Abstract
Objective
Birth weight is decreasing in the US and elsewhere, even among term singletons, although trends in most maternal characteristics should contribute to increased birth weight. Some studies have attributed this decline to the simultaneous decrease in gestational length.
Methods
Using data from Intermountain Healthcare, where a successful initiative reduced the number of early term (37–38 week) elective deliveries, we examined trends in birth weight, small-for-gestational-age (SGA), and large-for-gestational-age (LGA) among 219,694 singleton infants born July 2000 to December 2008 at 37–41 weeks gestation.
Results
Over the 8.5 years, births through scheduled deliveries at 37–38 weeks decreased (9.4% to 4.4%), but overall scheduled deliveries increased (29% to 34%) and mean gestational age at birth (39.1 weeks) did not change. Mean birth weight (3410g to 3383g) and LGA (9.0% to 7.4%) both decreased, whereas SGA increased (7.5% to 8.2%). In multivariable analyses adjusting for maternal and infant characteristics, birth weight decreased (36g; 95% CI: 31, 42), especially among infants born at 37–38 weeks (40g; 30, 49) or that had medical indications for urgent deliveries (48g; 34, 63). Odds of LGA decreased (0.84; 0.80, 0.88) and odds of SGA increased (1.14; 1.08, 1.20).
Conclusion
Even in a population where gestation length did not change, birth weight and fetal growth declined. Decrease in not only gestational length but in fetal growth as well is likely to be contributing to the widely observed recent decrease in birth weight.
Introduction
From the mid to late 20th century, average birth weight increased in many countries including the United States (US)(1). However, more recent data suggest a reversal in this trend (2). In the US, mean birth weight among term (37–41 weeks) singleton infants decreased from 3441 to 3389 grams from 1990 to 2005, and the entire distribution of birth weight shifted to the left (3, 4). Similar decreases in birth weight have been seen in many other countries (5–10).
Both gestational age at birth and fetal growth contribute to birth weight. Over the past decade, mean gestational age among term births in the US has decreased by 3 days (11, 12), and changes in obstetric practice that have permitted delivery “on demand” via scheduled induction of labor or cesarean delivery are likely to have played a substantial role (13–16). The extent to which fetal growth trends are contributing to the decline in birth weight is less clear.
Two recent studies – both using US birth certificate data but with different analytic approaches – reached different conclusions. Zhang et al. (4) found that though gestational age decreased from 1995 to 2003, fetal growth was still increasing. In contrast, Donahue et al. (3) concluded that gestational age and fetal growth had both declined from 1990 to 2005. Inconsistencies in these studies show that observing trends in fetal growth is difficult in a population where gestational age, which has a large impact on birth weight, is changing as well.
One way to isolate the contribution of fetal growth to birth weight trends is to study a population in which gestational age remained stable. We therefore examined trends in birth weight and fetal growth within the Intermountain Healthcare system in Utah, where in January 2001 clinical leaders developed and implemented guidelines to discourage elective early term deliveries (17, 18). We hypothesized that despite the decrease in early elective inductions, we would observe an ongoing decrease in birth weight and fetal growth.
Material and Methods
Design, Setting and Participants
We used data extracted from the electronic medical records of 21 hospitals affiliated with Intermountain Healthcare, a vertically integrated healthcare system in Utah and Southeast Idaho. All hospitals used the StorkBytes perinatal data program, an obstetric and delivery database application that captures maternal history, labor progression, delivery and postpartum data.
The initiative at Intermountain Healthcare has been described in detail elsewhere (17, 18). Briefly, beginning in January of 2001 hospital leaders launched a quality improvement program with the intent of limiting elective deliveries before 39 completed weeks of gestation.
We obtained information on 219,757 singleton term infants born from July 1, 2000 through December 31, 2008, which includes all data currently available in the StorkBytes system. We limited our analyses to singleton infants born at 37 to 41 completed weeks of gestation to ensure our findings would not be driven by trends in multiple births and preterm deliveries, though we included the small number of post-term deliveries (n=406) in sensitivity analyses. We excluded births with missing birth weight or with birth weight inconsistent with gestational age based on the method of Alexander et al.(19) (n=29), or that had missing data on gestational age (n=44). Thus we retained information on 219,694 infants and their mothers. We performed all analyses on this population.
We based our definition of gestational age on the clinical gestational age estimate, which incorporates information from the date of the last menstrual period (LMP) as well as other factors including ultrasound dating (20). We calculated z-scores, defined small for gestational age (SGA) as fetal growth less than the 10th percentile at each completed week of gestation, and large for gestational age (LGA) as greater than 90th percentile, according to reference data based on all US births that occurred in 1999–2000 (21). We also performed sensitivity analyses using the LMP-based gestational age, and gestational age specific percentile references by LMP, although in this study population only 0.2% (n=473) of births had discordant LMP-based and clinical-based gestational age.
For all analyses we also categorized maternal characteristics as seen in Table 1. We calculated maternal body mass index (BMI, kg/m2) from self-reported pre-pregnancy weight and height, and set categories as follows: obese [BMI >= 30 kg/m2], overweight [BMI 25–<30], normal [BMI 18.5–<25], underweight [BMI<18.5]. We based gestational weight gain categories on 2009 US Institute of Medicine guidelines (22).
Table 1.
Characteristics of 219,694 mothers and their infants born in the Intermountain Healthcare System, where an Intervention to Reduce Elective Early Term Elective Deliveries Was Implemented in January 2001.
| 2000 July–Dec n=11,139 |
2004 Jan–Dec n=25404 |
2008 Jan–Dec n=29582 |
||
|---|---|---|---|---|
| Maternal Characteristics | Mean (SD) or % | |||
|
| ||||
| Age (years) | 27.0(5.4) | 27.5(5.1) | 27.7(5.2) | |
| Married | 86.9% | 88.0% | 83.6% | |
| Received No Prenatal Care | 0.20% | 0.15% | 0.18% | |
|
| ||||
| Race/Ethnicity | White | 81.5% | 84.9% | 81.3% |
| Asian | 2.1% | 2.4% | 2.5% | |
| Black | 0.5% | 0.5% | 0.6% | |
| Hispanic | 8.9% | 10.6% | 14.5% | |
| Other | 7.0% | 1.6% | 1.1% | |
|
| ||||
| Parity | 0 | 37.3% | 34.6% | 33.7% |
| 1 | 27.6% | 28.8% | 27.8% | |
| 2+ | 35.1% | 36.7% | 38.4% | |
|
| ||||
| Smoking status | Smoked during pregnancy | 6.7% | 6.7% | 6.8% |
| Former Smoker | 6.6% | 7.5% | 7.6% | |
| Never Smoker | 85.7% | 85.8% | 85.6% | |
|
| ||||
| Pre-Gestational DM or GDM | 5.5% | 6.2% | 5.1% | |
|
| ||||
| Pre-Gestational HTN or PIH | 3.9% | 4.2% | 4.5% | |
|
| ||||
| Preeclampsia or Eclampsia | 1.0% | 1.7% | 2.0% | |
|
| ||||
| Weight status | Obese | 14.1% | 15.6% | 17.0% |
| Overweight | 20.8% | 21.7% | 22.7% | |
| Normal weight | 58.2% | 57.2% | 55.4% | |
| Underweight | 6.9% | 5.5% | 4.9% | |
|
| ||||
| Gestational Weight Gain | Excessive | 41.9% | 44.3% | 44.5% |
| Inadequate | 21.0% | 19.1% | 19.1% | |
| Adequate | 37.1% | 36.6% | 36.4% | |
|
| ||||
| Infant Characteristics | Mean (SD) or % | |||
|
| ||||
| Mean Gestational Age (weeks) | 39.1(1.0) | 39.1(1.0) | 39.1(1.0) | |
| Mean Birth weight (g) | 3410 (434) | 3404(430) | 3383(426) | |
| Mean Birth Length (inches) | 19.9(1.0) | 19.8(1.0) | 19.8(1.0) | |
| Large for Gestational Age | 9.0% | 8.7% | 7.4% | |
| Small for Gestational Age | 7.5% | 7.6% | 8.2% | |
| Macrosomia (>4500g) | 1.0% | 0.8% | 0.7% | |
| Low Birth weight (<2500) | 1.6% | 1.2% | 1.6% | |
| Early Term Birth (i.e. gestational age 37 or 38 weeks) | 36.2% | 33.5% | 32.2% | |
| Scheduled Delivery | 28.7% | 34.1% | 34.1% | |
| Delivery by Cesarean Section | 14.2% | 17.7% | 19.4% | |
| Scheduled Delivery During Early Term | 9.7% | 4.8% | 4.4% | |
We classified induction status, route of delivery, and delivery status by their indications. We categorized cesarean deliveries that could have been performed at a different time if chosen as ‘scheduled cesarean sections.’ This category included all elective cesarean sections, cesarean sections after elective inductions, and cesarean sections with ‘breech position’ or ‘repeated cesarean section’ being the indication. Similarly, ‘scheduled vaginal delivery’ included all vaginal deliveries initiated by elective inductions, as well as inductions with ‘post-term pregnancy’ being the indication. We defined ‘spontaneous vaginal deliveries’ as vaginal deliveries that did not start by induction or end in cesarean section. We categorized the remaining births, i.e. deliveries which were neither spontaneous nor scheduled, were considered to have had an indication for urgent delivery, as ‘indicated cesarean deliveries’ or ‘indicated vaginal deliveries.’
Several maternal characteristics known to contribute to fetal growth had some missing data. Maternal smoking during pregnancy was added to the database later on, and therefore smoking status is missing in 38% of the data including nearly all in 2000–2002 and most of 2003. Data were also missing in small numbers of mothers for race (n=513), marital status (n=1679), age (n=79), weight or height (n=4225), gestational weight gain (n=4868), and indication for cesarean delivery (n=66). As these variables were most likely missing at random (MAR) and not associated with the missing data itself, other maternal factors nor birth weight, we used multiple imputation using a Markov Chain Monte Carlo algorithm to impute missing values. We generated 10 imputed data sets, and combined results from each complete data set to produce inferential results.
The Harvard Pilgrim Health Care Institutional Review Board determined this study was exempt from review as the previously collected dataset did not contain any personal health information.
Statistical Analysis
We calculated the distributions of maternal and infant characteristics to observe their changes over the 8.5 year period. Next, as we expected change in indication as well as timing of birth due to the intervention, we plotted the percentages of births according to delivery route and indication, for early term, full term, and all term infants, as well as the distribution of gestational age in completed weeks, for every 6 month period. To estimate trends in birth outcomes over time, we performed linear regression analyses for birth weight and z-score, and multinomial logistic regression for SGA and LGA, using separate models for each outcome. We included each half-year of birth as a categorical variable to generate estimates for every 6-month period compared with the baseline time period of July-December 2000, and also included month of birth as a continuous predictor to examine the overall change in each outcome over the study period.
Finally we used multivariable-adjusted regression models to account for trends in maternal and neonatal characteristics as well as delivery methods, which also evolved over the 8.5 year study period. All models were adjusted for available maternal and newborn characteristics shown in Table 1. We again fit separate models including period of birth (in 6 month increments) as a categorical exposure, and also as a continuous exposure.
We performed our primary analyses using multiple imputation to address the problem of missing data, which was especially notable for smoking status. We also performed secondary analyses using four other methods to address missing data: 1) a complete case analysis restricting to births in 2004–2008 (n=130,538); 2) a complete case analysis for all years 2000–2008 (n=131,591); 3) a complete case analysis excluding ‘smoking status’ (n=211,780); and 4) a complete case analysis including “missing” smoking as a separate category (n=211,780). Additionally we performed stratified analyses on early-term or full-term births, as well as among scheduled or non-scheduled deliveries.
We also performed several additional sensitivity analyses. As the intervention could have led to an increased number of post-term deliveries, we repeated our analysis including the small number of births at >=42 weeks (n=406). We also repeated our analysis on birth weight including gestational length in days (instead of completed weeks) as a categorical variable, as well as including time of birth by each month (instead of by each half-year) as a continuous variable.
We performed all analyses using SAS version 9.3 (SAS Institute, Cary, NC).
Results
In Table 1 we present information on selected maternal and neonatal characteristics for three representative time periods (the last 6 months of 2000, 2004 and 2008). Over the period mean birth weight (3410g to 3383g), and LGA (9.0% to 7.4%) both decreased, whereas SGA increased (7.5% to 8.2%). An increasing proportion of infants were born to mothers with characteristics associated with lower fetal growth: non-white race, unmarried, had preeclampsia or other hypertensive disorders of pregnancy. On the other hand, an increasing proportion of neonates were also born to mothers with characteristics associated with higher fetal growth: older age, higher parity, diabetes before or during pregnancy, higher BMI, greater gestational weight gain. Average gestational age at birth stayed stable at approximately 39 weeks over the 8.5 years.
In Figure 1 we present trends in delivery type, separately for early term and full term infants. The intervention to reduce early term elective deliveries began in January 2001. The proportion of births born at early term decreased from a high of 36.2% in July-Dec 2000, to a low of 30.1% in Jan-June 2003, and thereafter remained in the range of 32–33% (Figure 1a). Among all term births, scheduled deliveries slightly increased from 29% to 34%. Scheduled early term births before 39 weeks of gestation dropped more than half from 9.7% to 4.4%, whereas scheduled births after 39 weeks increased from 19% to 29%. Cesarean section among term births increased from 14% to 20%. Cesarean section performed during early term increased minimally from 5.5% to 6.9%, but rose more markedly among full term births from 8% to 13%, with increases in both indicated and elective cesarean deliveries. The decrease in early term births and increase in late term births, is mostly due to the shift of births at 38 weeks to 39 weeks, rather than any change in the proportion of births at 37, 40 or 41 weeks.
Figure 1.
Trends over time in delivery route and scheduling among early term (a; 37–38 completed weeks of gestation) and full term (b; 39–41 completed weeks of gestation) infants. Data from 219,694 infants born in the Intermountain Healthcare System, where an intervention to reduce elective early term elective deliveries was implemented from January 2001.
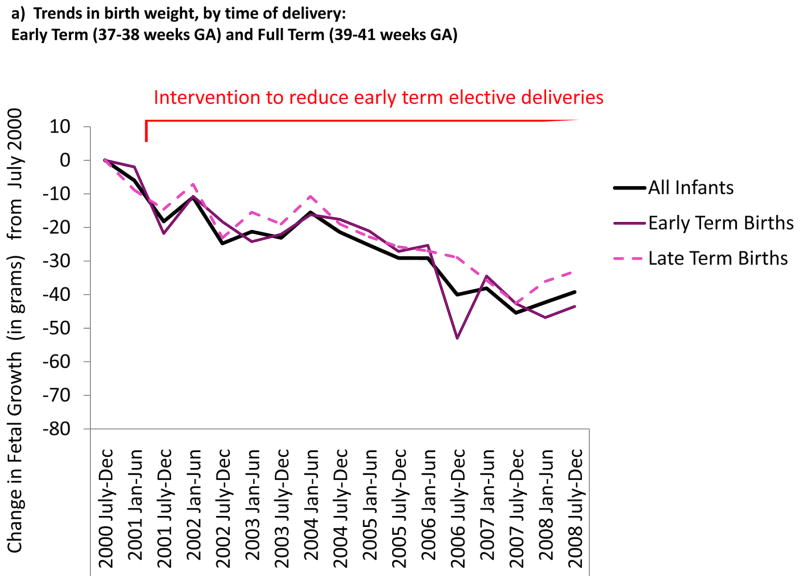
In Figure 2 we present the change in birth weight for each period of birth, adjusted for maternal and infant characteristics, compared to births July-December 2000. Birth weight decreased in all subgroups by duration of gestation and indication for delivery; i.e. both early term and full term infants, as well as scheduled, spontaneous, and medically indicated urgent deliveries.
Figure 2.
Change in birth weight, adjusted for maternal, infant, and delivery characteristics, from July–December 2000 to July–December 2008). Data from 219,694 infants born in the Intermountain Healthcare System where an intervention to reduce elective early term elective deliveries was implemented from January 2001.
In table 2 we present the estimated change in birth weight from July 2000 to December 2008, adjusted for delivery method and maternal characteristics. Over the 8.5 years, birth weight decreased by 36g (95% CI: −31, −42) and z-score by 0.08SD (95% CI: 0.07, 0.09). The estimated odds ratio for SGA was 1.12 (95% CI: 1.06, 1.19) and for LGA 0.77 (95% CI: 0.73, 0.82), for delivery in July-December 2008 compared to delivery in July-December 2000.
Table 2.
Changes in Birth weight, Fetal Growth (Birth weight for gestational age) and Odds of small (SGA) and large (LGA) for gestational age, among 219,694 Infants Born in the Intermountain Healthcare System (2000–2008)
| Change from July–December 2000 to July–December 2008 | ||||
|---|---|---|---|---|
| Birth weight (grams) | Fetal growth (z-score) | SGA Odds Ratio | LGA Odds Ratio | |
| All Infants | −36 (−42, −31) | −0.08 (−0.09, −0.07) | 1.12 (1.06, 1.19) | 0.77 (0.73, 0.82) |
| Early Term Births | −40 (−49, −30) | −0.09 (−0.11, −0.06) | 1.09 (0.99, 1.20) | 0.77 (0.70, 0.85) |
| Full Term Births | −32 (−38, −25) | −0.07 (−0.09, −0.06) | 1.13 (1.05, 1.21) | 0.79 (0.73, 0.84) |
| Scheduled Deliveries | −25 (−35, −16) | −0.06 (−0.08, −0.03) | 1.06 (0.95, 1.18) | 0.77 (0.70, 0.84) |
| Indicated Deliveries | −48 (−63, −34) | −0.11 (−0.14, −0.07) | 1.21 (1.08, 1.35) | 0.85 (0.75, 0.96) |
| Spontaneous Vaginal Deliveries | −40 (−47, −32) | −0.09 (−0.10, −0.07) | 1.13 (1.05, 1.23) | 0.75 (0.69, 0.82) |
In subgroup analyses, early term infants showed a larger decrease in birth weight (−40g; 95% CI:−30, −49) and z-score (−0.09 SD; 95% CI: −0.11, −0.06) than full term infants. Births with indications for urgent deliveries showed larger decrease in birth weight (−48g; 95% CI:−34, −63) and fetal growth (−0.11 units; 95% CI:−0.14, −0.07) compared to those born through scheduled deliveries or spontaneous vaginal deliveries.
As expected, in each model, all higher levels of maternal age, parity, pre-pregnancy BMI, or gestational weight gain, as well as taller height, longer gestational length (within term births), and diabetes before or during pregnancy, were all significantly associated with greater fetal growth. Also, non-white race and Hispanic ethnicity, smoking before or during pregnancy, preeclampsia, eclampsia, pre-pregnancy hypertension, hypertensive disorders of pregnancy, and being unmarried, were all significantly associated with less fetal growth (data not shown). The observed decreases in birth weight and LGA and increase in SGA persisted in all sensitivity analyses, whether altering the population or categorization of covariates.
Discussion
We observed a recent decline in birth weight among singleton term births in a hospital system that implemented a successful intervention to minimize early term elective deliveries. Previous analyses of birth weight trends have been complicated by the fact that gestation length decreased in parallel with birth weight, which may complicate efforts to isolate trends in fetal growth. For the present analysis, we studied a population in which mean gestational age did not change. Thus, any decrease in birth weight must result from reduced fetal growth.
Gestation length is notoriously difficult to get exactly right. We repeated our analyses using both LMP-based and clinical-based LMP, and using two reference datasets for our calculations of fetal growth based on either clinical or LMP dating. All methods showed similar results. Our results suggest that infants are becoming smaller independent of any trend in gestational length.
The cause of this decline in fetal growth remains unknown. Maternal and infant characteristics in our dataset that are known to contribute to fetal growth did not explain the observed decline in fetal growth, as adjustment for these variables did not eliminate the observed declines. This is concordant with the fact that trends of most maternal factors have not changed direction from the 1990s when birth weight was increasing. We also included maternal pre-pregnancy BMI, which was not available on US birth certificates until recently and thus not included in previous studies (1). However, obesity rates have continued to rise over recent decades, and maternal BMI is directly associated with fetal growth. We found, as expected, that adjusting for maternal BMI resulted in even stronger temporal decreases in birth weight.
Environmental, behavioral or nutritional factors not addressed on birth certificates may be contributing to the observed decrease in fetal growth. Unmeasured factors such as environmental toxicants including lead, mercury, and persistent organic pollutants, and also psychosocial stressors, may influence fetal growth (25, 26). Fetal growth has been also related to maternal prenatal intake of fatty acids (27–29), such as the omega-3 fatty acids found in fish, and trans fatty acids. Even though maternal BMI and presumably total energy intake is increasing, diet quality may declining, as studies show those obese have poorer micronutrient intake despite greater intake of total energy (30).
Our finding that recorded maternal and infant characteristics and also trends in obstetric care did explain the decrease in fetal growth calls for urgent research to identify attributable factors. Though a 36 gram decrease in a single infant may seem minimal, a recent study found that a 70g decrease in birth weight among girls born during an economic depression was associated with higher blood glucose (0.16 mmol/L, 95% CI: 0.07, 0.23) and greater odds of obesity 1.43 (95% CI: 1.01, 2.02) in adulthood. Furthermore, 10% increase in risk of being born SGA is a concern from public health perspectives. Numerous studies show SGA infants have increased risks for not only neonatal morbidity and mortality(31), but also for obesity, coronary heart disease, stroke, hypertension and type 2 diabetes(32–35) throughout the lifespan. Without elucidating the contributors to declining fetal growth, it is difficult to understand its implications and plan to intervene.
In addition, we note that although the Intermountain Healthcare initiative dramatically reduced early term elective deliveries, overall elective deliveries and use of cesarean section still increased. The American College of Obstetricians and Gynecologists recommended in 2007 that labor should be electively induced only after 39 weeks of gestation have been completed (36, 37). Our study suggests that though implementation of these guidelines may reduce early term elective deliveries, it is unlikely to reverse the climbing rates of elective deliveries in general.
There are several limitations to our study. As we used a pre-existing database and did not validate measures by chart review, there may have been misclassification or measurement error. However we anticipate any errors would have been consistent over time. Missing data were minimal except on smoking status. However, we found similar results using a number of approaches to account for these missing data. Our study was based on a multi-center healthcare system covering 23 hospitals in Utah and Southeast Idaho, and a greater proportion of mothers were white compared with many other regions. Therefore findings may not be generalizable to populations elsewhere. However, as decrease in fetal growth was consistent over racial/ethnic subgroups in the national data analysis by Donahue et al, we anticipate that this observed decrease is not unique to our population.
Increasing elective inductions and subsequently shorter gestational length most likely do not fully explain the widely observed decrease in birth weight in term infants. No commonly measured maternal or infant factors can account for this decrease in fetal growth. Future research focused on the determinants of declining fetal growth is needed.
Acknowledgments
This study was supported by a grant from the National Institutes of Health (K24 HD069408).
References
- 1.Kramer MS, Morin I, Yang H, Platt RW, Usher R, McNamara H, et al. Why are babies getting bigger? Temporal trends in fetal growth and its determinants. J Pediatr. 2002 Oct;141(4):538–42. doi: 10.1067/mpd.2002.128029. [DOI] [PubMed] [Google Scholar]
- 2.Oken E. Secular Trends in Birth weight. Nestlé Nutrition Institute Workshop Series; 2012. In Press. [DOI] [PubMed] [Google Scholar]
- 3.Donahue SM, Kleinman KP, Gillman MW, Oken E. Trends in birth weight and gestational length among singleton term births in the United States: 1990–2005. Obstet Gynecol. 2010 Feb;115(2 Pt 1):357–64. doi: 10.1097/AOG.0b013e3181cbd5f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Joseph KS, Kramer MS. Decreased term and postterm birth weight in the United States: impact of labor induction. Am J Obstet Gynecol. 2010 Aug;203(2):124, e1–7. doi: 10.1016/j.ajog.2010.03.044. [DOI] [PubMed] [Google Scholar]
- 5.Edwards NM, Audas RP. Trends of abnormal birth weight among full-term infants in Newfoundland and Labrador. Can J Public Health. Mar-Apr;101(2):138–42. doi: 10.1007/BF03404359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diouf I, Charles MA, Blondel B, Heude B, Kaminski M. Discordant time trends in maternal body size and offspring birth weight of term deliveries in France between 1972 and 2003: data from the French National Perinatal Surveys. Paediatr Perinat Epidemiol. 2011 May;25(3):210–7. doi: 10.1111/j.1365-3016.2010.01188.x. [DOI] [PubMed] [Google Scholar]
- 7.Lahmann PH, Wills RA, Coory M. Trends in birth size and macrosomia in Queensland, Australia, from 1988 to 2005. Paediatr Perinat Epidemiol. 2009 Nov;23(6):533–41. doi: 10.1111/j.1365-3016.2009.01075.x. [DOI] [PubMed] [Google Scholar]
- 8.Schack-Nielsen L, Molgaard C, Sorensen TI, Greisen G, Michaelsen KF. Secular change in size at birth from 1973 to 2003: national data from Denmark. Obesity (Silver Spring) 2006 Jul;14(7):1257–63. doi: 10.1038/oby.2006.143. [DOI] [PubMed] [Google Scholar]
- 9.Lu Y, Zhang J, Lu X, Xi W, Li Z. Secular trends of macrosomia in southeast China, 1994–2005. BMC Public Health. 11:818. doi: 10.1186/1471-2458-11-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohmi H, Hirooka K, Hata A, Mochizuki Y. Recent trend of increase in proportion of low birth weight infants in Japan. Int J Epidemiol. 2001 Dec;30(6):1269–71. doi: 10.1093/ije/30.6.1269. [DOI] [PubMed] [Google Scholar]
- 11.Martin JA, Kirmeyer S, Osterman M, Shepherd RA. Born a bit too early: recent trends in late preterm births. NCHS Data Brief. 2009 Nov;(24):1–8. [PubMed] [Google Scholar]
- 12.Davidoff MJ, Dias T, Damus K, Russell R, Bettegowda VR, Dolan S, et al. Changes in the gestational age distribution among U.S. singleton births: impact on rates of late preterm birth, 1992 to 2002. Semin Perinatol. 2006 Feb;30(1):8–15. doi: 10.1053/j.semperi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Murthy K, Grobman WA, Lee TA, Holl JL. Trends in induction of labor at early-term gestation. Am J Obstet Gynecol. 2011 May;204(5):435, e1–6. doi: 10.1016/j.ajog.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 14.ACOG. ACOG Committee Opinion No. 394, December 2007. Cesarean delivery on maternal request. Obstet Gynecol. 2007 Dec;110(6):1501. doi: 10.1097/01.AOG.0000291577.01569.4c. [DOI] [PubMed] [Google Scholar]
- 15.Reddy UM, Ko CW, Raju TN, Willinger M. Delivery indications at late-preterm gestations and infant mortality rates in the United States. Pediatrics. 2009 Jul;124(1):234–40. doi: 10.1542/peds.2008-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldenberg RL, McClure EM, Bhattacharya A, Groat TD, Stahl PJ. Women’s perceptions regarding the safety of births at various gestational ages. Obstet Gynecol. 2009 Dec;114(6):1254–8. doi: 10.1097/AOG.0b013e3181c2d6a0. [DOI] [PubMed] [Google Scholar]
- 17.Oshiro BT, Henry E, Wilson J, Branch DW, Varner MW. Decreasing elective deliveries before 39 weeks of gestation in an integrated health care system. Obstet Gynecol. 2009 Apr;113(4):804–11. doi: 10.1097/AOG.0b013e31819b5c8c. [DOI] [PubMed] [Google Scholar]
- 18.Oshiro BT, Berns SD. Quality improvement opportunities to prevent preterm births. Clin Perinatol. 2011 Sep;38(3):565–78. doi: 10.1016/j.clp.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996 Feb;87(2):163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 20.Mathews TJ, Minino AM, Osterman MJ, Strobino DM, Guyer B. Annual summary of vital statistics: 2008. Pediatrics. 2010 Jan;127(1):146–57. doi: 10.1542/peds.2010-3175. [DOI] [PubMed] [Google Scholar]
- 21.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003 Jul 8;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weight Gain During Pregnancy: Reexamining the Guidelines. National Academies Collection; 2009. [PubMed] [Google Scholar]
- 23.Ananth CV. Menstrual versus clinical estimate of gestational age dating in the United States: temporal trends and variability in indices of perinatal outcomes. Paediatr Perinat Epidemiol. 2007 Sep;21( Suppl 2):22–30. doi: 10.1111/j.1365-3016.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 24.Dietz PM, England LJ, Callaghan WM, Pearl M, Wier ML, Kharrazi M. A comparison of LMP-based and ultrasound-based estimates of gestational age using linked California livebirth and prenatal screening records. Paediatr Perinat Epidemiol. 2007 Sep;21( Suppl 2):62–71. doi: 10.1111/j.1365-3016.2007.00862.x. [DOI] [PubMed] [Google Scholar]
- 25.Karagas MR, Choi AL, Oken E, Horvat M, Schoeny R, Kamai E, et al. Evidence on the human health effects of low-level methylmercury exposure. Environ Health Perspect. 2012 Jun;120(6):799–806. doi: 10.1289/ehp.1104494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDiarmid MA, Gehle K. Preconception brief: occupational/environmental exposures. Matern Child Health J. 2006 Sep;10(5 Suppl):S123–8. doi: 10.1007/s10995-006-0089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al MD, van Houwelingen AC, Hornstra G. Long-chain polyunsaturated fatty acids, pregnancy, and pregnancy outcome. Am J Clin Nutr. 2000 Jan;71(1 Suppl):285S–91S. doi: 10.1093/ajcn/71.1.285s. [DOI] [PubMed] [Google Scholar]
- 28.Olsen SF, Hansen HS, Sorensen TI, Jensen B, Secher NJ, Sommer S, et al. Intake of marine fat, rich in (n-3)-polyunsaturated fatty acids, may increase birth weight by prolonging gestation. Lancet. 1986 Aug 16;2(8503):367–9. doi: 10.1016/s0140-6736(86)90055-3. [DOI] [PubMed] [Google Scholar]
- 29.Cohen JF, Rifas-Shiman SL, Rimm EB, Oken E, Gillman MW. Maternal trans fatty acid intake and fetal growth. Am J Clin Nutr. 2011 Nov;94(5):1241–7. doi: 10.3945/ajcn.111.014530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rifas-Shiman SL, Rich-Edwards JW, Kleinman KP, Oken E, Gillman MW. Dietary quality during pregnancy varies by maternal characteristics in Project Viva: a US cohort. J Am Diet Assoc. 2009 Jun;109(6):1004–11. doi: 10.1016/j.jada.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McIntire DD, Bloom SL, Casey BM, Leveno KJ. Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med. 1999 Apr;340(16):1234–8. doi: 10.1056/NEJM199904223401603. [DOI] [PubMed] [Google Scholar]
- 32.Forsén T, Eriksson JG, Tuomilehto J, Osmond C, Barker DJ. Growth in utero and during childhood among women who develop coronary heart disease: longitudinal study. BMJ. 1999 Nov;319(7222):1403–7. doi: 10.1136/bmj.319.7222.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singhal A, Lucas A. Early origins of cardiovascular disease: is there a unifying hypothesis? Lancet. 2004 May;363(9421):1642–5. doi: 10.1016/S0140-6736(04)16210-7. [DOI] [PubMed] [Google Scholar]
- 34.Barker DJ. Adult consequences of fetal growth restriction. Clin Obstet Gynecol. 2006 Jun;49(2):270–83. doi: 10.1097/00003081-200606000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003 Apr;11(4):496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 36.ACOG. ACOG Practice Bulletin No. 107: Induction of labor. Obstet Gynecol. 2009 Aug;114(2 Pt 1):386–97. doi: 10.1097/AOG.0b013e3181b48ef5. [DOI] [PubMed] [Google Scholar]
- 37.American College of Obstetrics and Gynecology. ACOG Committee Opinion No. 394, December 2007. Cesarean delivery on maternal request. Obstet Gynecol. 2007 Dec;110(6):1501. doi: 10.1097/01.AOG.0000291577.01569.4c. [DOI] [PubMed] [Google Scholar]