Abstract
Cyclin-dependent kinases (CDKs) play a central role in governing eukaryotic cell division. It is becoming clear that the transcription cycle of RNA polymerase II (RNAP II) is also regulated by CDKs; in metazoans, the cell cycle and transcriptional CDK networks even share an upstream activating kinase, which is itself a CDK. From recent chemical-genetic analyses we know that CDKs and their substrates control events both early in transcription (the transition from initiation to elongation) and late (3′ end formation and transcription termination). Moreover, mutual dependence on CDK activity might couple the “beginning” and “end” of the cycle, to ensure the fidelity of mRNA maturation and the efficient recycling of RNAP II from sites of termination to the transcription start site (TSS). As is the case for CDKs involved in cell cycle regulation, different transcriptional CDKs act in defined sequence on multiple substrates. These phosphorylations are likely to influence gene expression by several mechanisms, including direct, allosteric effects on the transcription machinery, co-transcriptional recruitment of proteins needed for mRNA-capping, splicing and 3′ end maturation, dependent on multisite phosphorylation of the RNAP II C-terminal domain (CTD) and, perhaps, direct regulation of RNA-processing or histone-modifying machinery. Here we review these recent advances, and preview the emerging challenges for transcription-cycle research.
Keywords: RNA polymerase II, RNA-processing, TFIIH, chemical genetics, cyclin-dependent kinase, positive transcription elongation factor b, promoter-proximal pausing, termination, transcription
Introduction
Eukaryotic cell division depends on specific cyclin-dependent kinases (CDKs) becoming active at the right level, time (cell cycle stage) and place within the cell. Orderly progression through the G1, S, G2 and M phases is ensured by intrinsic properties of the different CDKs and their substrates, which help determine the temporal order of target protein phosphorylation.1 The transcription cycle of RNA polymerase II (RNAP II) is likewise responsive to CDKs that act in defined sequence to regulate the stages of initiation, elongation and termination, but the precise roles of individual CDKs, and the mechanisms that order their functions, are still emerging.2 Two pivotal moments in the transcription cycle occur near the “beginning,” when the commitment to transcript elongation is made, and at the “end,” when a complex series of events leads to correct RNA 3′ end formation, transcription termination and efficient recycling of RNAP II to the transcription start site (TSS).3 Both transitions correlate with peaks of RNAP II cross-linking to chromatin,4,5 suggesting that each represents a kinetic barrier that must be overcome for RNAP II to progress to the next “phase” of the transcription cycle, and a potential “checkpoint” at which regulatory signals are integrated and gene expression decisions made. Recent work from our lab and others illuminates how CDKs—the class of enzymes responsible for so much of cell cycle coordination—orchestrate the initiation-elongation transition of RNAP II, and suggests how this transition might be coupled to the later steps of 3′ end maturation, termination and reinitiation.
In the Beginning: Promoter-Proximal Pausing and the Initiation-Elongation Transition
Shortly after initiating transcription, metazoan RNAP II frequently pauses in the promoter-proximal region; this is thought to act as a checkpoint to recruit other factors needed in elongation and RNA-processing, thereby coupling the two processes. Pausing depends on the DRB sensitivity-inducing factor (DSIF), a conserved heterodimer of Spt4 and Spt5 that binds to RNAP II. In order to enforce stable pausing of RNAP II in metazoans, DSIF recruits a negative elongation factor (NELF) that is absent in yeast. Importantly, although DSIF and NELF were identified biochemically as factors that inhibit elongation in vitro,6 the effects of pausing on gene expression may be generally positive: depletion of NELF in vivo decreases transcription of many genes.7
Promoter-proximal pausing appears to be an important regulatory step for thousands of metazoan genes, of particular importance for those that need to be turned on rapidly to control stimulus responses, or in all-or-none fashion to ensure the commitment to a specific differentiation program in stem cells.8 Another function of pausing uncovered in Drosophila is to establish the basal activity of signal-response networks.9 In mouse embryonic stem (ES) cells, promoter-proximal pausing occurs generally at both active and inactive genes.10 The notion of pausing as an obligate regulatory step, at which the transcription complex integrates signals telling it to elongate or not, also fits with the discovery that RNAP II can initiate transcription bidirectionally at many loci;5,11 regulated pause release might afford a way to favor sense over antisense transcription at these promoters.
The mechanism underlying RNAP II pausing is an interaction between ancient, conserved factors: the polymerase itself and the elongation factor DSIF, which has orthologs in both archaea (Spt4/5) and eubacteria (NusG). Recent work brought a third conserved player into the picture: the initiation factor TFIIE, which stimulates initiation by enhancing DNA melting and recruiting the initiation factor TFIIH.12 In archaea, the TFIIE ortholog TFE competes with Spt4/5 for mutually exclusive binding to the polymerase clamp region.13,14 RNA polymerase-binding affinities of Spt4/5 and TFE differ at different points of the transcription cycle: TFE binds to the initiating polymerase, preventing the binding of Spt4/5 that would inhibit initiation. Subsequent conformational changes of the transcription complex allow Spt4/5 to bind to the polymerase in the same region from which TFE was released, and promote efficient elongation.13 Similarly, in bacteria, the Spt5 homolog NusG competes with the transcription initiation factor sigma—which is structurally unrelated to TFE, but likewise able to promote pausing15—for binding to RNA polymerase.16 In both eubacteria and archaea, where there is no nuclear envelope physically separating transcription and translation, the exchange between initiation and elongation factors is likely to ensure the coupling of RNA and protein synthesis, rates of which are correlated over a broad range of growth conditions.17 NusG physically interacts with ribosomal protein S10,18 and so might help “feed” mRNA directly to the translation machinery. Furthermore, the inhibition of translation by antibiotics diminishes the efficiency of transcript elongation, which implies that binding of RNA polymerase to ribosomes can enhance transcription.17
In eukaryotes, the initiation-elongation switch also appears to be a coupling device, but between transcription and RNA processing. The mRNA maturation events of 5′ end capping, splicing and 3′ end polyadenylation are interconnected, and regulated by a CDK network that includes the essential family members, Cdk7 and Cdk9. Cdk7, together with its partners cyclin H and Mat1, forms a subcomplex within TFIIH; whereas Cdk9, with cyclin T1 or T2, constitutes positive transcription elongation factor b (P-TEFb). Several proteins thought to participate in pause establishment and release are substrates of both CDKs, suggesting a possible way to regulate these steps. Cdk7 phosphorylates the C-terminal domain (CTD) of Rpb1, the largest subunit of RNAP II, and other components of the preinitiation complex (PIC). The CTD consists of multiple heptad repeats that can be phosphorylated at sites within the consensus sequence 1-YSPTSPS-7, including the serine residues at positions 2, 5 and 7 (Ser2, Ser5 and Ser7). Phosphorylation of Ser5 and Ser7 predominates early in transcription, whereas Ser2 phosphorylation peaks toward the 3′ ends of genes.19,20 In accordance with this sequence, TFIIH-associated Cdk7 and its orthologs (Kin28 in budding yeast and Mcs6 in fission yeast) prefer to phosphorylate Ser5 and Ser7 in vitro.19,21-23 Moreover, an activity associated with human TFIIH phosphorylates the α subunit of TFIIE in vitro,12,24 and purified recombinant Cdk7 phosphorylates both TFIIEα and, with greater efficiency, TFIIEβ.25
Sequential Functions of Transcriptional CDKs Revealed by Chemical Genetics
Insights into the control of gene expression by CDKs have recently emerged from a chemical genetic approach: introduction into cells of an analog-sensitive (AS) kinase mutant with an expanded ATP-binding pocket, which allows specific inhibition by cell-permeable, bulky adenine analogs that do not affect wild type kinases.26 To uncover the roles of Cdk7 in vivo, we replaced both copies of the Cdk7 gene with Cdk7as in human colon carcinoma-derived HCT116 cells.27 Inhibition of Cdk7as with allele-specific small molecules did not compromise TFIIH integrity or chromatin recruitment, but decreased NELF recruitment at the 5′ end and attenuated promoter-proximal pausing by RNAP II.21 Furthermore, inhibition of Cdk7as caused reciprocal changes in occupancy by TFIIE and DSIF: crosslinking of TFIIE near the TSS was increased, whereas recruitment of Spt5 was decreased, suggesting that Cdk7 activity is required to promote exchange of the two factors and establish the paused state of RNAP II.25 A possible consequence of this requirement is the obligatory coupling of elongation to capping of the nascent pre-mRNA. RNAP II CTD phosphorylation by the TFIIH-associated kinase has been implicated in 5′ end capping in vivo and in vitro,28-30 and Ser5 phosphorylation specifically stimulates CTD-binding and activity of a capping enzyme.31 In budding yeast, allele-specific inhibition of the Cdk7 ortholog Kin28 by the AS kinase strategy caused a global reduction in 5′ end capping.32 Ser5-P also promotes recruitment of the nuclear cap-binding complex (CBC).33 Finally, NELF physically associates with the CBC,34 providing further evidence of a pausing-capping connection.
Cdk9 acts downstream of Cdk7 to promote RNAP II pause release and the switch to processive elongation. Phosphorylations catalyzed by P-TEFb—on the Rpb1 CTD, the C-terminal repeat (CTR) region of Spt5, and NELF—trigger release of NELF and conversion of DSIF into a transcription processivity factor.10,35 Like most CDKs, Cdk9 must be phosphorylated on its activation loop (T loop) to attain full activity. We recently identified Cdk7 as a Cdk9-activating kinase in human cells (Fig. 1).25 Therefore, the same kinase that ensures pausing also guarantees its transience, unless regulatory factors intervene to prevent the recruitment of Cdk9 or its activation. The latter mode of regulation might account for scenarios in which Cdk9 is recruited normally to chromatin but its activity—typically measured by quantifying Ser2 phosphorylation—is diminished.36,37 Direct interference with Cdk7 function is one obvious case in which Cdk9 recruitment is normal but its activation is blocked, and we indeed observed decreased Ser2-P:total Rpb1 crosslinking ratios upon selective inhibition of Cdk7as in human cells.25 The dependence of Cdk9 on Cdk7 for full activity also implies that a subset of the effects of Cdk7 inactivation might be due to impaired Cdk9 function, as discussed below. Finally, Cdk7 is also a CDK-activating kinase (CAK) for CDKs that drive cell cycle progression;27,38,39 the demonstration that it plays a similar role in the transcription cycle supports a unified model of the metazoan CDK network, and suggests potential ways to coordinate gene expression and cell division.
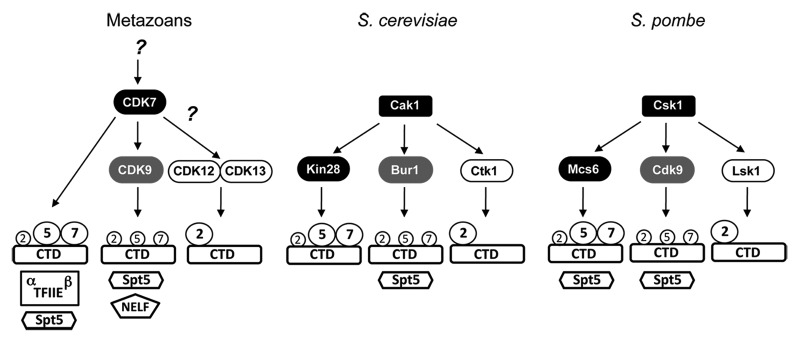
Figure 1. Transcriptional CDK networks of metazoans and yeast. In metazoans, the TFIIH-associated kinase Cdk7 directly activates Cdk9, catalytic subunit of P-TEFb. Still to be determined: the identity of a Cdk7-activating kinase active during transcription, and whether Cdk7 also activates Cdk12 and/or Cdk13. In both budding and fission yeast, the job of activating all transcriptional CDKs falls to a single-subunit CAK (Cak1 or Csk1, respectively). Substrate phosphorylation by transcriptional CDKs is conserved among eukaryotes; indicated preferences for different positions within the CTD repeats are based on an amalgamation of biochemical and genetic data, but in most cases have not been rigorously determined. The larger number of substrates shown for metazoan CDKs reflects either lack of conservation of individual substrates in yeast (e.g., NELF) or simply the fact that specific CDK-substrate relationships have not been tested (e.g., TFIIE with any CDK, or Spt5 with Kin28).
In contrast to the situation in metazoans, TFIIH-associated kinases do not directly activate P-TEFb orthologs in yeast (Fig. 1). In both budding and fission yeast, Cdk7 and Cdk9 orthologs are activated by a single-subunit CAK: Cak1 in S. cerevisiae40,41 and Csk1 in S. pombe.42,43 Perhaps due to the divergent organization of the CDK networks, monoubiquitylation of H2B (H2Bub1), a conserved histone modification that depends on Cdk9 in both metazoans and yeast, is also dependent on Cdk7 in human cells25 but independent of Cdk7/Mcs6 in S. pombe.44 Despite the absence of a direct, activator-effector relationship between Cdk7 and Cdk9 orthologs in yeast, recent work has uncovered two alternative mechanisms by which TFIIH and P-TEFb functions are ordered during the transcription cycle: 1) CTD phosphorylation by human Cdk7 or S. pombe Mcs6, probably on Ser7, “primes” a CTD substrate for subsequent phosphorylation by Cdk9;23,45,46 and 2) recruitment of P-TEFb to transcribed genes depends on TFIIH activity in both budding and fission yeast,45,47 but apparently not in human cells.25
The Initiation-Elongation Transition in Yeast: A Virtual Pause?
Discrete, promoter-proximal pausing by RNAP II has not been detected in yeast, possibly due to the lack of NELF. The divergent CDK network organization might be another contributing factor; in metazoans, pausing is a predicted output of the regulatory circuit linking Cdk7, Cdk9 and DSIF. Nevertheless, there is evidence for an analogous transition in which RNAP II is handed from initiation to elongation factors, while a scaffold complex consisting of initiation factors disengaged from RNAP II remains at the promoter.48 This transition is almost certain to be regulated by many of the same conserved factors described above. In budding yeast, a regulatory role in switching between initiation and elongation has been suggested for Ctk1, a non-essential CDK related most closely to metazoan Cdk12 and Cdk13:49 in cells lacking Ctk1, PIC formation is normal but several transcription initiation factors remain bound to RNAP II until termination. This seems to reflect a structural, i.e., non-catalytic, function of Ctk1: a kinase-dead mutant was not defective for dissociation of initiation factors from RNAP II.50 The functions of TFIIH and P-TEFb at the initiation-elongation transition have yet to be elucidated in yeast, but are more likely to be catalytic. Importantly, the “receiver” in the promoter-proximal RNAP II handoff—the Spt4/Spt5 heterodimer—is conserved in all eukaryotes. Although recruitment of Spt5 to several genes was unaffected by inhibition of Mcs6 (Cdk7) or Cdk9,45 Spt5 phosphorylation at a conserved position within its C-terminal repeats was abolished when Cdk9as was inactivated by allele-specific small molecules.44 Moreover, genetic evidence indicates that S. pombe Cdk9 promotes transcript elongation in large part through phosphorylation of Spt5.44,45
With or without a discrete pause, the coordinating or integrating functions of a regulated initiation-elongation transition appear to be conserved between metazoans and yeast. As noted above, chemical inhibition of Kin28 reduces mRNA-capping efficiency in budding yeast.51 In fission yeast, the last enzyme in the mRNA-capping pathway, the cap methyltransferase Pcm1, depends on a physical association with Cdk9 for its normal recruitment to chromatin, and normal Cdk9 recruitment in turn depends on Mcs6 activity.23,43,45 Finally, as described in the next section, CDK-dependent events that occur around the initiation-elongation transition influence later steps in mRNA maturation, mediated in part through conserved, covalent chromatin modifications, in both yeast and metazoan cells.
And in the End: CDKs Influence 3′ End Formation
Chromatin immunoprecipitation (ChIP) of RNAP II from mammalian cells typically reveals the highest accumulation around the TSS, and a smaller peak followed by a sharp drop-off at the polyadenylation site (Fig. 2).4,5 The peak and dropoff are likely to represent, respectively, a second “pause” of the transcription complex near the site of 3′-end cleavage and polyadenylation, and efficient termination by RNAP II in the immediate downstream region.50 Transcription termination is tightly regulated and coupled to cleavage and polyadenylation of the nascent transcript by a 3′ end processing machinery that recognizes specific sequence elements in the pre-mRNA.3 This coordination helps control gene expression and ensures insulation of neighboring genes.52 However, termination remains one of the least understood stages of the transcription cycle.
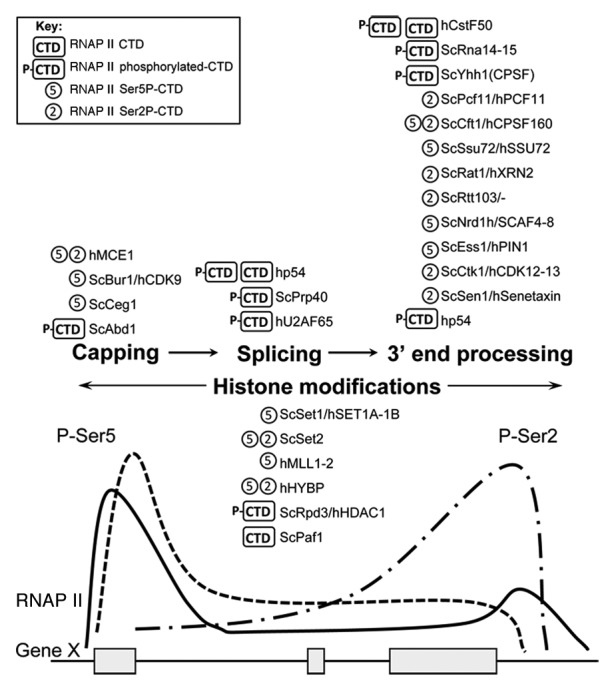
Figure 2. RNAP II CTD-binding preference of factors implicated in co-transcriptional events. The phosphorylation state of the RNAP II CTD changes during the transcription cycle, to promote recruitment of proteins responsible for histone modification and maturation of the nascent RNA transcript.52,67 Different factors can bind the CTD in 1) either unphosphorylated or phosphorylated form, 2) with a preference for the modified CTD but without discrimination among the phosphorylated positions, or 3) with phosphosite-specificity, as indicated in key at top left. Schematic at bottom shows distribution of total RNAP II (solid line) and RNAP II phosphorylated at Ser5 (P-Ser5) or Ser2 (P-Ser2) (dashed lines), along a typical metazoan gene.
In mammalian or yeast cells expressing RNAP II lacking the CTD, 3′ end processing is impaired.53,54 Several cleavage and polyadenylation factors are recruited dependent on Ser2 phosphorylation of elongating RNAP II (Fig. 2), and loss of the major Ser2 kinase Ctk1 causes aberrant transcription termination at specific genes in yeast.55 In human cells, Cdk7 activity is required for normal 3′ end maturation of individual RNAP II transcripts belonging to multiple classes: polyadenylated mRNA (a transfected β-globin reporter), non-polyadenylated mRNA (endogenous histone genes) and small nuclear RNA (snRNA).21,25 In addition, cleavage stimulation factor (CstF) and cleavage polyadenylation specificity factor (CPSF) both localize at the TSS (and this early recruitment is enhanced by treatment with the CDK inhibitor DRB, which accentuates pausing), even though their maximal occupancy occurs ~1 kb downstream of poly(A) sites.4
Cdk9 activity, and H2Bub1, are also required for correct 3′ end processing of histone mRNA (mRNA), which occurs through a poly(A)-independent pathway restricted to the DNA synthesis (S) phase of the cell cycle.56 H2Bub1 occurs predominantly in transcribed regions44,57 and depends on Cdk9 activity and Spt5 phosphorylation but may occur independent of RNAP II Ser2 phosphorylation.44,58 A simple explanation for why H2Bub1 and mRNA 3′ end formation depend on Cdk7 in metazoans but not in yeast might be the fact that Cdk9 is activated by Cdk7 in the former but not the latter.25,43 There is, however, another plausible explanation: inhibition of Cdk7 in human cells prevents promoter-proximal recruitment of NELF,21 which has also been implicated in histone mRNA 3′ end maturation.34
Taken together, these results suggest an important role for CDKs and their substrates in both early and late steps of transcription. This is consistent with an emerging model of the transcription cycle, in which rates of initiation are affected by the efficiency of termination: there are physical interactions between 5′ and 3′ ends of genes, and this gene “looping” depends on activities of Kin28 and the CTD Ser5 phosphatase Ssu72 in yeast.59,60 Gene looping appears to enforce directionality of RNAP II in budding yeast; when the normal circular conformation is disrupted in a thermo-sensitive ssu72 mutant, divergent transcription increases.61 Finally, mutation of the poly(A) site of a human β−globin reporter or knockdown of the processing/ termination factor Pcf11 decreases binding of RNAP II, TFIID and TFIIB at the TSS, suggesting that efficient termination facilitates recycling of transcription factors to the promoter.62
A Glimpse of the Future: CDKs in Control (of Gene Expression)
Recent advances have validated the paradigm of a transcription “cycle” (which might even operate on a circularized template in vivo), and established important roles for CDKs in governing its progression. It is far from clear, however, how CDK-dependent phosphorylations direct the complex, gene-specific RNA maturation events that occur co-transcriptionally at most or all genes transcribed by RNAP II. Much attention has focused, naturally, on the most obvious read-out of transcriptional CDK activity: the phosphorylation state of the RNAP II CTD.2 Although different constellations of phosphorylations at Ser2, Ser5 and Ser7 (and, perhaps, at Tyr1 and Thr4) undoubtedly play an important role in recruiting RNA-processing and chromatin-modifying machineries (Fig. 2),63 that is certainly not the end of the story. Results in both human and yeast systems have identified key steps in transcription that depend on the activities of specific CDKs, but may be independent of RNAP II phosphorylation.25,44 During cell division, dedicated CDKs phosphorylate scores, if not hundreds, of substrates64-66 to regulate cell cycle events by multiple modes. It is likely that the CDKs involved in transcription (Cdk7, Cdk8, Cdk9, Cdk12, Cdk13 and, possibly others) will also have many bona fide targets, and use an assortment of mechanisms to ensure proper coordination of RNA synthesis and maturation—including, but not limited to, regulated recruitment. The challenge for the next phase of transcription-cycle research will be to identify those targets and elucidate those mechanisms, which are likely to influence gene expression in both health and disease.
Acknowledgments
We thank Stéphane Larochelle, Ramon Amat, and other past and present members of the Fisher lab for helpful discussions. Work reviewed here was supported in part by grants GM056985 and GM076021 from the National Institutes of Health.
Glossary
Abbreviations:
- cyclin-dependent kinase
CDK
- positive transcription elongation factor b
P-TEFb
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/transcription/article/25146
References
- 1.Morgan D. The Cell Cycle: Principles of Control. Oxford University Press, 2006. [Google Scholar]
- 2.Buratowski S. Progression through the RNA polymerase II CTD cycle. Mol Cell. 2009;36:541–6. doi: 10.1016/j.molcel.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Proudfoot NJ. Ending the message: poly(A) signals then and now. Genes Dev. 2011;25:1770–82. doi: 10.1101/gad.17268411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glover-Cutter K, Kim S, Espinosa J, Bentley DL. RNA polymerase II pauses and associates with pre-mRNA processing factors at both ends of genes. Nat Struct Mol Biol. 2008;15:71–8. doi: 10.1038/nsmb1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Core LJ, Waterfall JJ, Lis JT. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science. 2008;322:1845–8. doi: 10.1126/science.1162228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Renner DB, Yamaguchi Y, Wada T, Handa H, Price DH. A highly purified RNA polymerase II elongation control system. J Biol Chem. 2001;276:42601–9. doi: 10.1074/jbc.M104967200. [DOI] [PubMed] [Google Scholar]
- 7.Gilchrist DA, Nechaev S, Lee C, Ghosh SKB, Collins JB, Li L, et al. NELF-mediated stalling of Pol II can enhance gene expression by blocking promoter-proximal nucleosome assembly. Genes Dev. 2008;22:1921–33. doi: 10.1101/gad.1643208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA. A chromatin landmark and transcription initiation at most promoters in human cells. Cell. 2007;130:77–88. doi: 10.1016/j.cell.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilchrist DA, Fromm G, dos Santos G, Pham LN, McDaniel IE, Burkholder A, et al. Regulating the regulators: the pervasive effects of Pol II pausing on stimulus-responsive gene networks. Genes Dev. 2012;26:933–44. doi: 10.1101/gad.187781.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahl PB, Lin CY, Seila AC, Flynn RA, McCuine S, Burge CB, et al. c-Myc regulates transcriptional pause release. Cell. 2010;141:432–45. doi: 10.1016/j.cell.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seila AC, Calabrese JM, Levine SS, Yeo GW, Rahl PB, Flynn RA, et al. Divergent transcription from active promoters. Science. 2008;322:1849–51. doi: 10.1126/science.1162253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohkuma Y. Multiple functions of general transcription factors TFIIE and TFIIH in transcription: possible points of regulation by trans-acting factors. J Biochem. 1997;122:481–9. doi: 10.1093/oxfordjournals.jbchem.a021777. [DOI] [PubMed] [Google Scholar]
- 13.Grohmann D, Nagy J, Chakraborty A, Klose D, Fielden D, Ebright RH, et al. The initiation factor TFE and the elongation factor Spt4/5 compete for the RNAP clamp during transcription initiation and elongation. Mol Cell. 2011;43:263–74. doi: 10.1016/j.molcel.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein BJ, Bose D, Baker KJ, Yusoff ZM, Zhang X, Murakami KS. RNA polymerase and transcription elongation factor Spt4/5 complex structure. Proc Natl Acad Sci U S A. 2011;108:546–50. doi: 10.1073/pnas.1013828108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zenkin N, Kulbachinskiy A, Yuzenkova Y, Mustaev A, Bass I, Severinov K, et al. Region 1.2 of the RNA polymerase sigma subunit controls recognition of the -10 promoter element. EMBO J. 2007;26:955–64. doi: 10.1038/sj.emboj.7601555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sevostyanova A, Svetlov V, Vassylyev DG, Artsimovitch I. The elongation factor RfaH and the initiation factor sigma bind to the same site on the transcription elongation complex. Proc Natl Acad Sci U S A. 2008;105:865–70. doi: 10.1073/pnas.0708432105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Proshkin S, Rahmouni AR, Mironov A, Nudler E. Cooperation between translating ribosomes and RNA polymerase in transcription elongation. Science. 2010;328:504–8. doi: 10.1126/science.1184939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burmann BM, Schweimer K, Luo X, Wahl MC, Stitt BL, Gottesman ME, et al. A NusE:NusG complex links transcription and translation. Science. 2010;328:501–4. doi: 10.1126/science.1184953. [DOI] [PubMed] [Google Scholar]
- 19.Akhtar MS, Heidemann M, Tietjen JR, Zhang DW, Chapman RD, Eick D, et al. TFIIH kinase places bivalent marks on the carboxy-terminal domain of RNA polymerase II. Mol Cell. 2009;34:387–93. doi: 10.1016/j.molcel.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komarnitsky P, Cho EJ, Buratowski S. Different phosphorylated forms of RNA polymerase II and associated mRNA processing factors during transcription. Genes Dev. 2000;14:2452–60. doi: 10.1101/gad.824700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glover-Cutter K, Larochelle S, Erickson B, Zhang C, Shokat K, Fisher RP, et al. TFIIH-associated Cdk7 kinase functions in phosphorylation of C-terminal domain Ser7 residues, promoter-proximal pausing, and termination by RNA polymerase II. Mol Cell Biol. 2009;29:5455–64. doi: 10.1128/MCB.00637-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hengartner CJ, Myer VE, Liao SM, Wilson CJ, Koh SS, Young RA. Temporal regulation of RNA polymerase II by Srb10 and Kin28 cyclin-dependent kinases. Mol Cell. 1998;2:43–53. doi: 10.1016/S1097-2765(00)80112-4. [DOI] [PubMed] [Google Scholar]
- 23.St Amour CV, Sansó M, Bösken CA, Lee KM, Larochelle S, Zhang C, et al. Separate domains of fission yeast Cdk9 (P-TEFb) are required for capping enzyme recruitment and primed (Ser7-phosphorylated) Rpb1 carboxyl-terminal domain substrate recognition. Mol Cell Biol. 2012;32:2372–83. doi: 10.1128/MCB.06657-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yankulov KY, Bentley DL. Regulation of CDK7 substrate specificity by MAT1 and TFIIH. EMBO J. 1997;16:1638–46. doi: 10.1093/emboj/16.7.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larochelle S, Amat R, Glover-Cutter K, Sansó M, Zhang C, Allen JJ, et al. Cyclin-dependent kinase control of the initiation-to-elongation switch of RNA polymerase II. Nat Struct Mol Biol. 2012;19:1108–15. doi: 10.1038/nsmb.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knight ZA, Shokat KM. Features of selective kinase inhibitors. Chem Biol. 2005;12:621–37. doi: 10.1016/j.chembiol.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Larochelle S, Merrick KA, Terret M-E, Wohlbold L, Barboza NM, Zhang C, et al. Requirements for Cdk7 in the assembly of Cdk1/cyclin B and activation of Cdk2 revealed by chemical genetics in human cells. Mol Cell. 2007;25:839–50. doi: 10.1016/j.molcel.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho EJ, Takagi T, Moore CR, Buratowski S. mRNA capping enzyme is recruited to the transcription complex by phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev. 1997;11:3319–26. doi: 10.1101/gad.11.24.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schroeder SC, Schwer B, Shuman S, Bentley D. Dynamic association of capping enzymes with transcribing RNA polymerase II. Genes Dev. 2000;14:2435–40. doi: 10.1101/gad.836300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moteki S, Price D. Functional coupling of capping and transcription of mRNA. Mol Cell. 2002;10:599–609. doi: 10.1016/S1097-2765(02)00660-3. [DOI] [PubMed] [Google Scholar]
- 31.Ho CK, Shuman S. Distinct roles for CTD Ser-2 and Ser-5 phosphorylation in the recruitment and allosteric activation of mammalian mRNA capping enzyme. Mol Cell. 1999;3:405–11. doi: 10.1016/S1097-2765(00)80468-2. [DOI] [PubMed] [Google Scholar]
- 32.Kanin EI, Kipp RT, Kung C, Slattery M, Viale A, Hahn S, et al. Chemical inhibition of the TFIIH-associated kinase Cdk7/Kin28 does not impair global mRNA synthesis. Proc Natl Acad Sci U S A. 2007;104:5812–7. doi: 10.1073/pnas.0611505104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong C-M, Qiu H, Hu C, Dong J, Hinnebusch AG. Yeast cap binding complex impedes recruitment of cleavage factor IA to weak termination sites. Mol Cell Biol. 2007;27:6520–31. doi: 10.1128/MCB.00733-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narita T, Yung TMC, Yamamoto J, Tsuboi Y, Tanabe H, Tanaka K, et al. NELF interacts with CBC and participates in 3′ end processing of replication-dependent histone mRNAs. Mol Cell. 2007;26:349–65. doi: 10.1016/j.molcel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Yamada T, Yamaguchi Y, Inukai N, Okamoto S, Mura T, Handa H. P-TEFb-mediated phosphorylation of hSpt5 C-terminal repeats is critical for processive transcription elongation. Mol Cell. 2006;21:227–37. doi: 10.1016/j.molcel.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 36.Meyer KD, Lin SC, Bernecky C, Gao Y, Taatjes DJ. p53 activates transcription by directing structural shifts in Mediator. Nat Struct Mol Biol. 2010;17:753–60. doi: 10.1038/nsmb.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaaf CA, Kwak H, Koenig A, Misulovin Z, Gohara DW, Watson A, et al. Genome-wide control of RNA polymerase II activity by cohesin. PLoS Genet. 2013;9:e1003382. doi: 10.1371/journal.pgen.1003382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merrick KA, Larochelle S, Zhang C, Allen JJ, Shokat KM, Fisher RP. Distinct activation pathways confer cyclin-binding specificity on Cdk1 and Cdk2 in human cells. Mol Cell. 2008;32:662–72. doi: 10.1016/j.molcel.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schachter MM, Merrick KA, Larochelle S, Hirschi A, Zhang C, Shokat KM, et al. A cdk7-cdk4 T-loop phosphorylation cascade promotes g1 progression. Mol Cell. 2013;50:250–60. doi: 10.1016/j.molcel.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Espinoza FH, Farrell A, Nourse JL, Chamberlin HM, Gileadi O, Morgan DO. Cak1 is required for Kin28 phosphorylation and activation in vivo. Mol Cell Biol. 1998;18:6365–73. doi: 10.1128/mcb.18.11.6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao S, Prelich G. Activation of the Bur1-Bur2 cyclin-dependent kinase complex by Cak1. Mol Cell Biol. 2002;22:6750–8. doi: 10.1128/MCB.22.19.6750-6758.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee KM, Saiz JE, Barton WA, Fisher RP. Cdc2 activation in fission yeast depends on Mcs6 and Csk1, two partially redundant Cdk-activating kinases (CAKs) Curr Biol. 1999;9:441–4. doi: 10.1016/S0960-9822(99)80194-8. [DOI] [PubMed] [Google Scholar]
- 43.Pei Y, Du H, Singer J, Stamour C, Granitto S, Shuman S, et al. Cyclin-dependent kinase 9 (Cdk9) of fission yeast is activated by the CDK-activating kinase Csk1, overlaps functionally with the TFIIH-associated kinase Mcs6, and associates with the mRNA cap methyltransferase Pcm1 in vivo. Mol Cell Biol. 2006;26:777–88. doi: 10.1128/MCB.26.3.777-788.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sansó M, Lee KM, Viladevall L, Jacques P-É, Pagé V, Nagy S, et al. A positive feedback loop links opposing functions of P-TEFb/Cdk9 and histone H2B ubiquitylation to regulate transcript elongation in fission yeast. PLoS Genet. 2012;8:e1002822. doi: 10.1371/journal.pgen.1002822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Viladevall L, St Amour CV, Rosebrock A, Schneider S, Zhang C, Allen JJ, et al. TFIIH and P-TEFb coordinate transcription with capping enzyme recruitment at specific genes in fission yeast. Mol Cell. 2009;33:738–51. doi: 10.1016/j.molcel.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Czudnochowski N, Bösken CA, Geyer M. Serine-7 but not serine-5 phosphorylation primes RNA polymerase II CTD for P-TEFb recognition. Nat Commun. 2012;3:842. doi: 10.1038/ncomms1846. [DOI] [PubMed] [Google Scholar]
- 47.Qiu H, Hu C, Hinnebusch AG. Phosphorylation of the Pol II CTD by KIN28 enhances BUR1/BUR2 recruitment and Ser2 CTD phosphorylation near promoters. Mol Cell. 2009;33:752–62. doi: 10.1016/j.molcel.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y, Kung C, Fishburn J, Ansari AZ, Shokat KM, Hahn S. Two cyclin-dependent kinases promote RNA polymerase II transcription and formation of the scaffold complex. Mol Cell Biol. 2004;24:1721–35. doi: 10.1128/MCB.24.4.1721-1735.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bartkowiak B, Liu P, Phatnani HP, Fuda NJ, Cooper JJ, Price DH, et al. CDK12 is a transcription elongation-associated CTD kinase, the metazoan ortholog of yeast Ctk1. Genes Dev. 2010;24:2303–16. doi: 10.1101/gad.1968210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahn SH, Keogh M-C, Buratowski S. Ctk1 promotes dissociation of basal transcription factors from elongating RNA polymerase II. EMBO J. 2009;28:205–12. doi: 10.1038/emboj.2008.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanin EI, Kipp RT, Kung C, Slattery M, Viale A, Hahn S, et al. Chemical inhibition of the TFIIH-associated kinase Cdk7/Kin28 does not impair global mRNA synthesis. Proc Natl Acad Sci U S A. 2007;104:5812–7. doi: 10.1073/pnas.0611505104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuehner JN, Pearson EL, Moore C. Unravelling the means to an end: RNA polymerase II transcription termination. Nat Rev Mol Cell Biol. 2011;12:283–94. doi: 10.1038/nrm3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCracken S, Fong N, Yankulov K, Ballantyne S, Pan G, Greenblatt J, et al. The C-terminal domain of RNA polymerase II couples mRNA processing to transcription. Nature. 1997;385:357–61. doi: 10.1038/385357a0. [DOI] [PubMed] [Google Scholar]
- 54.Licatalosi DD, Geiger G, Minet M, Schroeder S, Cilli K, McNeil JB, et al. Functional interaction of yeast pre-mRNA 3′ end processing factors with RNA polymerase II. Mol Cell. 2002;9:1101–11. doi: 10.1016/S1097-2765(02)00518-X. [DOI] [PubMed] [Google Scholar]
- 55.Ahn SH, Kim M, Buratowski S. Phosphorylation of serine 2 within the RNA polymerase II C-terminal domain couples transcription and 3′ end processing. Mol Cell. 2004;13:67–76. doi: 10.1016/S1097-2765(03)00492-1. [DOI] [PubMed] [Google Scholar]
- 56.Pirngruber J, Shchebet A, Schreiber L, Shema E, Minsky N, Chapman RD, et al. CDK9 directs H2B monoubiquitination and controls replication-dependent histone mRNA 3′-end processing. EMBO Rep. 2009;10:894–900. doi: 10.1038/embor.2009.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Minsky N, Shema E, Field Y, Schuster M, Segal E, Oren M. Monoubiquitinated H2B is associated with the transcribed region of highly expressed genes in human cells. Nat Cell Biol. 2008;10:483–8. doi: 10.1038/ncb1712. [DOI] [PubMed] [Google Scholar]
- 58.Xiao Y, Yang YH, Burckin TA, Shiue L, Hartzog GA, Segal MR. Analysis of a splice array experiment elucidates roles of chromatin elongation factor Spt4-5 in splicing. PLoS Comput Biol. 2005;1:e39. doi: 10.1371/journal.pcbi.0010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ansari A, Hampsey M. A role for the CPF 3′-end processing machinery in RNAP II-dependent gene looping. Genes Dev. 2005;19:2969–78. doi: 10.1101/gad.1362305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Sullivan JM, Tan-Wong SM, Morillon A, Lee B, Coles J, Mellor J, et al. Gene loops juxtapose promoters and terminators in yeast. Nat Genet. 2004;36:1014–8. doi: 10.1038/ng1411. [DOI] [PubMed] [Google Scholar]
- 61.Tan-Wong SM, Wijayatilake HD, Proudfoot NJ. Gene loops function to maintain transcriptional memory through interaction with the nuclear pore complex. Genes Dev. 2009;23:2610–24. doi: 10.1101/gad.1823209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mapendano CK, Lykke-Andersen S, Kjems J, Bertrand E, Jensen TH. Crosstalk between mRNA 3′ end processing and transcription initiation. Mol Cell. 2010;40:410–22. doi: 10.1016/j.molcel.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 63.Egloff S, Dienstbier M, Murphy S. Updating the RNA polymerase CTD code: adding gene-specific layers. Trends Genet. 2012;28:333–41. doi: 10.1016/j.tig.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 64.Blethrow JD, Glavy JS, Morgan DO, Shokat KM. Covalent capture of kinase-specific phosphopeptides reveals Cdk1-cyclin B substrates. Proc Natl Acad Sci U S A. 2008;105:1442–7. doi: 10.1073/pnas.0708966105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chi Y, Welcker M, Hizli AA, Posakony JJ, Aebersold R, Clurman BE. Identification of CDK2 substrates in human cell lysates. Genome Biol. 2008;9:R149. doi: 10.1186/gb-2008-9-10-r149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ubersax JA, Woodbury EL, Quang PN, Paraz M, Blethrow JD, Shah K, et al. Targets of the cyclin-dependent kinase Cdk1. Nature. 2003;425:859–64. doi: 10.1038/nature02062. [DOI] [PubMed] [Google Scholar]
- 67.Hsin J-P, Manley JL. The RNA polymerase II CTD coordinates transcription and RNA processing. Genes Dev. 2012;26:2119–37. doi: 10.1101/gad.200303.112. [DOI] [PMC free article] [PubMed] [Google Scholar]


