Abstract
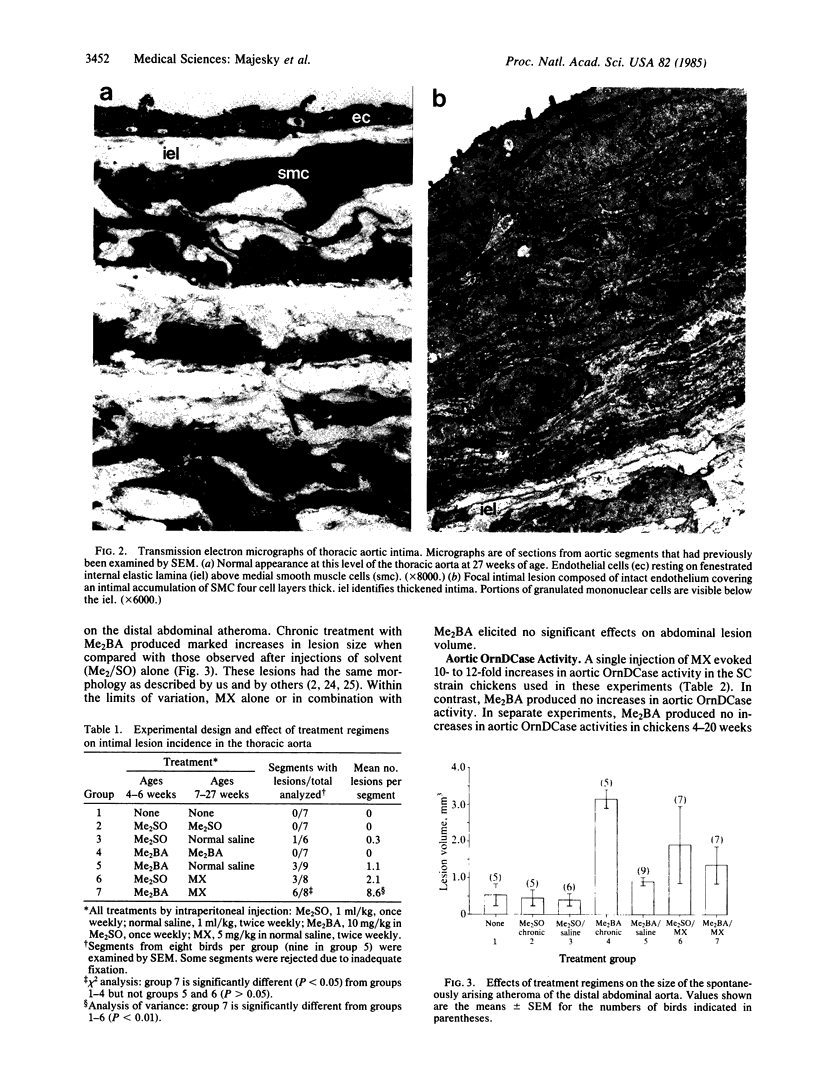
Human atherosclerotic fibrous plaques display a clonal character similar to many benign neoplasms. We report here that chickens treated with an initiation-promotion sequence developed focal intimal smooth muscle lesions in the thoracic aorta that resemble early forms of atherosclerosis. Scanning electron microscopy revealed small mound-like lesions protruding from an intact endothelium in birds treated with an initiating dose of 7,12-dimethylbenz[a]anthracene (Me2BA) followed by twice weekly injections of the alpha 1-selective adrenergic agonist methoxamine for 20 weeks. Intimal lesion foci were composed of densely packed modified smooth muscle cells, abundant extracellular matrix, and occasional mononuclear cells (possibly monocytes). There was no ultrastructural evidence of lipid accumulation or alteration of the underlying media. These intimal lesions appeared in aortic segments of treated chickens in a pattern similar to that observed in classical experiments of multistage tumorigenesis in epidermis and other tissues. The treatment with Me2BA followed by methoxamine produced more focal lesions per thoracic segment and more segments per group with lesions than did treatment with either Me2BA or methoxamine alone. Thoracic intimal foci were absent from untreated and vehicle-treated groups. In contrast, the growth of a spontaneously arising atheroma in the distal abdominal aorta was not demonstrably affected by the initiation-promotion regimen. Likewise, weekly injections of Me2BA for 23 weeks, while greatly enhancing abdominal atheroma growth, produced no thoracic lesions. These results provide evidence that focal proliferation of intimal smooth muscle cells, a critical early event in atherogenesis, can be produced by an initiation-promotion treatment sequence.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert R. E., Vanderlaan M., Burns F. J., Nishizumi M. Effect of carcinogens on chicken atherosclerosis. Cancer Res. 1977 Jul;37(7 Pt 1):2232–2235. [PubMed] [Google Scholar]
- BOUTWELL R. K. SOME BIOLOGICAL ASPECTS OF SKIN CARCINOGENISIS. Prog Exp Tumor Res. 1964;4:207–250. doi: 10.1159/000385978. [DOI] [PubMed] [Google Scholar]
- Benditt E. P., Barrett T., McDougall J. K. Viruses in the etiology of atherosclerosis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6386–6389. doi: 10.1073/pnas.80.20.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Benditt J. M. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1753–1756. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P. Evidence for a monoclonal origin of human atherosclerotic plaques and some implications. Circulation. 1974 Oct;50(4):650–652. doi: 10.1161/01.cir.50.4.650. [DOI] [PubMed] [Google Scholar]
- Bond J. A., Gown A. M., Yang H. L., Benditt E. P., Juchau M. R. Further investigations of the capacity of polynuclear aromatic hydrocarbons to elicit atherosclerotic lesions. J Toxicol Environ Health. 1981 Feb;7(2):327–335. doi: 10.1080/15287398109529983. [DOI] [PubMed] [Google Scholar]
- Boutwell R. K. The function and mechanism of promoters of carcinogenesis. CRC Crit Rev Toxicol. 1974 Jan;2(4):419–443. doi: 10.3109/10408447309025704. [DOI] [PubMed] [Google Scholar]
- Day N. E., Brown C. C. Multistage models and primary prevention of cancer. J Natl Cancer Inst. 1980 Apr;64(4):977–989. [PubMed] [Google Scholar]
- Doll R., Peto R. Mortality in relation to smoking: 20 years' observations on male British doctors. Br Med J. 1976 Dec 25;2(6051):1525–1536. doi: 10.1136/bmj.2.6051.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber E., Cameron R. The sequential analysis of cancer development. Adv Cancer Res. 1980;31:125–226. doi: 10.1016/s0065-230x(08)60658-2. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J. The origin and development of human tumors studied with cell markers. N Engl J Med. 1974 Jul 4;291(1):26–35. doi: 10.1056/NEJM197407042910109. [DOI] [PubMed] [Google Scholar]
- Gartler S. M. Patterns of cellular proliferation in normal and tumor cell populations. Am J Pathol. 1977 Mar;86(3):685–692. [PMC free article] [PubMed] [Google Scholar]
- HAUST M. D., MORE R. H., MOVAT H. Z. The role of smooth muscle cells in the fibrogenesis of arteriosclerosis. Am J Pathol. 1960 Oct;37:377–389. [PMC free article] [PubMed] [Google Scholar]
- Hicks R. M., Wakefield J., Chowaniec J. Evaluation of a new model to detect bladder carcinogens or co-carcinogens; results obtained with saccharin, cyclamate and cyclophosphamide. Chem Biol Interact. 1975 Sep;11(3):225–233. doi: 10.1016/0009-2797(75)90101-5. [DOI] [PubMed] [Google Scholar]
- Jänne J., Pösö H., Raina A. Polyamines in rapid growth and cancer. Biochim Biophys Acta. 1978 Apr 6;473(3-4):241–293. doi: 10.1016/0304-419x(78)90015-x. [DOI] [PubMed] [Google Scholar]
- Kannel W. B., McGee D., Gordon T. A general cardiovascular risk profile: the Framingham Study. Am J Cardiol. 1976 Jul;38(1):46–51. doi: 10.1016/0002-9149(76)90061-8. [DOI] [PubMed] [Google Scholar]
- Linder D., Gartler S. M. Glucose-6-phosphate dehydrogenase mosaicism: utilization as a cell marker in the study of leiomyomas. Science. 1965 Oct 1;150(3692):67–69. doi: 10.1126/science.150.3692.67. [DOI] [PubMed] [Google Scholar]
- Maudsley D. V. Regulation of polyamine biosynthesis. Biochem Pharmacol. 1979;28(2):153–161. doi: 10.1016/0006-2952(79)90496-9. [DOI] [PubMed] [Google Scholar]
- Minick C. R., Fabricant C. G., Fabricant J., Litrenta M. M. Atheroarteriosclerosis induced by infection with a herpesvirus. Am J Pathol. 1979 Sep;96(3):673–706. [PMC free article] [PubMed] [Google Scholar]
- Moolgavkar S. H., Day N. E., Stevens R. G. Two-stage model for carcinogenesis: Epidemiology of breast cancer in females. J Natl Cancer Inst. 1980 Sep;65(3):559–569. [PubMed] [Google Scholar]
- Moss N. S., Benditt E. P. The ultrastructure of spontaneous and experimentally induced arterial lesions. II. The spontaneous plaque in the chicken. Lab Invest. 1970 Sep;23(3):231–245. [PubMed] [Google Scholar]
- Narisawa T., Magadia N. E., Weisburger J. H., Wynder E. L. Promoting effect of bile acids on colon carcinogenesis after intrarectal instillation of N-methyl-N'-nitro-N-nitrosoguanidine in rats. J Natl Cancer Inst. 1974 Oct;53(4):1093–1097. doi: 10.1093/jnci/53.4.1093. [DOI] [PubMed] [Google Scholar]
- O'Brien T. G. The induction of ornithine decarboxylase as an early, possibly obligatory, event in mouse skin carcinogenesis. Cancer Res. 1976 Jul;36(7 Pt 2):2644–2653. [PubMed] [Google Scholar]
- Pearson T. A., Dillman J. M., Solex K., Heptinstall R. H. Clonal markers in the study of the origin and growth of human atherosclerotic lesions. Circ Res. 1978 Jul;43(1):10–18. doi: 10.1161/01.res.43.1.10. [DOI] [PubMed] [Google Scholar]
- Penn A., Batastini G., Soloman J., Burns F., Albert R. Dose-dependent size increases of aortic lesions following chronic exposure to 7,12-dimethylbenz(a)anthracene. Cancer Res. 1981 Feb;41(2):588–592. [PubMed] [Google Scholar]
- Peraino C., Fry R. J., Staffeldt E. Reduction and enhancement by phenobarbital of hepatocarcinogenesis induced in the rat by 2-acetylaminofluorene. Cancer Res. 1971 Oct;31(10):1506–1512. [PubMed] [Google Scholar]
- Reddy A. L., Fialkow P. J. Papillomas induced by initiation-promotion differ from those induced by carcinogen alone. Nature. 1983 Jul 7;304(5921):69–71. doi: 10.1038/304069a0. [DOI] [PubMed] [Google Scholar]
- Reidy M. A., Schwartz S. M. Endothelial regeneration. III. Time course of intimal changes after small defined injury to rat aortic endothelium. Lab Invest. 1981 Apr;44(4):301–308. [PubMed] [Google Scholar]
- Ross R., Glomset J. A. The pathogenesis of atherosclerosis (second of two parts). N Engl J Med. 1976 Aug 19;295(8):420–425. doi: 10.1056/NEJM197608192950805. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Snyder S. H. Amine synthesis in regenerating rat liver: extremely rapid turnover of ornithine decarboxylase. Mol Pharmacol. 1969 May;5(3):253–262. [PubMed] [Google Scholar]
- Scribner J. D., Süss R. Tumor initiation and promotion. Int Rev Exp Pathol. 1978;18:137–198. [PubMed] [Google Scholar]
- Takigawa M., Verma A. K., Simsiman R. C., Boutwell R. K. Polyamine biosynthesis and skin tumor promotion: inhibition of 12-O-tetradecanoylphorbol-13-acetate-promoted mouse skin tumor formation by the irreversible inhibitor of ornithine decarboxylase alpha-difluoromethylornithine. Biochem Biophys Res Commun. 1982 Apr 14;105(3):969–976. doi: 10.1016/0006-291x(82)91065-8. [DOI] [PubMed] [Google Scholar]
- Thomas W. A., Kim D. N. Biology of disease. Atherosclerosis as a hyperplastic and/or neoplastic process. Lab Invest. 1983 Mar;48(3):245–255. [PubMed] [Google Scholar]
- Thomas W. A., Reiner J. M., Janakidevi K., Florentin R. A., Lee K. T. Population dynamics of arterial cells during atherogenesis. X. Study of monotypism in atherosclerotic lesions of black women heterozygous for glucose-6-phosphate dehydrogenase (G-6-PD). Exp Mol Pathol. 1979 Dec;31(3):367–386. doi: 10.1016/0014-4800(79)90038-8. [DOI] [PubMed] [Google Scholar]
- Weeks C. E., Herrmann A. L., Nelson F. R., Slaga T. J. alpha-Difluoromethylornithine, an irreversible inhibitor of ornithine decarboxylase, inhibits tumor promoter-induced polyamine accumulation and carcinogenesis in mouse skin. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6028–6032. doi: 10.1073/pnas.79.19.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witschi H., Williamson D., Lock S. Enhancement of urethan tumorigenesis in mouse lung by butylated hydroxytoluene. J Natl Cancer Inst. 1977 Feb;58(2):301–305. doi: 10.1093/jnci/58.2.301. [DOI] [PubMed] [Google Scholar]