ε4+ | 0.27 | 0.001 | 0.07 | 0.08 | |
|
| |||||
| DP | −0.09 | 0.26 | |||
|
| |||||
| NP | 0.58 | 0.001 | |||
The secondary model (S-2) characterized simple relations of age and APOE with the latent factors. Regression paths from NP and DP to NFT (dashed lines in Figure 1) were not included in this model, but residual correlations of NFT with NP and DP were estimated. Since this was a re-parameterization, model fit was the same as for S-1. Standardized regression coefficients that represent simple relations of NP, DP, and NFT latent variables with age and APOE are also included in Table 5 (Simple Effect column).
The two structural models verify that both NFT factors had simple, direct relations with age and APOE (Simple Effect, Table 5), were strongly related to NP (but not DP) (Multivariate Effect, Table 5), but were not related to age and APOE independent of NP (Multivariate Effect, Table 5). The simple regression coefficient describing the effect of age on neocortical NFT was diminished by 86% in the multivariate model that accounted for the effect of NP on neocortical NFT (standardized coefficient of 0.22 versus 0.03). The corresponding coefficient for APOE decreased by 85%. The APOE effect on medial temporal NFT diminished by 68%, while the effect of age decreased 29%. About 15% of the variance in neocortical NFT was explained by age and APOE alone, but this increased dramatically to 59% when NP (and DP) were added as predictors. For medial temporal NFT, age and APOE alone explained 25% of the variance, and this increased to 46% when NP and DP were added. These findings support a mediating effect of NP on the relations of age and APOE with NFT. The mediating effect of NP on the age – medial temporal NFT relation was smaller, and there still was an age effect independent of NP.
Results Summary
The best fitting measurement model included separable neocortical and medial temporal dimensions of NFT, but single dimensions of NP and DP. Both NFT factors were strongly related to NP, and NP was related to age and APOE. Results supported the hypothesis that NP mediated the association of APOE with both NFT factors and mediated the association of age with neocortical NFT. NP partially mediated the relation between age and medial temporal NFT, but age still was related to medial temporal NFT independent of NP. DP was not related to NFT independent of NP.
Discussion
Results of this study support the presence of two processes underlying amyloid plaques and neurofibrillary tangles, the characteristic neuropathological features of AD. The dominant process is a classic AD process characterized by neuritic plaques and neurofibrillary tangles throughout the brain. This process, as would be expected, was related in this study to two major risk factors for AD, older age and APOE ε4. Age and APOE were associated with neuritic (amyloid) plaques, neuritic plaques in turn were associated with neurofibrillary tangles in the neocortex and medial temporal lobes, and there was evidence that neuritic plaques mediated the relations of the risk factors with tangles, especially neocortical neurofibrillary tangles. Results also supported a second process that contributes to medial temporal neurofibrillary tangles. This process also was age related, but results suggest that its relation to age falls outside the causal pathway from age and APOE to Aβ and neuritic plaques to neurofibrillary tangles.
This study used latent variables to summarize expert ratings of AD neuropathology from multiple brain regions, and then used these summary variables to test hypotheses about neuropathology processes in AD. Results have important implications for understanding the biologic cascade involved in the development and progression of AD neuropathology. Several alternative explanations to account for results of this study merit consideration.
The simplest explanation is that Aβ neuritic plaques represent the primary neurotoxic input and have a causal effect with respect to downstream development of neurofibrillary tangles. While this explanation is consistent with the strong association of neuritic plaques with both neocortical and medial temporal tangles, it nevertheless is problematic in two important ways. First, accounting for the early presence of medial temporal tangles in the absence of neuritic plaques presents difficulties (Longstreth et al., 2009). There is a widely accepted view that the neurofibrillary pathology of AD begins in the medial temporal lobes and subsequently spreads to neocortex. This is the fundamental observation underlying the well-established Braak & Braak staging of AD (Braak & Braak, 1991; Braak et al., 1993), and is consistent with the identification of two factors accounting for neurofibrillary tangles in this study. The amyloid cascade hypothesis posits that Aβ is a prerequisite for development of neurofibrillary tangles, and therefore would argue that Aβ is present preceding or at least concurrent with the development of tangles. Consequently, explaining the early presence of isolated medial temporal tangles in the context of a lack of a corresponding regional/temporal distribution of plaques presents a challenge within the context of the amyloid cascade hypothesis (Longstreth et al., 2009).
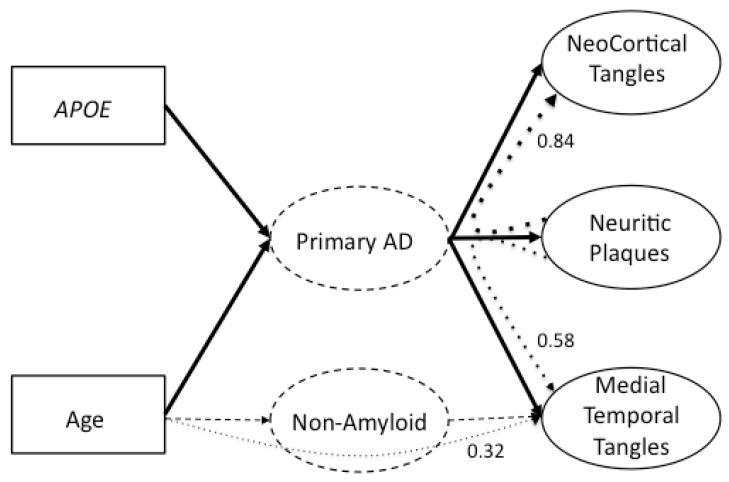
The strong relation of neuritic plaques to neurofibrillary tangles is this study could result from these two features being correlated outcomes of a more primary AD process. This model is presented graphically in Figure 2. The primary AD process is presented as a hypothesized intervening variable between risk factors, neuritic plaques, and neurofibrillary tangles. The solid lines show the hypothesized causal paths from risk factors through primary AD to plaques and tangles. Note that plaques and tangles are related through their common relations with primary AD. This model can explain some of the major findings of this study, specifically a single neuritic plaque factor but separable medial temporal and neocortical neurofibrillary tangle factors, and the relationship of APOE and neuritic plaques to neocortical and medial temporal tangles. However, this explanation cannot account for the curved, dotted lines in Figure 2, which show the effect of age on medial temporal tangles independent of neuritic plaques and the stronger relation of neocortical versus medial temporal tangles with neuritic plaques.
Figure 2.
Conceptual model to explain how risk factors (age and APOE) relate to neuritic plaque and neurofibrillary tangle neuropathology. Solid boxes are observed variables in this study, and solid ovals are latent variables in this study measured by the observed neuropathology ratings. Curved dotted lines represent paths tested in this study, and values are the estimated standardized regression coefficients for these paths. Dashed ovals in the center are hypothesized processes to explain study results. Primary AD is a postulated, amyloid based, causative agent for neuritic plaques and neurofibrillary tangles. The mechanisms of Primary AD require future clarification, but correlations of plaques with tangles are hypothesized to result from their common relations with Primary AD. A non-amyloid process, or processes, is required to explain the association of age with medial temporal neurofibrillary tangles that cannot be explained by the amyloid path.
A two-process model for plaque and tangle neuropathology is better equipped to account for the overall pattern of results in this study. The primary AD process in Figure 2 could account for neuritic plaques and neocortical tangles, and could make a major contribution to medial temporal tangles. However, it appears that one or more nonamyloid processes also contribute to medial temporal tangles. Neurofibrillary tangles are associated with a number of non-AD conditions (Dickson, 2009; Nelson, Abner, et al., 2009). Tau mutations (Klein et al., 2004) and chronic traumatic encephalopathy (Baugh et al., 2012; Yoshiyama et al., 2005) are but two of the potential etiologies that require further investigation. An implication of the two-process conceptualization is that neurofibrillary tangles in the medial temporal lobes, often considered a defining feature of early AD, may not always be associated with AD. This conclusion is supported in part by the new NIA-AA guidelines on the neuropathologic diagnosis of AD (Hyman et al., 2012; Montine et al., 2012). While not explicitly addressed in the text, the table in Hyman et al. clearly indicates that a pathologic diagnosis of AD requires amyloid deposition such that the sole presence of neurofibrillary pathology is not even sufficient for the diagnosis of possible AD. How this is reconciled with Braak Staging for AD remains to be determined.
Diffuse plaques in this study were related to both age and APOE, and were strongly associated with neuritic plaques. While diffuse plaques were correlated with NFT, they were not related to NFT independent of effects of neuritic plaques. Overall, results of this study provide further evidence that diffuse plaques are not central to the neuropathological changes of AD.
This study has many strengths but also has important limitations. The large sample with well-characterized neuropathology is a strength, as is the broad variability of neuropathology from persons with cognitive abilities proximate to death that ranged from NCI, to MCI, to dementia. The availability of two independent samples characterized with the same neuropathologic methods allowed for replicating results across two different groups of study participants. The major limitation of this study is that data are essentially correlational, and the primary hypotheses of interest are causal. Causal conclusions from correlational data must be made with caution, even correlations with genotype data that presumably has an effect prior to the development of pathology. Ultimately, these findings must be supported by broader lines of evidence that can more directly address causal relationships.
An additional limitation is that this study examined a limited set of variables relevant to AD, and did not address broader questions of how other pathologies such as cerebral amyloid angiopathy (CAA), cerebrovascular disease (CVD) and Lewy bodies might relate to AD findings, and ultimately, clinical and cognitive outcomes. This is important because we and others have shown a relation of APOE with CAA and CVD (Schneider et al., 2005), and that mixed pathologies are strongly related to cognition (Dowling et al., 2011; Schneider, Arvanitakis, Leurgans, & Bennett, 2009). In addition, we and others have found that indices of neural reserve, including neuron and synaptic density, also contribute to cognition (Honer et al., 2012; R. S. Wilson et al., 2013). Future analyses should incorporate these variables into the multi-process model to develop a more complete understanding of the relationships between APOE, AD and other post-mortem indices related to cognitive decline and dementia.
This study has important implications for how we think about AD in general, and the conduct of biological investigations of AD in particular as it suggests the existence of at least two processes leading to tangle formation in the medial temporal lobe. The medial temporal lobe is the most extensively studied brain region as a result of the general view that it appears to be the site of the earliest lesions of AD. This may in fact be less true than we think and it may be much more prudent to study AD processes in the neocortex. It is noteworthy that the CERAD neuropathology criteria for AD published in 1991 came to a similar conclusion, defining AD by the presence of neuritic plaques in the neocortex (Mirra et al., 1991).
Acknowledgements
This work was supported by grants from the National Institute on Aging P30 AG10161 (Bennett PI), R01 AG15819 (Bennett PI) and R13 AG030995 (Mungas PI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors (Dr. Dan Mungas, Dr. Rochelle Tractenberg, Dr. Julie Schneider, Dr. Paul Crane, and Dr. David Bennett) have no actual or potential conflicts of interest that could inappropriately influence this work.
The data contained in the manuscript being submitted have not been previously published, have not been submitted elsewhere and will not be submitted elsewhere while under consideration at Neurobiology of Aging.
This manuscript describes data analysis of deidentified data that was collected under approved Institutional Review Board protocols.
All authors have reviewed the contents of the manuscript being submitted, approve of its contents and validate the accuracy of the data.f its contents and validate the accuracy of the data.
Disclosure: The authors have no conflicts to disclose that relate to this study.
References
- Baugh CM, Stamm JM, Riley DO, Gavett BE, Shenton ME, Lin A, Nowinski CJ, Cantu RC, McKee AC, Stern RA. Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging and Behavior. 2012;6:244–254. doi: 10.1007/s11682-012-9164-5. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah RC, Kelly JF, Fox JH, Cochran EJ, Arends D, Treinkman AD, Wilson RS. Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27:169–176. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurology. 2006;5:406–412. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Archives of Neurology. 2004;61:378–384. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Aggarwal NT, Arnold SE, Cochran EJ, Berry-Kravis E, Bienias JL. Apolipoprotein E epsilon4 allele, AD pathology, and the clinical expression of Alzheimer’s disease. Neurology. 2003;60:246–252. doi: 10.1212/01.wnl.0000042478.08543.f7. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- Bentler PM. Comparative fit indices in structural models. Psychological Bulletin. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA. Mild cognitive impairment: risk of Alzheimer disease and rate of cognitive decline. Neurology. 2006;67:441–445. doi: 10.1212/01.wnl.0000228244.10416.20. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. European Neurology. 1993;33:403–408. doi: 10.1159/000116984. [DOI] [PubMed] [Google Scholar]
- Browne M, Cudek R. Alternate ways of assessing model fit. In: Bollen K, Long J, editors. Testing structural equation models. Sage, Thousand Oaks; CA: 1993. pp. 136–162. [Google Scholar]
- Buchman AS, Boyle PA, Wilson RS, Beck TL, Kelly JF, Bennett DA. Apolipoprotein E e4 allele is associated with more rapid motor decline in older persons. Alzheimer’s Disease and Associated Disorders. 2009;23:63–69. doi: 10.1097/wad.0b013e31818877b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW. Neuropathology of non-Alzheimer degenerative disorders. International Journal of Clinical and Experimental Pathology. 2009;3:1–23. [PMC free article] [PubMed] [Google Scholar]
- Dowling NM, Tomaszewski Farias S, Reed BR, Sonnen JA, Strauss ME, Schneider JA, Bennett DA, Mungas D. Neuropathological associates of multiple cognitive functions in two community-based cohorts of older adults. Journal of the International Neuropsychological Society. 2011;17:602–614. doi: 10.1017/S1355617710001426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Isla T, Spires T, De Calignon A, Hyman BT. Neuropathology of Alzheimer’s disease. Handbook of Clinical Neurology. 2008;89:233–243. doi: 10.1016/S0072-9752(07)01222-5. [DOI] [PubMed] [Google Scholar]
- Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. [Review] Trends in Pharmacological Science. 1991;12(10):383–388. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. [Review] Science. 1992;256(5054):184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Honer WG, Barr AM, Sawada K, Thornton AE, Morris MC, Leurgans SE, Schneider JA, Bennett DA. Cognitive reserve, presynaptic proteins and dementia in the elderly. Translational Psychiatry. 2012;2:e114. doi: 10.1038/tp.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman BT. The neuropathological diagnosis of Alzheimer’s disease: clinical-pathological studies. Neurobiology of Aging. 1997;18:S27–32. doi: 10.1016/s0197-4580(97)00066-3. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurology. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA, Bancher C. Senile dementia with tangles (tangle predominant form of senile dementia) Brain Pathology. 1998;8:367–376. doi: 10.1111/j.1750-3639.1998.tb00160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein WL, Stine WB, Jr., Teplow DB. Small assemblies of unmodified amyloid beta-protein are the proximate neurotoxin in Alzheimer’s disease. Neurobiology of Aging. 2004;25:569–580. doi: 10.1016/j.neurobiolaging.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Longstreth WT, Jr., Sonnen JA, Koepsell TD, Kukull WA, Larson EB, Montine TJ. Associations between microinfarcts and other macroscopic vascular findings on neuropathologic examination in 2 databases. Alzheimer’s Disease and Associated Disorders. 2009;23:291–294. doi: 10.1097/WAD.0b013e318199fc7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Trojanowski JQ, Vinters HV, Hyman BT. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathologica. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray ME, DeJesus-Hernandez M, Rutherford NJ, Baker M, Duara R, Graff-Radford NR, Wszolek ZK, Ferman TJ, Josephs KA, Boylan KB, Rademakers R, Dickson DW. Clinical and neuropathologic heterogeneity of c9FTD/ALS associated with hexanucleotide repeat expansion in C9ORF72. Acta Neuropathol. 2011;122:673–690. doi: 10.1007/s00401-011-0907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. Seventh Edition Muthén & Muthén; Los Angeles, CA: 1998-2012. [Google Scholar]
- Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Santacruz K, Smith CD, Patel E, Markesbery WR. Brains with medial temporal lobe neurofibrillary tangles but no neuritic amyloid plaques are a diagnostic dilemma but may have pathogenetic aspects distinct from Alzheimer disease. Journal of Neuropathology and Experimental Neurology. 2009;68:774–784. doi: 10.1097/NEN.0b013e3181aacbe9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. Journal of Neuropathology and Experimental Neurology. 2009;68:1–14. doi: 10.1097/NEN.0b013e3181919a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Tran L, Lambert MP, Glabe CG, Klein WL, LaFerla FM. Temporal profile of amyloid-beta (Abeta) oligomerization in an in vivo model of Alzheimer disease. A link between Abeta and tau pathology. The Journal of Biological Chemistry. 2006;281:1599–1604. doi: 10.1074/jbc.M507892200. [DOI] [PubMed] [Google Scholar]
- Petrovitch H, Ross GW, Steinhorn SC, Abbott RD, Markesbery W, Davis D, Nelson J, Hardman J, Masaki K, Vogt MR, Launer L, White LR. AD lesions and infarcts in demented and non-demented Japanese-American men. Annals of Neurology. 2005;57:98–103. doi: 10.1002/ana.20318. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Annals of Neurology. 2009;66:200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Bienias JL, Wilson RS, Berry-Kravis E, Evans DA, Bennett DA. The apolipoprotein E epsilon4 allele increases the odds of chronic cerebral infarction [corrected] detected at autopsy in older persons. Stroke. 2005;36:954–959. doi: 10.1161/01.STR.0000160747.27470.2a. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. The molecular pathology of Alzheimer’s disease. [Review] Neuron. 1991;6(4):487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- Sonnen JA, Larson EB, Crane PK, Haneuse S, Li G, Schellenberg GD, Craft S, Leverenz JB, Montine TJ. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Annals of Neurology. 2007;62:406–413. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- Sonnen JA, Montine KS, Quinn JF, Kaye JA, Breitner JC, Montine TJ. Biomarkers for cognitive impairment and dementia in elderly people. Lancet Neurology. 2008;7:704–714. doi: 10.1016/S1474-4422(08)70162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DR, Griffin WS, Braak H. Parenchymal and vascular Abeta-deposition and its effects on the degeneration of neurons and cognition in Alzheimer’s disease. Journal of Cellular and Molecular Medicine. 2008;12:1848–1862. doi: 10.1111/j.1582-4934.2008.00411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troncoso JC, Zonderman AB, Resnick SM, Crain B, Pletnikova O, O’Brien RJ. Effect of infarcts on dementia in the Baltimore longitudinal study of aging. Annals of Neurology. 2008;64:168–176. doi: 10.1002/ana.21413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker LR, Lewis C. A reliability coefficient for maximum likelihood factor analysis. Psychometrika. 1973;38:1–10. [Google Scholar]
- Wilson R, Barnes L, Bennett D. Assessment of lifetime participation in cognitively stimulating activities. Journal of Clinical and Experimental Neuropsychology. 2003;25:634–642. doi: 10.1076/jcen.25.5.634.14572. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA. Individual differences in rates of change in cognitive abilities of older persons. Psychology and Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- Wilson RS, Mendes De Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, Bennett DA. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. Journal of American Medical Association. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Nag S, Boyle PA, Hizel LP, Yu L, Buchman AS, Schneider JA, Bennett DA. Neural reserve, neuronal density in the locus ceruleus, and cognitive decline. Neurology. 2013;80:1202–1208. doi: 10.1212/WNL.0b013e3182897103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama Y, Uryu K, Higuchi M, Longhi L, Hoover R, Fujimoto S, McIntosh T, Lee VM, Trojanowski JQ. Enhanced neurofibrillary tangle formation, cerebral atrophy, and cognitive deficits induced by repetitive mild brain injury in a transgenic tauopathy mouse model. Journal of Neurotrauma. 2005;22:1134–1141. doi: 10.1089/neu.2005.22.1134. [DOI] [PubMed] [Google Scholar]