Abstract
Objective
Circadian rhythms are intrinsic timekeeping mechanisms that allow for adaptation to cyclic environmental changes. Increasing evidence suggests that circadian rhythms may influence progression of a variety of diseases as well as effectiveness and toxicity of drugs commonly used in the intensive care unit. In this perspective, we provide a brief review of the molecular mechanisms of circadian rhythms and its relevance to critical care.
Data Sources, Study Selection, Data Extraction, and Data Synthesis
Articles related to circadian rhythms and organ systems in normal and disease conditions were searched through the PubMed library with the goal of providing a concise review.
Conclusions
Critically ill patients may be highly vulnerable to disruption of circadian rhythms as a result of the severity of their underlying diseases as well as the intensive care unit environment where noise and frequent therapeutic/diagnostic interventions take place. Further basic and clinical research addressing the importance of circadian rhythms in the context of critical care is warranted to develop a better understanding of the complex pathophysiology of critically ill patients as well as to identify novel therapeutic approaches for these patients.
Keywords: clock gene, diurnal variation, inflammation
During evolution, organisms have adapted to the external environment. A major universal external environmental factor is the cyclic changes in light and darkness occurring with a period of 24 hrs as a consequence of the earth’s rotation around the sun (1). To cope with these changes, plants and animals developed a universal intrinsic timekeeping system with a period close to 24 hrs, called circadian rhythms (from the Latin term circa, around, and diem, day). This system allows the organism to “anticipate” upcoming environmental changes and hence has inherent advantages. It is known that numerous vital physiological, biochemical, and behavioral processes, including body temperature, feeding behavior, hormone secretion, and glucose homeostasis, are influenced by circadian rhythms in humans.
The hypothalamus–pituitary–adrenal axis, primarily responsible for the regulation of stress responses, exhibits a diurnal variation in healthy subjects (2, 3), and impairment of this variation has biological implications (4–6). Animal studies have demonstrated that mice exposed to chronic jet lag have increased tumor progression (7). Animals with a point mutation in casein kinase-1ε, a gene involved in circadian rhythms, have a shortened lifespan and develop extensive fibrosis, impaired myocardial contractility, and severe renal dysfunction in association with massive cellular apoptosis (8). There are also implications in humans; for example, epidemiologic studies suggest that shift workers may have an increased risk of developing breast cancer (9).
In this critical care perspective, we provide a brief review of the fundamental mechanisms underlying circadian rhythms and highlight its relevance in critically ill patients.
The Circadian Rhythm Network
The circadian rhythms are part of a hierarchical network with a “master clock” located in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus, controlling the day/night rhythm of the organism’s physiological and behavioral functions. The central clock is synchronized with geophysical time mainly through photic cues perceived by the retina and transmitted by electrical signals to neurons in the SCN. The synchronization mechanism known as Zeitgeber (German for “time giver”) is defined as an environmental cue that entrains an organism’s internal timekeeping system. A fundamental Zeitgeber is light. There are also nonphotic regulators of circadian rhythms, including eating/drinking patterns, environmental temperature, pharmacologic manipulations, or social interactions (10, 11). Circadian physiology and behavior act through neuronal and humoral cues that entrain the central pacemaker to a 24-hr period and local oscillators in peripheral organs and tissues (10). As a result of altered light/dark cycles, eating and drinking patterns, social interactions, and pharmacologic treatments, the entrainment of circadian processes by different Zeitgebers may be hindered or absent in the intensive care unit (ICU) environment. The rhythmicity of the sleep–wake cycle and locomotor and adrenocortical activity are lost in animals with lesions in the SCN (12). The SCN integrates the light/dark cycles from the environment and sends both neuronal and humoral signals to other brain regions and the periphery (12). The SCN is therefore responsible for appropriate timing of various physiological functions. Although it may be the central hub for coordinating circadian rhythms, other centers located in peripheral organs such as the liver, spleen, lung, and heart act as “peripheral clocks” controlling further cyclic biologic functions. The relationships between central and peripheral clocks are not yet clearly understood (13–15). Using a luciferase period 2 (PER2) fusion protein as a real-time reporter of dynamic circadian rhythms in mice, it has been demonstrated that peripheral tissues express self-sustained circadian oscillations, suggesting the existence of organ-specific synchronizers of circadian rhythms at the cellular and tissue levels (16).
Molecular Basis of Circadian Rhythms
The period of the underlying molecular mechanisms generating circadian rhythms is approximately 24 hrs in the absence of synchronizing input; the network can adapt to a limited range of day lengths (17–19). There are a number of well-studied genes that appear to be important for initiating and sustaining circadian rhythms, including circadian locomotor output cycles kaput (CLOCK), a gene encoding proteins that affect the persistence and length of a circadian cycle (20); brain and muscle aryl hydrocarbon receptor nuclear translocator-like (BMAL1), a basic-helix–loop-helix transcription factor; period (PER1, PER2, PER3), a negative element in the circadian transcriptional loop by interacting with other circadian regulatory proteins and transporting them to the nucleus; cryptochrome (CRY1 and CRY2), a negative element inhibiting CLOCK-mediated transcription in maintaining period length and circadian rhythmicity; and orphan nuclear receptor (Rev-ErbA), an α-thyroid hormone receptor splice variant (21, 22) playing an important role in the regulation of CLOCK and BMAL1 gene expression (23, 24). Oscillation of expression of these genes (25) (Fig. 1) can result in circadian variations of organ functions (Table 1).
Figure 1.
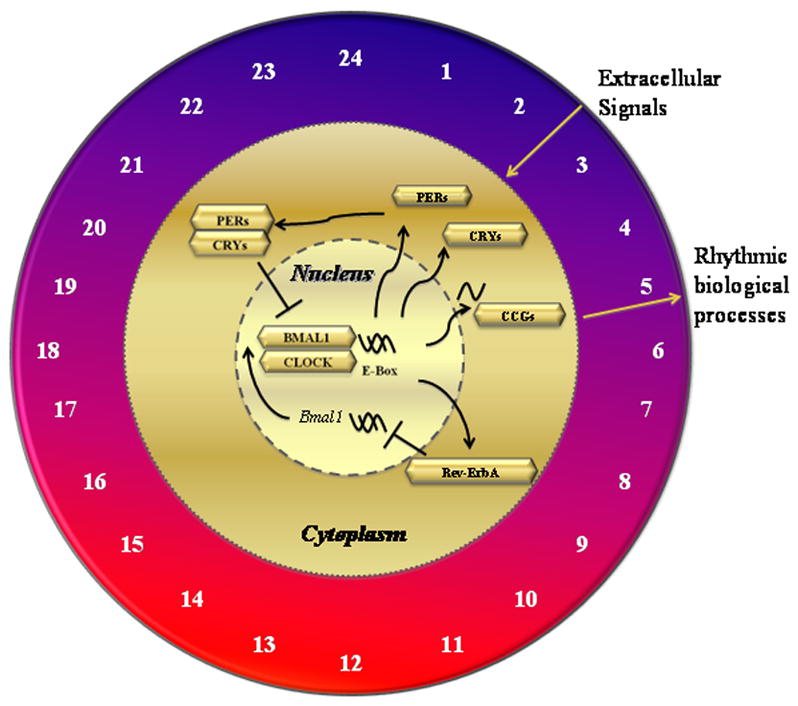
Molecular mechanisms of circadian rhythms in critical illness. A variety of biologic reactions are involved in the pathogenesis of critical illness, which are influenced by circadian rhythm clock of cells. Circadian locomotor output cycles kaput (CLOCK) dimerizes with brain and muscle aryl hydrocarbon receptor nuclear translocator-like (BMALI) in the nucleus and transactivates gene expression of orphan nuclear receptor (Rev-ErbA), period (PER), and cryptochrome (CRY), producing proteins that accomplish various circadian physiological actions. PER and CRY reside in the cytoplasm and form a protein complex that traffics back into the nucleus, downregulating CLOCK and BMALI expression, thus closing a negative feedback loop. Various external stimuli such as environmental disturbances and lipopolysaccharide challenge can alter circadian rhythms in critically ill patients, which may influence development and/or progression of various diseases. The number of timing on the clock face (ie., 6 hrs coincides with the onset of the light period) play a significant role in regulating the human cycle of the clock genes.
Table 1.
Selected physiological and pathological aspects influenced by circadian rhythm.
| System | Aspect | Circadian Rhythm-Associated Physiological Variations | Clinical Implications | References |
|---|---|---|---|---|
|
| ||||
| Central nervous system | Sleep | Sleep/wake cycles | Sleep pattern is altered in patients admitted to the intensive care unit, which is related to environmental factors as well as underlying diseases | 83–86 |
| Mortality rate increased in septic animals with altered circadian light/dark cycle | 96 | |||
| Melatonin | Secretion patterns | Circadian profiles of melatonin in circulation are altered in critically ill patients | 26,19,91–95,97 | |
|
| ||||
| Immune | Immune cells | Circulating monocytes, natural killer cells and lymphocytes counts | Circadian rhythm of circulating leukocytes can be altered by lipopolysaccharide in human volunteers | 82 |
| Immune cell function (production of cytolitic factors and cytokines) | 31,33 | |||
| Gene expression of pattern recognition molecules | 43 | |||
|
| ||||
| Coagulation | Platelets | Number of circulating platelets Platelet aggregration Number of circulating platelets | 46–48 | |
| Coagulation factors | Plasma levels of VIIa, fibrinogen, antithrombin III, protein C, tissue factor inhibitors, plasminogen activator inhibitor type 1 | 50 | ||
|
| ||||
| Cardiovascular | Cardiovascular function, cardiac rhythm | Blood pressure, heart rate, regulation on atrial fibrillation, ventricular tachycardia/fibrillation | Profiles of blood pressure and heart rate are altered by disturbed circadian rhythm in intensive care unit patients | 97 |
| Decreased cardiac compensation by altered circadian rhythm in murine model of cardiac hypertrophy | 53 | |||
|
| ||||
| Respiratory | Lung | Lung mechanics and gas exchange | 56–58,37 | |
| Surfactant protein D alveolar levels | 60 | |||
|
| ||||
| Metabolic | Kidney | Renal blood flow, glomerular filtration rate, tubular resorption and secretion, erythropoietin secretion | Disturbance of renal circadian rhythms is a risk factor for hypertension, polyuria, contributing to renal fibrosis in humans and animals | 65–67 |
| Liver | Hepatic gluconeogenesis and glucose metabolism | 63,64 | ||
| Metabolism of drugs such as corticosteroids, antibiotics, chemotherapeutics | 75–81 | |||
Melatonin, secreted by the pineal gland in the brain, is another important element that helps to regulate other hormones and maintains the body’s circadian rhythms. Melatonin has both hypnotic and sleep/wake control properties (26). It is secreted into the systemic circulation in response to signals from the SCN and provides a feedback mechanism to the central clock that regulates circadian rhythms (27). Melatonin is part of a complex multilayered system of interactions between central and peripheral clocks and is one of the best known examples of how the SCN acts on peripheral clocks to regulate the circadian rhythms (19).
Circadian Rhythms and the Immune System
The number of circulating immune cells such as monocytes, natural killer cells, and lymphocytes are different during the day than at night. Nighttime sleep reduces circulating monocytes, natural killer cells, and all phenotypes of lymphocytes compared with nights when sleep is disturbed. However, counts of natural killer cells and lymphocytes are significantly higher in the afternoon and evening of the day after sleep than after a night of interrupted sleep (28, 29). The circadian rhythm of the immune system is regulated both by central and local intrinsic circadian clocks that operate autonomously; specific examples of clock genes include PER1, PER2, BMAL1, CLOCK, and the D site of the albumin promoter binding protein (30). A study by Keller et al (31) recently reported that the local circadian clock operating in splenic macrophages of mice regulates cytokine production in response to endotoxin stimulation. In addition, disruption of rhythm clock genes in natural killer cells using RNA interference resulted in a decrease in the levels of the cytolytic factors such as granzyme B and perforin and an increase in interferon-γ in rats (32), but the overall biologic/clinical implications of this phenomenon are yet to be determined.
Cytokines are important signaling molecules and biomarkers whose expression demonstrates a day/night rhythm (33). Interferon-γ and tumor necrosis factor-α have been shown to be regulated by CLOCK genes in a circadian fashion (30). Mice with impaired circadian rhythms resulting from a point mutation of the PER2 gene do not have diurnal variations of interferon-γ at both the gene and protein levels (32). Mice deficient in PER2 gene (PER2_/_) are more resistant to lipopolysaccharide-induced endotoxic shock than wild-type mice (34). The levels of serum proinflammatory cytokines interferon-γ and interleukin- 1_ are dramatically decreased in those mice and a defective natural killer cell function may be responsible for the depressed cytokine profile (34). Serum interleukin-6, an important molecule in relation to host defense mechanisms and acute phase reactions, exhibits a day/night variation in healthy subjects (35). In rheumatoid arthritis, interleukin-6 levels peak in the morning and decline in the afternoon, corresponding to the clinical manifestations of the disease (36). In patients with obstructive sleep apnea, serum interleukin-6 concentration is elevated and its diurnal variation is lost when compared with healthy subjects (37). Interestingly, the diurnal variation is resumed after treatment with continuous positive airway pressure ventilation (37).
Defensins are a family of antimicrobial peptides contributing to host defense mechanisms and play a role in modulating the innate immune system to infections (38–41). Human α-defensins are stored in the primary granules of immune cells, including neutrophils, monocytes, natural killer cells, and dendritic cells. Beta-defensins are constitutively expressed in epithelial cells and are important in controlling host defense and immune responses (38–40). Increased expression of α-defensin-1 has been shown to be mediated by CLOCK-BMAL1 but is depressed when CRY1 was coexpressed in the cells (42). Pattern recognition molecules are receptors expressed on the cell surface; they are capable of binding to micro-organisms through bacterial carbohydrates (e.g., lipopolysaccharide or mannose), nucleic acids (bacterial or viral DNA/RNA), bacterial peptides (flagellin), peptidoglycans and lipoteichoic acids from Gram-positive bacteria, N-formylmethionine, lipoproteins as well as fungal glucans. The digestive tract is one of the major ports of entry for pathogens and consequently expresses a variety of pattern recognition receptors as part of the host defense mechanisms. In mouse jejunum and Paneth-enriched crypt cells, messenger RNA levels of pattern recognition molecules such as Tolllike receptors and nucleotide-binding oligomerization domains demonstrate circadian oscillations (43). These findings suggest that the expression of pattern recognition molecules exhibit a circadian rhythm controlling the immune host defense mechanisms in response to gastrointestinal pathogens.
Circadian Rhythms in the Coagulation System
Inflammation and coagulation play an important role in the pathogenesis of cardiovascular events and sepsis. The increased risk of cardiovascular events in the morning may be explained by circadian changes in hemostasis (44, 45). The number of circulating platelets and their ability to aggregate has been shown to follow a circadian rhythm. For example, the highest number of platelets is found in the afternoon, but an increase in platelet aggregation largely occurs in the morning (46–48). These fluctuations in hemostatic activity related to variation in platelet aggregability appear to be governed by the endogenous circadian clock, because they are abolished in Clock mutant mice (46).
Components of the coagulation system, including factor VIIa and fibrinogen, are higher in the morning, but so are components of coagulation inhibitors such as protein C, antithrombin III, and tissue factor inhibitors. Plasminogen activator inhibitor type 1, a major physiological regulator of fibrinolysis, exhibits circadian variations in its expression with a peak in the early morning. Rev-ErbA suppresses plasminogen activator inhibitor type 1 gene expression and is therefore a major determinant of the circadian plasminogen activator inhibitor type 1 variation and may influence the susceptibility to myocardial infarction in the early morning (49). Experimental findings in mice suggest that ketogenic status increases hypofibrinolytic risk by inducing abnormal circadian expression of plasminogen activator inhibitor type 1 (50).
Circadian Rhythms in the Cardiovascular and Respiratory System
Under normal conditions, approximately 13% of genes exhibit significant differences in temporal expression patterns in murine heart (51). Some of these genes are rhythmically expressed between day and night, whereas others show abrupt changes from light-to-dark or dark-to-light transitions. Many of these genes play an important role in cardiac remodeling (51), both the diurnal variations of heart rate and the episodes of bradycardia decrease, whereas myocardial oxygen consumption and fatty acid oxidation rate increase in mice with mutation of the cardiomyocyte CLOCK gene (52). Disruption of circadian rhythms in rhythm disruptive cycles (10 hr light/10 hr darkness vs. 12 hr light/12 hr darkness) leads to decreased cardiac compensation in a murine model of pressure overload cardiac hypertrophy (53). The circadian rhythm disturbance is associated with altered expression of PER2 and BMAL and down regulation of some crucial genes regulating hypertrophic pathways. Restoration of the normal 24-hr circadian rhythms reverses or attenuates the abnormal pathology (53). Furthermore, oscillations in myocardial triglyceride levels, net triglyceride synthesis, and lipolysis have been shown to be decreased in mice with impaired expression of CLOCK protein (54). It has been suggested that CLOCK inactivates lipase during the active/awake phase at both the transcriptional and posttranslational levels suggesting that the peripheral circadian rhythm clock can directly regulate triglyceride turnover (54).
The levels of circulating lipoprotein a, cholesterol, triglycerides, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol display a significant circadian rhythm. Interestingly, the peak levels of lipoprotein and fibrinogen that occur in the morning coincide with the peak frequency of myocardial infarction and stroke, whereas the peak in platelet count appears to coincide with late afternoon peak in sudden cardiac death and fatal stroke (55).
Respiratory control, lung mechanics, and gas exchange also appear to fluctuate in a circadian fashion, independent of daily activity (56). Healthy adults have small daily variations in tidal volume, minute ventilation, and mean inspiratory flow (57). Under pathologic conditions, the diurnal variations become more apparent. For example, hypoxia or hypercapnia induces significant hyperventilatory responses that vary throughout 24-hr cycles (58).
Surfactant protein D, synthesized by alveolar epithelial type 2 cells and Clara cells, plays an important role in innate host defense (59). Surfactant protein D secretion varies significantly with peaks occurring at 10 AM and decreasing to its nadir at 10 PM (60). However, the relationship between diurnal variations of surfactant protein D levels and the susceptibility of developing acute lung injury has not been addressed.
Circadian Rhythms in the Hepatic and Renal Systems and Metabolism
Investigators have reported that peak serum levels of bilirubin occurred 10.6 hrs after falling asleep during nighttime in healthy volunteers. The intraindividual diurnal variation was higher during the day compared with the night sleep condition, suggesting that bilirubin sampling should be restricted to the morning after a normal night’s sleep to minimize diurnal variations (61). In disease conditions, the peak plasma melatonin/cortisol times can be further delayed in patients with cirrhosis compared with healthy control subjects (62). Hepatic gluconeogenesis and glucose metabolism is regulated by the circadian rhythms through changes in circulating glucagon and epinephrine that trigger cAMP-mediated phosphorylation of cAMP response element-binding protein and dephosphorylation of the cAMP response element-binding protein-regulated transcription coactivator-2 (63). cAMP response element-binding protein activity is modulated by Cry1 and Cry2 in the liver (64). Elevation of Cry1 expression lowers blood glucose concentrations and improves insulin sensitivity by blocking glucagon-mediated increases in intracellular cAMP concentrations (63). Circadian modulation of renal function was first described in the 19th century, and glomerular filtration rate, renal blood flow, tubular resorption, tubular secretion, and electrolyte excretion exhibit robust circadian oscillations (65, 66). Disturbance of the renal circadian rhythms is associated with a high risk of hypertension and renal fibrosis (67–69). A number of studies provide increasing evidence of the involvement of a molecular clock in the generation of renal excretory rhythms in renal epithelial cells (68). As a result of space limitations, we do not discuss this issue in detail, but there are several recent excellent review articles that provide mechanistic insights linking the circadian clock to kidney function (67, 70). The impact of circadian rhythms on liver and kidney function is associated with changes in metabolism of endogenous and exogenous biologic substances. For example, erythropoietin, a hematopoietic growth factor, is produced primarily in the kidneys (71) and shows important circadian rhythms (67). Noteworthy, the effects of erythropoietin therapy can be influenced by the time of the day at which it is administered (72). Furthermore, the endogenous secretion of glucocorticoids is governed by circadian rhythms in men (73, 74). There is a growing body of evidence showing that both pharmacokinetics and pharmacodynamics of drugs are influenced by the circadian rhythms (75). The timing of drug administration, including corticosteroids (76, 77), antibiotics (76), and medications for hypertension (78), can significantly alter the drugs’ effectiveness and toxicity (79). Significant differences in pharmacokinetics are observed when a single dose of gentamicin is administered at different times of the day (80). A lower total body clearance and higher serum gentamicin concentration are observed when the gentamicin is given at 10 PM rather than at 9 AM, suggesting that circadian rhythms and rest–activity routine should be taken into account to minimize toxicity and enhance effectiveness (80). These findings may also be relevant to other drugs that demonstrate circadian pharmacokinetic rhythms and narrow therapeutic windows. A study by Decousus et al (81) demonstrated a circadian variation in various coagulation tests, including activated partial thromboplastin time, thrombin time, and coagulation factor Xa inhibition. Maximum values of these tests were found at night and minimum values in the morning. Therefore, the metabolism and effectiveness of heparin therapy in patients with venous thromboembolism largely depends on the circadian clock. The time of the day at which hypertension medications are administered not only influences the effectiveness of blood pressure control, but also cardiovascular disease morbidity (78).
Circadian Rhythms in the ICU
A study by Haimovich and colleagues (82) has recently demonstrated that intravenous infusion of endotoxin in human volunteers results in dramatic alteration of circadian clock gene expression in peripheral blood leukocytes. The authors pointed out that the realignment of the central and peripheral clocks may be involved in the modulation of the course of the systemic inflammatory response syndrome in human.
Critically ill patients in the ICU have more frequent sleep deprivation and sleep disturbances than patients on a general ward (83–86). The normal rhythmic 24-hr profiles of physiological parameters such as blood pressure, heart rate, body temperature, spontaneous motor activity, and the levels of melatonin and cortisol are altered in ICU patients. Sleep deprivation and the inability to sleep are described by survivors as major sources of anxiety and stress during stays in the ICU (87–90). Furthermore, an impaired circadian rhythm of melatonin secretion has been reported in sedated and mechanically ventilated patients in the ICU (91). There are striking abnormalities in urinary 6-sulfatoxymelatonin excretion observed in septic ICU patients but not in nonseptic ICU patients, suggesting a role of severe sepsis per se and/or concomitant medication in the pathogenesis of the abolished circadian rhythms of melatonin secretion (92).
Several factors can contribute to sleep disruption in critically patients such as noise, patient care interactions, mechanical ventilation, pain, medications, artificial light, fatigue, stress, delirium, impaired cognition, altered physiology, and critical illness (87–90). It has been suggested that a reduction of plasma melatonin levels associated with the loss of circadian rhythms in critically ill patients receiving mechanical ventilation may contribute to sleep deprivation (91, 93–95). Patients with sepsis demonstrate loss of the circadian rhythms in melatonin levels compared with nonseptic patients (92). Experimentally, survival of septic animals is markedly decreased with an altered circadian light/dark cycle, providing further evidence for the negative impact of disruption of circadian rhythms (96).
In a prospective, observational study involving 24 critically ill sedated patients, Paul and Lemmer (97) showed that the 24-hr profiles of blood melatonin, cortisol, blood pressure, heart rate, body temperature, and spontaneous motor activity were greatly disturbed/abolished compared with the well-known rhythmic 24-hr patterns in healthy control subjects. These alterations were more pronounced in patients with brain injury.
In critically ill patients, blood glucose values and the incidence of hyperglycemia have a circadian rhythm. Investigators have shown that morning blood glucose may not be an accurate surrogate of blood glucose control over the daily cycle in the ICU (98, 99), although there is still controversy surrounding the concept of tight glycemic control in critically ill (98, 100, 101).
Although much is known about the impact of circadian rhythms in the development of a broad range of human diseases, relatively little attention has been paid to critically ill patients in the ICU. It remains unclear if the circadian rhythm alterations observed in critically ill patients represent a compensatory response or whether they are in and of themselves pathologic. However, further studies are warranted to determine whether there is any use in restoring the circadian rhythms in all critically ill patients, what therapeutic goals should be targeted, and how it would be achieved. This is an exciting time for critical care physicians to define the role of circadian rhythms in the management of critically illness. A clinical trial is underway at the University of Chicago (http://clinicaltrials.gov/ct2/show/NCT01276652) to determine whether the sleep and circadian rhythms of critically ill patients undergoing mechanical ventilation can be improved through practical strategies that can be used at the bedside.
CONCLUSION
Circadian rhythms are complex but important physiological phenomena that help synchronize biologic functions with the external environment. In addition to being closely related to the sleep/wake cycle, circadian rhythms are involved not only in inflammatory responses, but also in modulation of therapeutic efficiency. More research is warranted to determine the molecular mechanisms underlying circadian rhythms as well as the impact of these rhythms on the use of therapeutic agents in the context of critical care medicine.
Acknowledgments
Supported by Canadian Institutes of Health Research to Dr. Slutsky and Dr. Zhang (MOP8558, MOP 77818, MOP69042), Ontario Thoracic Society and McLaughlin Foundation to Dr. Zhang, and German Research Foundation to Dr. Spieth (DFG SP 1222/3–1). Dr. Spieth is recipient of the University of Toronto Eli Lilly Critical Care Medicine Fellowship Award.
Footnotes
The authors have not disclosed any potential conflicts of interest.
References
- 1.Shibata S, Tahara Y, Hirao A. The adjustment and manipulation of biological rhythms by light, nutrition, and abused drugs. Adv Drug Deliv Rev. 2010;62:918–927. doi: 10.1016/j.addr.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Spengler CM, Shea SA. Endogenous circadian rhythm of pulmonary function in healthy humans. Am J Respir Crit Care Med. 2000;162:1038–1046. doi: 10.1164/ajrccm.162.3.9911107. [DOI] [PubMed] [Google Scholar]
- 3.van Eekelen AP, Kerkhof GA, van Amsterdam JG. Circadian variation in cortisol reactivity to an acute stressor. Chronobiol Int. 2003;20:863–878. doi: 10.1081/cbi-120024212. [DOI] [PubMed] [Google Scholar]
- 4.Marcheva B, Ramsey KM, Affinati A, et al. Clock genes and metabolic disease. J Appl Physiol. 2009;107:1638–1646. doi: 10.1152/japplphysiol.00698.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahar S, Sassone-Corsi P. Metabolism and cancer: The circadian clock connection. Nat Rev Cancer. 2009;9:886–896. doi: 10.1038/nrc2747. [DOI] [PubMed] [Google Scholar]
- 6.Paschos GK, FitzGerald GA. Circadian clocks and vascular function. Circ Res. 2010;106:833–841. doi: 10.1161/CIRCRESAHA.109.211706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filipski E, Delaunay F, King VM, et al. Effects of chronic jet lag on tumor progression in mice. Cancer Res. 2004;64:7879–7885. doi: 10.1158/0008-5472.CAN-04-0674. [DOI] [PubMed] [Google Scholar]
- 8.Martino TA, Oudit GY, Herzenberg AM, et al. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1675–1683. doi: 10.1152/ajpregu.00829.2007. [DOI] [PubMed] [Google Scholar]
- 9.Hansen J. Light at night, shiftwork, and breast cancer risk. J Natl Cancer Inst. 2001;93:1513–1515. doi: 10.1093/jnci/93.20.1513. [DOI] [PubMed] [Google Scholar]
- 10.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 11.Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev. 2010;90:1063–1102. doi: 10.1152/physrev.00009.2009. [DOI] [PubMed] [Google Scholar]
- 12.Lucas RJ, Freedman MS, Lupi D, et al. Identifying the photoreceptive inputs to the mammalian circadian system using transgenic and retinally degenerate mice. Behav Brain Res. 2001;125:97–102. doi: 10.1016/s0166-4328(01)00274-1. [DOI] [PubMed] [Google Scholar]
- 13.Duguay D, Cermakian N. The crosstalk between physiology and circadian clock proteins. Chronobiol Int. 2009;26:1479–1513. doi: 10.3109/07420520903497575. [DOI] [PubMed] [Google Scholar]
- 14.Teboul M, Grechez-Cassiau A, Guillaumond F, et al. How nuclear receptors tell time. J Appl Physiol. 2009;107:1965–1971. doi: 10.1152/japplphysiol.00515.2009. [DOI] [PubMed] [Google Scholar]
- 15.Cuninkova L, Brown SA. Peripheral circadian oscillators: Interesting mechanisms and powerful tools. Ann N Y Acad Sci. 2008;1129:358–370. doi: 10.1196/annals.1417.005. [DOI] [PubMed] [Google Scholar]
- 16.Yoo SH, Yamazaki S, Lowrey PL, et al. PERIOD2:LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Nat Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meijer JH, Michel S, Vanderleest HT, et al. Daily and seasonal adaptation of the circadian clock requires plasticity of the SCN neuronal network. Eur J Neurosci. 2010;32:2143–2151. doi: 10.1111/j.1460-9568.2010.07522.x. [DOI] [PubMed] [Google Scholar]
- 18.Edery I. Circadian rhythms in a nutshell. Physiol Genomics. 2000;3:59–74. doi: 10.1152/physiolgenomics.2000.3.2.59. [DOI] [PubMed] [Google Scholar]
- 19.Zhang EE, Kay SA. Clocks not winding down: Unravelling circadian networks. Nat Rev Mol Cell Biol. 2010;11:764–776. doi: 10.1038/nrm2995. [DOI] [PubMed] [Google Scholar]
- 20.Ishida N, Kaneko M, Allada R. Biological clocks. Proc Nat Acad Sci U S A. 1999;96:8819–8820. doi: 10.1073/pnas.96.16.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyajima N, Horiuchi R, Shibuya Y, et al. Two erbA homologs encoding proteins with different T3 binding capacities are transcribed from opposite DNA strands of the same genetic locus. Cell. 1989;57:31–39. doi: 10.1016/0092-8674(89)90169-4. [DOI] [PubMed] [Google Scholar]
- 22.Lazar MA, Hodin RA, Darling DS, et al. A novel member of the thyroid/steroid hormone receptor family is encoded by the opposite strand of the rat c-erbA alpha transcriptional unit. Mol Cell Biol. 1989;9:1128–1136. doi: 10.1128/mcb.9.3.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lazar MA, Hodin RA, Cardona G, et al. Gene expression from the c-erbA alpha/Rev ErbA alpha genomic locus. Potential regulation of alternative splicing by opposite strand transcription. J Biol Chem. 1990;265:12859–12863. [PubMed] [Google Scholar]
- 24.Munroe SH, Lazar MA. Inhibition of c-erbA mRNA splicing by a naturally occurring antisense RNA. J Biol Chem. 1991;266:22083–22086. [PubMed] [Google Scholar]
- 25.Matsuo T, Yamaguchi S, Mitsui S, et al. Control mechanism of the circadian clock for timing of cell division in vivo. Science. 2003;302:255–259. doi: 10.1126/science.1086271. [DOI] [PubMed] [Google Scholar]
- 26.Arendt J. Melatonin, circadian rhythms, and sleep. N Engl J Med. 2000;343:1114–1116. doi: 10.1056/NEJM200010123431510. [DOI] [PubMed] [Google Scholar]
- 27.Foulkes NS, Whitmore D, Sassone-Corsi P. Rhythmic transcription: the molecular basis of circadian melatonin synthesis. Biol Cell. 1997;89:487–494. doi: 10.1016/s0248-4900(98)80004-x. [DOI] [PubMed] [Google Scholar]
- 28.Lange T, Dimitrov S, Born J. Effects of sleep and circadian rhythm on the human immune system. Ann N Y Acad Sci. 2010;1193:48–59. doi: 10.1111/j.1749-6632.2009.05300.x. [DOI] [PubMed] [Google Scholar]
- 29.Born J, Lange T, Hansen K, et al. Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol. 1997;158:4454–4464. [PubMed] [Google Scholar]
- 30.Arjona A, Sarkar DK. Circadian oscillations of clock genes, cytolytic factors, and cytokines in rat NK cells. J Immunol. 2005;174:7618–7624. doi: 10.4049/jimmunol.174.12.7618. [DOI] [PubMed] [Google Scholar]
- 31.Keller M, Mazuch J, Abraham U, et al. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci U S A. 2009;106:21407–21412. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arjona A, Sarkar DK. The circadian gene mPer2 regulates the daily rhythm of IFNgamma. J Interferon Cytokine Res. 2006;26:645–649. doi: 10.1089/jir.2006.26.645. [DOI] [PubMed] [Google Scholar]
- 33.Seruga B, Zhang H, Bernstein LJ, et al. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8:887–899. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Malkani G, Shi X, et al. The circadian clock Period 2 gene regulates gamma interferon production of NK cells in host response to lipopolysaccharide-induced endotoxic shock. Infect Immunol. 2006;74:4750–4756. doi: 10.1128/IAI.00287-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sothern RB, Roitman-Johnson B, Kanabrocki EL, et al. Circadian characteristics of circulating interleukin-6 in men. J Allergy Clin Immunol. 1995;95:1029–1035. doi: 10.1016/s0091-6749(95)70104-4. [DOI] [PubMed] [Google Scholar]
- 36.Arvidson NG, Gudbjornsson B, Elfman L, et al. Circadian rhythm of serum interleukin-6 in rheumatoid arthritis. Ann Rheum Dis. 1994;53:521–524. doi: 10.1136/ard.53.8.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burioka N, Miyata M, Fukuoka Y, et al. Day–night variations of serum interleukin-6 in patients with severe obstructive sleep apnea syndrome before and after continuous positive airway pressure (CPAP) Chronobiol Int. 2008;25:827–834. doi: 10.1080/07420520802384101. [DOI] [PubMed] [Google Scholar]
- 38.Khine AA, Del Sorbo L, Vaschetto R, et al. Human neutrophil peptides induce interleukin-8 production through the P2Y6 signaling. :2936–2942. doi: 10.1182/blood-2005-06-2314. [DOI] [PubMed] [Google Scholar]
- 39.Vaschetto R, Grinstein J, Del Sorbo L, et al. Role of human neutrophil peptides in the initial interaction between lung epithelial cells and CD4+ lymphocytes. J Leukoc Biol. 2007;81:1022–1031. doi: 10.1189/jlb.0706435. [DOI] [PubMed] [Google Scholar]
- 40.Voglis S, Quinn K, Tullis E, et al. Human neutrophil peptides and phagocytic deficiency in bronchiectatic lungs. Am J Respir Crit Care Med. 2009;180:159–166. doi: 10.1164/rccm.200808-1250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Syeda F, Liu HY, Tullis E, et al. Differential signaling mechanisms of HNP-induced IL-8 production in human lung epithelial cells and monocytes. J Cell Physiol. 2008;214:820–827. doi: 10.1002/jcp.21279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sherman H, Froy O. Expression of human beta-defensin 1 is regulated via c-Myc and the biological clock. Mol Immunol. 2008;45:3163–3167. doi: 10.1016/j.molimm.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Froy O, Chapnik N. Circadian oscillation of innate immunity components in mouse small intestine. Mol Immunol. 2007;44:1954–1960. doi: 10.1016/j.molimm.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 44.Kapiotis S, Jilma B, Quehenberger P, et al. Morning hypercoagulability and hypofibrinolysis. Diurnal variations in circulating activated factor VII, prothrombin fragment F1+2, and plasmin-plasmin inhibitor complex. Circulation. 1997;96:19–21. doi: 10.1161/01.cir.96.1.19. [DOI] [PubMed] [Google Scholar]
- 45.Andreotti F, Davies GJ, Hackett DR, et al. Major circadian fluctuations in fibrinolytic factors and possible relevance to time of onset of myocardial infarction, sudden cardiac death and stroke. Am J Cardiol. 1988;62:635–637. doi: 10.1016/0002-9149(88)90669-8. [DOI] [PubMed] [Google Scholar]
- 46.Ohkura N, Oishi K, Sudo T, et al. CLOCK regulates circadian platelet activity. Thromb Res. 2009;123:523–527. doi: 10.1016/j.thromres.2008.03.009. [DOI] [PubMed] [Google Scholar]; Crit Care Med. 2012;40(1–251) [Google Scholar]
- 47.Wiens L, Lutze G, Luley C, et al. Platelet count and platelet activation: Impact of a fat meal and day time. Platelets. 2007;18:171–173. doi: 10.1080/09537100600930946. [DOI] [PubMed] [Google Scholar]
- 48.Chrusciel P, Goch A, Banach M, et al. Circadian changes in the hemostatic system in healthy men and patients with cardiovascular diseases. Med Sci Monit. 2009;15:RA203–208. doi: 10.12659/msm.878203. [DOI] [PubMed] [Google Scholar]
- 49.Wang J, Yin L, Lazar MA. The orphan nuclear receptor Rev-erb alpha regulates circadian expression of plasminogen activator inhibitor type 1. J Biol Chem. 2006;281:33842–33848. doi: 10.1074/jbc.M607873200. [DOI] [PubMed] [Google Scholar]
- 50.Oishi K, Uchida D, Ohkura N, et al. Ketogenic diet disrupts the circadian clock and increases hypofibrinolytic risk by inducing expression of plasminogen activator inhibitor-1. Arterioscler Thromb Vasc Biol. 2009;29:1571–1577. doi: 10.1161/ATVBAHA.109.190140. [DOI] [PubMed] [Google Scholar]
- 51.Martino T, Arab S, Straume M, et al. Day/night rhythms in gene expression of the normal murine heart. J Mol Med. 2004;82:256–264. doi: 10.1007/s00109-003-0520-1. [DOI] [PubMed] [Google Scholar]
- 52.Bray MS, Shaw CA, Moore MW, et al. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am J Physiol Heart Circ Physiol. 2008;294:H1036–1047. doi: 10.1152/ajpheart.01291.2007. [DOI] [PubMed] [Google Scholar]
- 53.Martino TA, Tata N, Belsham DD, et al. Disturbed diurnal rhythm alters gene expression and exacerbates cardiovascular disease with rescue by resynchronization. Hypertension. 2007;49:1104–1113. doi: 10.1161/HYPERTENSIONAHA.106.083568. [DOI] [PubMed] [Google Scholar]
- 54.Tsai JY, Kienesberger PC, Pulinilkunnil T, et al. Direct regulation of myocardial triglyceride metabolism by the cardiomyocyte circadian clock. J Biol Chem. 2010;285:2918–2929. doi: 10.1074/jbc.M109.077800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bremner WF, Sothern RB, Kanabrocki EL, et al. Relation between circadian patterns in levels of circulating lipoprotein (a), fibrinogen, platelets, and related lipid variables in men. Am Heart J. 2000;139:164–173. doi: 10.1016/s0002-8703(00)90324-7. [DOI] [PubMed] [Google Scholar]
- 56.Mortola JP. Breathing around the clock: An overview of the circadian pattern of respiration. Eur J Appl Physiol. 2004;91:119–129. doi: 10.1007/s00421-003-0978-0. [DOI] [PubMed] [Google Scholar]
- 57.Adamczyk W, Tafil-Klawe M, Siekierka M, et al. Daily pattern of breathing in healthy young men. J Physiol Pharmacol. 2008;59(Suppl 6):115–122. [PubMed] [Google Scholar]
- 58.Saiki C, Mortola JP. Hypoxia abolishes the morning–night differences of metabolism and ventilation in 6-day-old rats. Can J Physiol Pharmacol. 1995;73:159–164. doi: 10.1139/y95-024. [DOI] [PubMed] [Google Scholar]
- 59.Hartl D, Griese M. Surfactant protein D in human lung diseases. Eur J Clin Invest. 2006;36:423–435. doi: 10.1111/j.1365-2362.2006.01648.x. [DOI] [PubMed] [Google Scholar]
- 60.Hoegh SV, Sorensen GL, Tornoe I, et al. Long-term stability and circadian variation in circulating levels of surfactant protein D. Immunobiology. 2010;215:314–320. doi: 10.1016/j.imbio.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 61.Larsson A, Hassan M, Ridefelt P, et al. Circadian variability of bilirubin in healthy men during normal sleep and after an acute shift of sleep. Chronobiol Int. 2009;26:1613–1621. doi: 10.3109/07420520903398534. [DOI] [PubMed] [Google Scholar]
- 62.Montagnese S, Middleton B, Mani AR, et al. On the origin and the consequences of circadian abnormalities in patients with cirrhosis. Am J Gastroenterol. 2010;105:1773–1781. doi: 10.1038/ajg.2010.86. [DOI] [PubMed] [Google Scholar]
- 63.Screaton RA, Conkright MD, Katoh Y, et al. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell. 2004;119:61–74. doi: 10.1016/j.cell.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 64.Zhang EE, Liu Y, Dentin R, et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med. 2010;16:1152–1156. doi: 10.1038/nm.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Voogel AJ, Koopman MG, Hart AA, et al. Circadian rhythms in systemic hemodynamics and renal function in healthy subjects and patients with nephrotic syndrome. Kidney Int. 2001;59:1873–1880. doi: 10.1046/j.1523-1755.2001.0590051873.x. [DOI] [PubMed] [Google Scholar]
- 66.Pons M, Forpomes O, Espagnet S, et al. Relationship between circadian changes in renal hemodynamics and circadian changes in urinary glycosaminoglycan excretion in normal rats. Chronobiol Int. 1996;13:349–358. doi: 10.3109/07420529609012659. [DOI] [PubMed] [Google Scholar]
- 67.Firsov D, Bonny O. Circadian regulation of renal function. Kidney Int. 2010;78:640–645. doi: 10.1038/ki.2010.227. [DOI] [PubMed] [Google Scholar]
- 68.Gumz ML, Stow LR, Lynch IJ, et al. The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J Clin Invest. 2009;119:2423–2434. doi: 10.1172/JCI36908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koch BC, Nagtegaal JE, Kerkhof GA, et al. Circadian sleep–wake rhythm disturbances in end-stage renal disease. Nat Rev Nephrol. 2009;5:407–416. doi: 10.1038/nrneph.2009.88. [DOI] [PubMed] [Google Scholar]
- 70.Stow LR, Gumz ML. The circadian clock in the kidney. J Am Soc Nephrol. 2011;22:598–604. doi: 10.1681/ASN.2010080803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Donnelly S. Why is erythropoietin made in the kidney? The kidney functions as a critmeter. Am J Kidney Dis. 2001;38:415–425. doi: 10.1053/ajkd.2001.26111. [DOI] [PubMed] [Google Scholar]
- 72.Lee MS, Lee JS, Lee JY. Prevention of erythropoietin- associated hypertension. Hypertension. 2007;50:439–445. doi: 10.1161/HYPERTENSIONAHA.107.090423. [DOI] [PubMed] [Google Scholar]
- 73.Pollmacher T, Mullington J, Korth C, et al. Diurnal variations in the human host response to endotoxin. J Infect Dis. 1996;174:1040–1045. doi: 10.1093/infdis/174.5.1040. [DOI] [PubMed] [Google Scholar]
- 74.Nader N, Chrousos GP, Kino T. Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: Potential physiological implications. FASEB J. 2009;23:1572–1583. doi: 10.1096/fj.08-117697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sukumaran S, Almon RR, DuBois DC, et al. Circadian rhythms in gene expression: Relationship to physiology, disease, drug disposition and drug action. Adv Drug Deliv Rev. 2010;62:904–917. doi: 10.1016/j.addr.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Beauchamp D, Labrecque G. Chronobiology and chronotoxicology of antibiotics and aminoglycosides. Adv Drug Deliv Rev. 2007;59:896–903. doi: 10.1016/j.addr.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 77.Debono M, Ghobadi C, Rostami-Hodjegan A, et al. Modified-release hydrocortisone to provide circadian cortisol profiles. J Clin Endocrinol Metab. 2009;94:1548 –1554. doi: 10.1210/jc.2008-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hermida RC, Ayala DE, Mojon A, et al. Influence of circadian time of hypertension treatment on cardiovascular risk: Results of the MAPEC study. Chronobiol Int. 2010;27:1629–1651. doi: 10.3109/07420528.2010.510230. [DOI] [PubMed] [Google Scholar]
- 79.Paschos GK, Baggs JE, Hogenesch JB, et al. The role of clock genes in pharmacology. Annu Rev Pharmacol Toxicol. 2010;50:187–214. doi: 10.1146/annurev.pharmtox.010909.105621. [DOI] [PubMed] [Google Scholar]
- 80.Dickson CJ, Schwartzman MS, Bertino JS., Jr Factors affecting aminoglycoside disposition: effects of circadian rhythm and dietary protein intake on gentamicin pharmacokinetics. Clin Pharmacol Ther. 1986;39:325–328. doi: 10.1038/clpt.1986.47. [DOI] [PubMed] [Google Scholar]
- 81.Decousus HA, Croze M, Levi FA, et al. Circadian changes in anticoagulant effect of heparin infused at a constant rate. BMJ (Clin Res Ed) 1985;290:341–344. doi: 10.1136/bmj.290.6465.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haimovich B, Calvano J, Haimovich AD, et al. In vivo endotoxin synchronizes and suppresses clock gene expression in human peripheral blood leukocytes. Crit Care Med. 2010;38:751–758. doi: 10.1097/CCM.0b013e3181cd131c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Drouot X, Cabello B, d’Ortho MP, et al. Sleep in the intensive care unit. Sleep Med Rev. 2008;12:391–403. doi: 10.1016/j.smrv.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 84.Freedman NS, Gazendam J, Levan L, et al. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 2001;163:451–457. doi: 10.1164/ajrccm.163.2.9912128. [DOI] [PubMed] [Google Scholar]
- 85.Cooper AB, Thornley KS, Young GB, et al. Sleep in critically ill patients requiring mechanical ventilation. Chest. 2000;117:809–818. doi: 10.1378/chest.117.3.809. [DOI] [PubMed] [Google Scholar]
- 86.Gabor JY, Cooper AB, Crombach SA, et al. Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med. 2003;167:708–715. doi: 10.1164/rccm.2201090. [DOI] [PubMed] [Google Scholar]
- 87.Toublanc B, Rose D, Glerant JC, et al. Assist-control ventilation vs. low levels of pressure support ventilation on sleep quality in intubated ICU patients. Intensive Care Med. 2007;33:1148–1154. doi: 10.1007/s00134-007-0659-2. [DOI] [PubMed] [Google Scholar]
- 88.Davydow DS, Gifford JM, Desai SV, et al. Depression in general intensive care unit survivors: A systematic review. Intensive Care Med. 2009;35:796–809. doi: 10.1007/s00134-009-1396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rotondi AJ, Chelluri L, Sirio C, et al. Patients’ recollections of stressful experiences while receiving prolonged mechanical ventilation in an intensive care unit. Crit Care Med. 2002;30:746–752. doi: 10.1097/00003246-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 90.Alexopoulou C, Kondili E, Vakouti E, et al. Sleep during proportional-assist ventilation with load-adjustable gain factors in critically ill patients. Intensive Care Med. 2007;33:1139–1147. doi: 10.1007/s00134-007-0630-2. [DOI] [PubMed] [Google Scholar]
- 91.Olofsson K, Alling C, Lundberg D, et al. Abolished circadian rhythm of melatonin secretion in sedated and artificially venti- 252 Crit Care Med 2012 Vol. 40, No. 1 lated intensive care patients. Acta Anaesthesiol Scand. 2004;48:679–684. doi: 10.1111/j.0001-5172.2004.00401.x. [DOI] [PubMed] [Google Scholar]
- 92.Mundigler G, Delle-Karth G, Koreny M, et al. Impaired circadian rhythm of melatonin secretion in sedated critically ill patients with severe sepsis. Crit Care Med. 2002;30:536–540. doi: 10.1097/00003246-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 93.Frisk U, Olsson J, Nylen P, et al. Low melatonin excretion during mechanical ventilation in the intensive care unit. Clin Sci (Lond) 2004;107:47–53. doi: 10.1042/CS20030374. [DOI] [PubMed] [Google Scholar]
- 94.Shilo L, Dagan Y, Smorjik Y, et al. Patients in the intensive care unit suffer from severe lack of sleep associated with loss of normal melatonin secretion pattern. Am J Med Sci. 1999;317:278–281. doi: 10.1097/00000441-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 95.Ibrahim MG, Bellomo R, Hart GK, et al. A double-blind placebo-controlled randomized pilot study of nocturnal melatonin in tracheostomised patients. Crit Care Resusc. 2006;8:187–191. [PubMed] [Google Scholar]
- 96.Carlson DE, Chiu WC. The absence of circadian cues during recovery from sepsis modifies pituitary–adrenocortical function and impairs survival. Shock. 2008;29:127–132. doi: 10.1097/shk.0b013e318142c5a2. [DOI] [PubMed] [Google Scholar]
- 97.Paul T, Lemmer B. Disturbance of circadian rhythms in analgosedated intensive care unit patients with and without craniocerebral injury. Chronobiol Int. 2007;24:45–61. doi: 10.1080/07420520601142569. [DOI] [PubMed] [Google Scholar]
- 98.Egi M, Bellomo R, Stachowski E, et al. Circadian rhythm of blood glucose values in critically ill patients. Crit Care Med. 2007;35:416–421. doi: 10.1097/01.CCM.0000253814.78836.43. [DOI] [PubMed] [Google Scholar]
- 99.Smith SM, Oveson KE, Strauss W, et al. Ultradian variation of blood glucose in intensive care unit patients receiving insulin infusions. Diabetes Care. 2007;30:2503–2505. doi: 10.2337/dc07-0865. [DOI] [PubMed] [Google Scholar]
- 100.Rudic RD, McNamara P, Curtis AM, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]


