Abstract
The transmembrane protein Crumbs/Crb is a key regulator of apico-basal epithelial cell polarity, both in Drosophila and in vertebrates. In most cases studied so far, the apical localisation of Drosophila Crumbs depends on the interaction of its C-terminal amino acids with the scaffolding protein Stardust. Consequently, embryos lacking either Crumbs or Stardust develop a very similar phenotype, characterised by the loss of epithelial tissue integrity and cell polarity in many epithelia. An exception is the hindgut, which is not affected by the loss of either gene. The hindgut is a single layered epithelial tube composed of two cell populations, the boundary cells and the principal cells. Here we show that Crumbs localisation in the principal cells depends on Stardust, similarly to other embryonic epithelia. In contrast, localisation of Crumbs in the boundary cells does not require Stardust and is independent of its PDZ domain- and FERM-domain binding motifs. In line with this, the considerable upregulation of Crumbs in boundary cells is not followed by a corresponding upregulation of its canonical binding partners. Our data are the first to suggest a mechanism controlling apical Crumbs localisation, which is independent of its conserved FERM- and PDZ-domain binding motifs.
Introduction
A hallmark of epithelial cell polarity is the separation of the plasma membrane into an apical side facing the outside or a lumen, and a baso-lateral side, which makes contact with the neighbouring cells and/or the basal membrane. The zonula adherens (ZA), an adhesion belt surrounding the apex of epithelial cells, marks the boundaries between them. The apico-basal subdivision of the plasma membrane becomes manifest by the uneven distribution of various proteins, many of which serve membrane domain-specific functions. Proper targeting of proteins to and their maintenance in the respective membrane is of utmost importance for epithelial development and homeostasis. Mechanisms controlling these processes include exo- and endocytosis, protein-protein and protein-lipid interactions to stabilise proteins in the membrane, or recycling and degradation of proteins. In addition, the synthesis of the right amounts of membrane-specific proteins, their modifications and proper targeting are important regulators of apico-basal polarity [reviewed in [1], [2], [3], [4], [5]].
One of the key regulators of epithelial polarity in the Drosophila embryo is the Crumbs protein complex, the core components of which are the transmembrane protein Crumbs (Crb) and the scaffolding proteins Stardust (Sdt), DLin-7 and DPATJ. Other components, such as DPar6, a member of the Par protein group, or Yurt, a negative regulator of Crb, can be transiently recruited into the complex [reviewed in [6], [7]]. crb and sdt mutant embryos are unable to maintain apico-basal polarity in many of their epithelia. This eventually results in a complete breakdown of tissue integrity due to a failure to position and maintain the ZA, followed by apoptosis in some tissues, e.g. the epidermis [8], [9], [10], [11]. Similar defects in epithelial integrity are observed in mice lacking Crb2 or Crb3 [12], [13]. Conversely, overexpression of Drosophila Crb can lead to an expansion of the apical membrane domain, both in embryonic epithelial cells [14] and in photoreceptor cells [15], [16], [17]. These results suggest that the amount of Crb has to be tightly regulated in order to maintain the proper size and differentiation of the apical membrane.
So far, little is known about the mechanisms that ensure the proper levels of Crb and other members of the complex at the apical membrane and hence the balance between apical and baso-lateral membrane domains. Exo84, a component of the exocyst, and the retromer, which controls recycling of Crb, as well as Cdc42 and Rab11 are essential for localising and maintaining Crb on the apical surface [18], [19], [20], [21], [22]. In most epithelial tissues of the Drosophila embryo a direct interaction between the C-terminal ERLI motif of the short cytoplasmic tail of Crb and the PDZ (PSD-95/Discs-large/ZO-1)-domain of Sdt is essential for the localisation of both proteins in the subapical region (SAR), a portion of the apical plasma membrane just apical to the ZA. Loss of either crb or sdt results in the loss of the respective other protein from the apical membrane and thus to a very similar mutant embryonic phenotype [9], [23], [24].
Strikingly, the Drosophila embryonic hindgut does not show any obvious defect in polarity or morphogenesis in crb or sdt mutant embryos, although it expresses the Crb complex from early on. The hindgut is a single layered epithelial tube, which is subdivided – from anterior to posterior - into the small intestine, the large intestine and the rectum [reviewed in [25]]. The large intestine is additionally patterned along the dorso-ventral axis, with the dorsal and ventral compartments separated by a single row of epithelial cells, called the boundary cells (BCs). These three compartments can be distinguished by the morphology of their cells and different gene expression patterns, but their specific functions later on are only partially understood. While the engrailed-expressing dorsal cells become specialized for water and ion absorption [26], a specific function of the engrailed-negative, Delta-expressing ventral cells has not yet been described. The BCs not only express several transcription factors distinct from those expressed in the dorsal and ventral compartment, but also exhibit much higher levels of Crb on their apical surface in comparison to the neighbouring, principal cells (PCs) [27], [28], [29]. In addition, BCs are more elongated than PCs and develop more pronounced apical microvilli [29]. Hence, the Drosophila large intestine provides an ideal system not only to study pattern formation, but also to unravel the requirement for cell-type specific differentiation and morphogenesis of epithelial cells in a single epithelia tube. In particular, the previously demonstrated link between Crb abundance and apical differentiation motivated us to study in more detail the requirement of this polarity regulator for BC differentiation. Here we show that BCs use a so far not described, Sdt-independent mechanism to accumulate Crb on the apical surface.
Materials and Methods
Flies were kept at 25°C. The following stocks/mutant alleles were used: OregonR as wild-type control, crb11A22 [30], crbGX24 [31], crb8F105 [30], [32] foscrbY10A,ΔERLI; crbGX24 [33], UAS-crb30.12e [14] called UAS-crbfull here, en-Gal4 [34]. Mutant stocks were balanced over TM3, Twist-Gal4, UAS-EGFP (Bloomington Stock Center).
Immunohistochemistry
Embryo collection, fixation and antibody staining were conducted as previously described [33]. The following primary antibodies were used: rabbit anti-Baz (1∶100) [35], rat anti-Crb 2.8 (1∶1000) [36], rabbit anti-Crb intra (1∶400 raised against the peptide NKRATRGTYSPSAQE; unpublished), rabbit anti-DPATJ (1∶1000) [36], mouse anti-Dlg 4F3 (1∶400; Developmental Studies Hybridoma Bank [DSHB]), rabbit anti-Lgl (1∶100) [37], rabbit anti-DLin-7 (1∶100) [38], rat anti-DPar6 (1∶500; kindly provided by A. Wodarz), rabbit anti-PKCζ C20 (1∶400; Santa Cruz Biotechnology), rabbit anti-Sdt-PDZ (1∶500) [39], rabbit anti-Sas (1∶500; kindly provided by E. Organ and D. Cavener), rabbit anti-Scrib (kindly provided by D. Bilder), mouse anti-α-Spectrin SA9 (1∶400; DSHB). Secondary antibodies used in this study were conjugated to Alexa Flour 488, −568 and −647 (Life Technologies). Stained embryos were mounted in glycerine propyl gallate (75% glycerol, 50 mg/ml propyl gallate).
Cryosections were prepared from fixed and stained embryos. For cryopreservation specimens were first incubated in 10% sucrose for 30 min at room temperature, and then in 25% sucrose over night at 4°C. Embryos were embedded in tissue-freezing medium (NEG50, Thermo Scientific), frozen on dry ice and stored at −80°C. Cryosections (10 μm) were made with a Microm Cryo-Star HM560M, collected on coated glass slides (Marienfeld) and mounted in DABCO-containing (Sigma) Mowiol (Calbiochem).
Images were taken with a LSM Zeiss 510 using a Zeiss Plan-Achromat 63x lens. All quantifications were performed using Fiji software [40]. To measure the fluorescence intensity of Crb in BCs and PCs a region of interest (ROI) was defined around the respective cell as well as in an area without fluorescent objects, which was used for background subtraction. Whole cell signal corrected per area was calculated using the following formula: (whole cell signal – area of selected cell x mean fluorescence of background readings) / area of selected cell. For the analysis of each cell type 18 regions of six individual hindguts were selected. The statistical significance was assessed by two-sided Student's t-test in Microsoft Excel. Colocalization analyses were performed using the JACOP plugin of the Fiji software [41].
For colocalization analysis in the BCs a section was used that showed a lateral view of the hindgut. The ROI was drawn on the apical surface of individual BCs. For colocalization analysis in PCs a section was used that showed a longitudinal section through the hindgut tube. The ROI was drawn on the SAR of the PCs. For the analysis of each cell type 12 regions of four individual hindguts were selected. Box graphs and statistical analysis were performed using Microsoft Excel. For image processing and analysis Fiji and Adobe Photoshop CS5 were used and Adobe Illustrator CS5 for image assembly.
Transmission Electron Microscopy
Sections were prepared as described in [42] with modifications. In brief, fixation of devitellinized embryos in 0.1 M phosphate buffer (pH 7.2) was performed in 2.5% glutaraldehyde, followed by fixation in 1% osmium tetroxide/2% glutaraldehyde, followed by 2% OsO4. After dehydration embryos were embedded in Araldite. Semithin sections (2.5 μm) and ultra thin sections (70 nm) were prepared with the Leica Ultracut UCT microtome. Ultrathin sections were contrasted and analysed with a FEI Tecnai 12 Bio Twin. Microvilli length was measured using Fiji software and statistical analysis was performed using Microsoft Excel. For image processing and analysis Fiji software and Adobe Photoshop CS5 were used and Adobe Illustrator CS5 for image assembly. The statistical significance was assessed by two-sided Student's t-test in Microsoft Excel.
Results
Crb, but not other members of the Crb complex is upregulated in the BCs
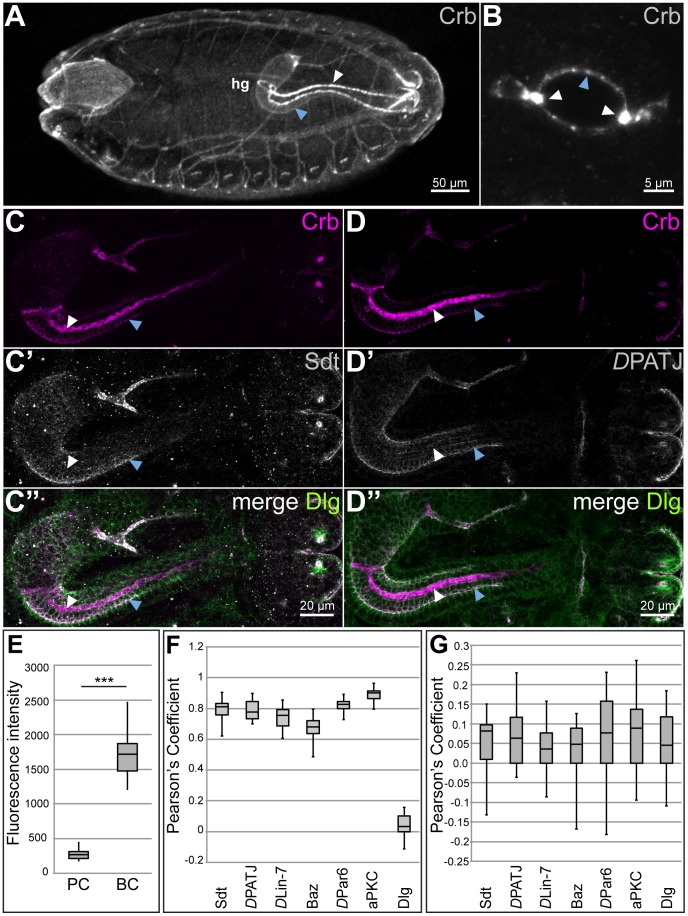
crb RNA and protein have been shown to be strongly upregulated in BCs of the Drosophila embryonic hindgut [27], [28], [29]. Crb protein is spread across the apical pole of the BCs in stage 15 (Fig. 1A, B, white arrowheads) and older (stage 16) embryos [33]. In contrast, the majority of the hindgut cells, which we will call principle cells (PCs) from now on, express low levels of Crb, which is restricted to the subapical region (SAR), apical to the zonula adherens (ZA), as in most other embryonic epithelia (Fig. 1A, B, blue arrowheads). Crb is about 6.5 fold more abundant in BCs than in PCs (Fig. 1E).
Figure 1. Crb, but not the Crb complex members Sdt and DPATJ, is enriched in the BCs of the embryonic hindgut.
(A–D″) Confocal microscope images of stage 15 wild-type embryos. (A) Dorsal view of a whole mount embryo stained with anti-Crb, showing apical localisation in the PCs (blue arrowhead) and strong enrichment in the BCs (white arrowhead) of the hindgut (hg). Anterior is left. (B) Cross section through the large intestine stained with anti-Crb to show strong apical accumulation of Crb in the BCs (white arrowheads) and SAR localisation in the PCs (blue arrowhead). (C–D″) Confocal microscope images of embryonic hindguts stained with anti-Crb (magenta in C, C″, D and D″), anti-Sdt (grey in C′ and C″) and anti-DPATJ (grey in D′ and D″) as well as anti-Dlg (green in C″ and D″). BCs (white arrowheads) accumulate Crb (C, C″, D, D″) but not Sdt (C′ and C″) or DPATJ (D′, and D″). PCs (C–D″, blue arrowheads) localise Crb (C, C″, D, D″), Sdt (C′ and C″) and DPATJ (D′ and D″) in the SAR. (E) Box Plot showing the fluorescence intensity of anti-Crb staining in PCs and BCs of stage 15 wild-type embryos. The line within the box represents the median value; the whiskers represent the maximum and minimum values; *** indicate p-value <0.001, assessed by two-sided Student's t-test.(F, G) Box Plot showing the Pearson's correlation coefficient of Crb and Sdt, DPATJ, DLin-7, Baz, DPar6, aPKC and Dlg in PCs (F) and BCs (G) of stage 15 wild-type embryos. The line within the box represents the median value; the whiskers represent the maximum and minimum values. Note the difference in the scale of the Pearson's correlation coefficient in F and G.
The short cytoplasmic domain of Crb is required to localise other members of the Crb complex to the SAR by direct interaction between the C-terminal ERLI motif of Crb and the PDZ domain of Sdt. Consequently, in many epithelia loss of crb results in loss of the scaffolding core components of the Crb complex, Sdt, DPATJ and DLin-7. Furthermore, overexpression of the membrane bound intracellular domain of Crb can recruit other Crb complex members to ectopic sites, but only in the presence of an intact PDZ-domain binding motif [17], [43]. Therefore we asked, whether upregulated Crb in the BCs also results in the upregulation of Sdt, DPATJ and DLin-7. Unlike Crb, none of the three proteins is upregulated in the BCs, but all show a similar level of expression as in PCs (Fig. 1C′, C″ and 1D′, D″ and data not shown) and co-localise with Crb, as demonstrated by determining the Pearson's Correlation Coefficient (Fig. 1F). None of them is spread on the apical pole of the BCs, but all are restricted to the SAR. This result is in contrast to data published previously (although not shown) [27], arguing that DPATJ (in this paper still called Discs Lost, Dlt) is upregulated in the BCs. From our data we conclude that, unlike in most epithelial cells, in which the regulation of the amount of Crb and Sdt seem to be tightly coupled [23], [24], [44], the increased level of Crb in the BCs is not associated with a corresponding increase of other core components of the complex.
Loca lisation of other polarity regulators is not altered in the BCs
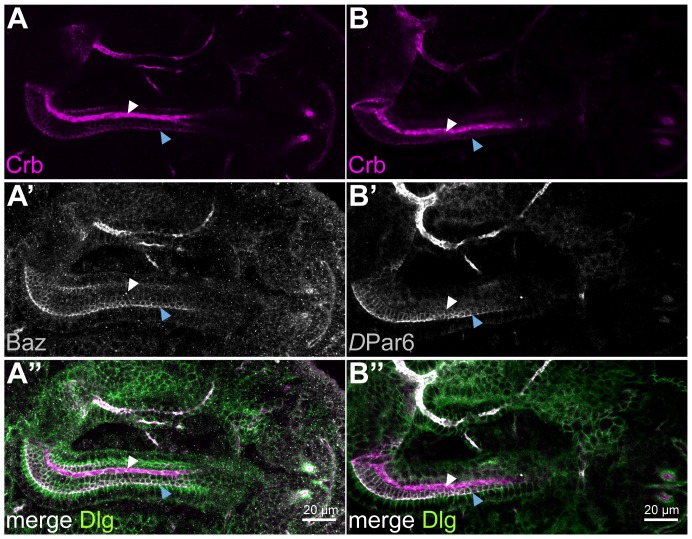
Beside the Crb complex, other proteins and protein complexes are required for the establishment and maintenance of apico-basal polarity in epithelial cells. Amongst these are the scaffolding proteins Bazooka (Baz), the Drosophila orthologue of Par3, DPar6 and the atypical protein kinase C (aPKC). Baz often, but not always, forms a complex with the DPar6/aPKC heterodimer at the SAR, occasionally overlapping with the ZA [45], [46], [47], [48]. Several results suggest a close connection between members of the Crb and the Baz/DPar6/aPKC complexes [see [7], [49] for recent reviews]. For example, the single PDZ-domain of DPar6/Par6 can directly bind to the C-terminus of Crb/CRB3 [50], [51]. In the BCs of the hindgut, however, neither DPar6 nor Baz or aPKC are upregulated. All three are restricted to the SAR as in PCs (Fig. 2A′-A″, B′-B″ and data not shown), where they co-localise with Crb (Fig. 1F).
Figure 2. Localisation of apical and baso-lateral polarity proteins is not altered in BCs.
Confocal microscope images of stage 15 wild-type embryonic hindguts (BCs: white arrowheads, PCs: blue arrowheads). Anterior is left. (A–A″) Hindgut stained for the polarity markers Crb (magenta), Baz (grey) and Dlg (green). Only Crb is upregulated in the BCs (A and A″), while Baz (A′ and A″) and Dlg (A″) show the same amount and localisation as in the PCs. (B–B″) Hindgut stained for the polarity markers Crb (magenta) and DPar6 (green). Crb is enriched in the BCs (B and B″) but DPar6 localises only to the SAR as in the PCs (B′ and B″).
Members of the conserved Scribble (Scrib) module, including the multi PDZ-domain protein Scrib, the membrane-associated guanylate kinase (MAGUK) Discs large (Dlg) and the WD40-domain protein Lethal giant larvae (Lgl), localise at the baso-lateral membrane of many epithelial cells and antagonise the function of apical regulators [52], [53], [54] [see [55], [56] for recent reviews]. In the hindgut, all three proteins are localised at the baso-lateral membrane, both in BCs and PCs (Fig. 2A″ and data not shown). To summarise, only the transmembrane protein Crb, but none of the other known polarity regulators tested, is upregulated in the BCs of the embryonic hindgut.
Crb stabilisation in the apical membrane domain of the BCs is independent of Sdt
In most embryonic epithelial cells Crb and Sdt are strongly dependent on each other with respect to their amount, localisation and stability [23], [24], [44]. As a consequence, loss of either crb or sdt results in the same mutant phenotype [9], [30], [57]. Given the observation that the upregulation of Crb is not reflected by an upregulation of Sdt in the BCs of the hindgut, we asked whether stabilization of Crb in these cells depends on Sdt at all. Therefore, we studied localization of Crb in embryos mutant for sdtK85, a complete loss of function allele [39]. In the BCs of homozygous sdtK85 embryos, Crb protein is still apically localised and strongly upregulated. In contrast, Crb is not detectable in the SAR in the PCs of the hindgut, as has been described for most epithelia (Fig. 3A–A″; compare with Fig. 1D). The hindgut tube remains single layered, as visualized by α-Spectrin staining, and cells maintain proper apico-basal polarity, as shown by apical localisation of Stranded-at-Second (Sas), a marker for the apical membrane [14] (Fig. 3). In conclusion, Sdt is not required for apico-basal polarity of the hindgut epithelium, or for the stabilisation of Crb in the apical membrane of the BCs.
Figure 3. Apical localisation of Crb in BCs is independent of its known interaction partner Sdt.
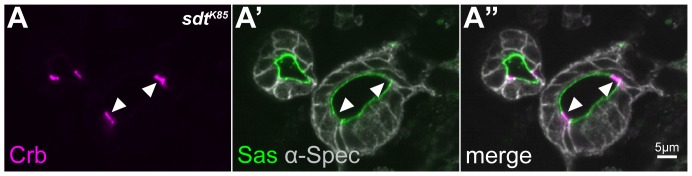
Confocal microscopy images of a cross section through the large intestine of a stage 15 homozygous sdtK85 mutant embryo stained with anti-Crb (magenta), anti-α-Spectrin (grey) and anti-Sas (green). Crb is upregulated and apically localised in the BCs (white arrowheads), but is not detectable in the SAR of the PCs (A, A″). Sas, an apical marker of the monolayered epithelial tube, is reduced in the BCs (A′, A″).
Crb stabilisation in the apical membrane domain is independent of interactions via its PDZ- and FERM-domain binding motifs
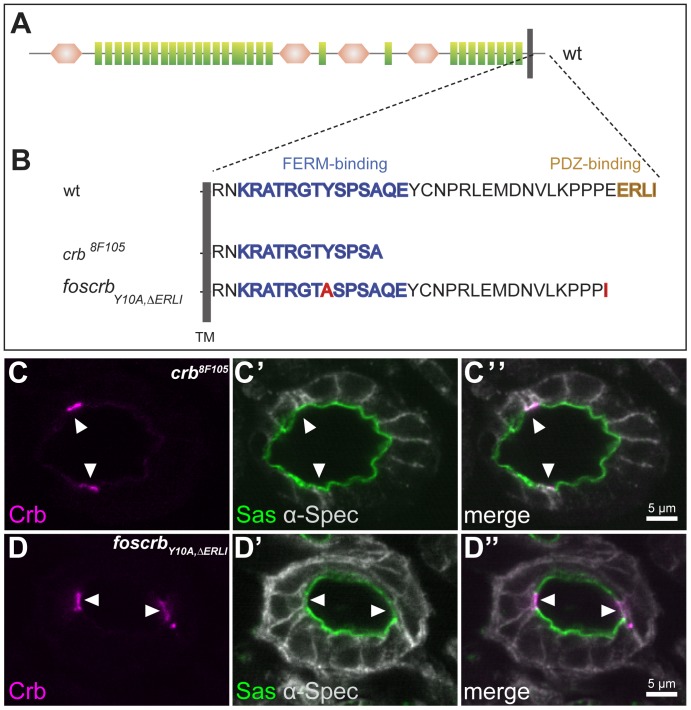
The cytoplasmic domain of Crb contains two protein-protein interaction domains, a C-terminal PDZ-domain binding motif, -ERLI, and a FERM (protein 4.1/ezrin/radixin/moesin)-domain binding motif [43], [58]. Given the observation that the apical enrichment of Crb in the BCs is independent of Sdt, we were interested to know whether another protein containing a PDZ-domain could be involved in Crb stabilisation in these cells. To address this question, we studied Crb localisation in the allele crb8F105. A point mutation in this allele induces a premature stop codon, resulting in the synthesis of a truncated Crb protein that lacks the last 23 amino acids of the intracellular domain, including the PDZ-domain binding motif (Fig. 4B). Yet, the phenotype of homozygous crb8F105 mutant embryos resembles that of complete loss of function alleles [32]. The truncated Crb protein produced in homozygous mutant crb8F105 embryos is not apically localised in the majority of epithelial cells [32], including the PCs (Fig. 4C–C″). In contrast, the truncated protein is still enriched in the apical pole of the BCs (Fig. 4C–C″, white arrowheads).
Figure 4. Localisation of Crb in BCs is independent from its known protein binding motifs.
(A) Schematic representation of the Crb protein and its variants used in this study. Green rectangles: EGF-like repeats, brown hexagons: laminin A G like domains, grey bar: transmembrane domain (TM). (B) Amino acid sequences of the cytoplasmic tails of wild-type and mutant Crb proteins used in this study. Blue: FERM domain-binding motif, brown: PDZ domain-binding motif. Red: point mutations. (C–D″) Confocal microscopy images of cross sections through the large intestine of stage 15 homozygous crb8F105 (C–C″) and foscrbY10A,ΔERLI (D–D″) embryos stained with anti-Crb (magenta), anti-α-Spectrin (grey) and anti-Sas (green). Crb is upregulated and apically localised in the BCs (white arrowheads), but is not detectable in the PCs.
The second well-established protein-protein interaction domain of the cytoplasmic tail of Crb is a conserved FERM-domain binding motif. In Drosophila, two binding partners have been identified so far, the FERM proteins Yurt and Expanded [58], [59]. To address whether the accumulated Crb protein in the BCs is stabilised via interactions through its FERM-domain binding site, we studied the localisation of Crb in embryos lacking endogenous crb, but containing a transgene encoding a Crb protein with mutations in the FERM-domain binding and PDZ-domain binding motifs, called foscrbY10A,ΔERLI. The Crb protein encoded by this transgene carries an exchange of a conserved tyrosine residue in the FERM-domain binding motif by an alanine (Y10A). In addition, the PDZ-domain binding motif is removed (ΔERLI) (Fig. 4B). This mutant protein is unable to rescue any defect of crb mutant embryos, and the phenotype of foscrbY10A,ΔERLI; crb mutant embryos resembles that of embryos with no functional crb gene, in that epithelial integrity is lost in most embryonic tissues [33]. In the hindgut, the mutant Crb protein is accumulated and stabilised in the apical membrane domain of the BCs (Fig. 4D–D″), while no localised signal was detected in the PCs. As in other crb alleles, the hindgut maintains its monolayered tubular structure and proper apico-basal polarity in crb8F105 and foscrbY10A,ΔERLI; crb mutant embryos, as revealed by proper apical localisation of Sas (Fig. 4C′, C″, D′, D″, green staining).
From these results we conclude, that, unlike in most embryonic epithelia, the PDZ-domain binding motif of Crb is not required for its stabilisation and enrichment on the apical membrane of the BCs. This observation excludes Sdt, DPar6 and other PDZ-domain containing proteins as candidates for its stabilisation. Similarly, apical accumulation of Crb in BCs does not depend on an intact FERM-domain binding motif. These results suggest that the BCs use a different way to stabilise Crb on the apical surface, which is independent of its known interactors.
Loss of crb in BCs affects the length of microvilli
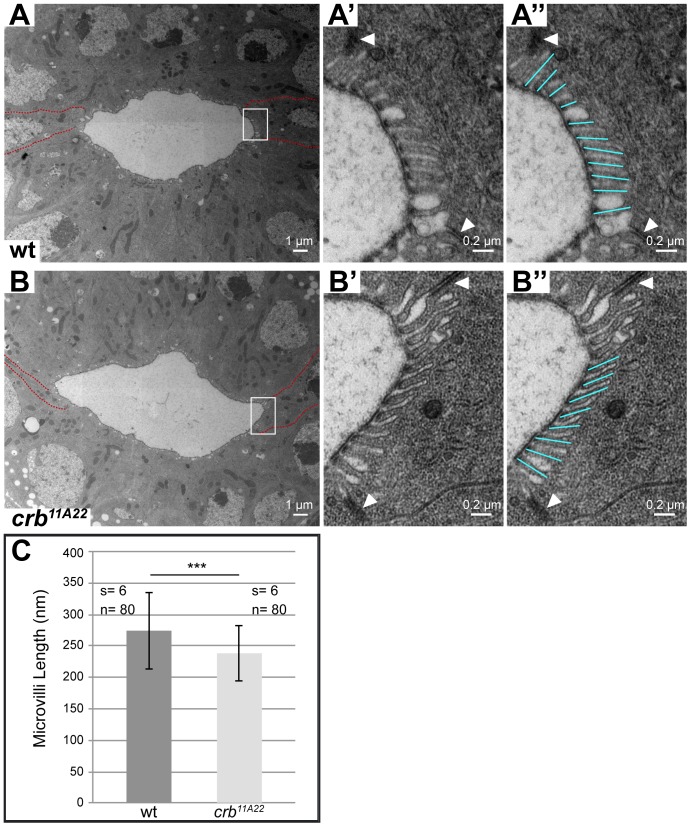
While loss of Crb results in a reduction of the apical membrane in some cells, its overexpression can induce an expansion of the apical membrane. Therefore, we asked whether the high level of Crb in the BCs is responsible for the increased length of microvilli observed in these cells. Increase in microvilli length becomes obvious from stage 14 onwards [26], [27], [60]. In fact, the length of the microvilli in the BCs of stage 16 crb11A22 mutant embryos is slightly, but significantly reduced compared to that in wild-type BCs (Fig. 5).
Figure 5. Loss of Crb from the BCs alters the apical membrane structure.
(A–B″) Electron micrographs of cross sections through the large intestines of stage 16 wild-type (A–A″) and homozygous mutant crb11A22 (B–B″) embryos. In A and B, BCs are outlined by red lines, the rectangles indicate areas enlarged in A′, A″, B′ and B″. White arrowheads in A′, A″, B′ and B″ point to the adherens junctions between the BCs and PCs. BCs form longer and more regular microvilli than the PCs in wild-type (A–A″) and homozygous crb11A22 mutant embryos (B–B″). (C) Graph showing the mean length of microvilli in the BCs of stage 16 wild-type and crb11A22 mutant embryos ± standard deviation. s refers to the number of embryos analysed; n refers to the number of microvilli analysed. ***indicate p-value <0.001, assessed by two-sided Student's t-test.
To find out whether overexpression of Crb has an effect on the differentiation of the apical surface of the PCs, we overexpressed full-length Crb protein in the dorsal PCs of the hindgut using en-Gal4. Similarly as already reported [14], [15], [16], [17] overexpressed Crb becomes ectopically localised and recruits other apical proteins (e. g. DPar6) to ectopic sites in epithelial cells of the large intestine (Fig. 6). The epithelium is disorganised, making any quantification of microvilli impossible.
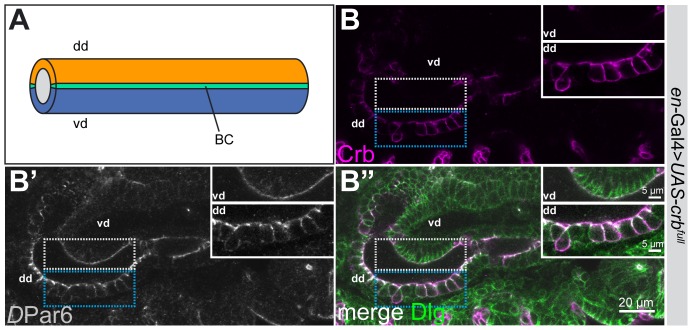
Figure 6. Crb overexpression in PCs leads to an expansion of the apical membrane domain.
(A) Schematic representation of the large intestine with the dorsal domain (dd) in orange, the ventral domain (vd) in blue and the BCs in green. (B–B″) Confocal microscope images of a stage 15 embryonic hindgut expressing UAS-crbfull under the control of en-GAL4 in the PCs of the dorsal domain. The hindgut is stained with anti-Crb (magenta), anti-DPar6 (grey) and anti-Dlg (green). The insets show higher magnifications of the vd (outlined by the grey dotted line), which serves as control tissue and the dd (outlined by the blue dotted line) where the altered apico-basal polarity in cells overexpressing Crb is highlighted (due to the very strong overexpression of Crb in the dd, the gain of the microscope was strongly reduced).
Discussion
In most Drosophila tissues studied so far, apical localisation of Crb depends on Sdt, mediated by direct interaction between the C-terminus of Crb and the PDZ-domain of Sdt [23], [24], [39], [61]. This interaction is conserved in vertebrates [62], [63]. Here we show that neither the C-terminal PDZ-domain binding motif nor an intact FERM-domain binding motif of the cytoplasmic domain of Crb is required for proper localisation of Crb in the BCs of the hindgut. A Crb protein lacking the C-terminal ERLI motif, as in foscrbY10A,ΔERLI;crbGX24 [33] or in crb8F105 [32] still accumulates apically in the BCs. Unlike cells of the Malpighian tubules, which show apical localisation of the Crb8F105 protein at early (stage 11), but not at late stages (stage 16) [64], the mutant protein remains apically in the BCs even at late stages. This suggests that stabilisation of Crb does not require the interaction with a PDZ-domain containing protein in these cells, thus also excluding DPar6. Since all (verified and predicted) Sdt isoforms contain a PDZ domain (Flybase) and all sdt alleles described so far are protein null in the embryo, as revealed by using an antibody directed against the PDZ domain [39], we find it rather unlikely that an unknown isoform of Sdt is involved in the stabilisation of Crb in the BCs. Furthermore, an intact FERM-domain binding domain in Crb is not required for apical localisation of Crb, as revealed by proper apical enrichment of Crb in the BCs of foscrbY10A,ΔERLI;crbGx24 embryos. We assume that the Y10A exchange abolishes the FERM-binding function. This conclusion is based on data showing that a tyrosine residue at position 10 in the FERM-domain binding motif of the adhesion molecule ICAM-2 is part of the peptide that participates in intimate interactions with the FERM-domain of radixin. Exchange of this tyrosine by alanine caused a 16 fold reduction in the binding affinity to the FERM-domain of radixin [65].
So far, we can only speculate whether the 14 amino acids still present in the truncated cytoplasmic domain of the Crb protein encoded by crb8F105 [32] contain a yet undefined apical targeting and/or retention sequence. If so, this sequence is acting in a cell-type specific way, since the mutant Crb protein, which lacks both the PDZ- and FERM- domain binding motif is still apically localised in BCs, but not in PCs. All predicted Drosophila Crb isoforms contain the same cytoplasmic tail composed of 37 amino acids, but we cannot exclude the possibility that an alternative form is expressed in BCs. The human CRB3 gene encodes two isoforms due to alternative splicing, one of which, CRB-CLPI, contains an alternative C-terminus that lacks the conserved –ERLI motif. This isoform is specifically localised in cilia of fully differentiated Madin-Darbine canine kidney (MDCK) cells, but not in newly polarised cells still lacking cilia [66].
Various mechanisms have been described that are involved in the stabilisation/retention of proteins on the apical surface. For example, the stability of the apical Cystic Fibrosis Transmembrane Conductance Regulator (CFTR), a cyclic AMP-regulated chloride channel with an important role in the control of the volume of the lung airway surface liquid, can be regulated by various interactions mediated by its cytoplasmic domains. Beside the interaction of its C-terminus with the PDZ domain of EBP50 (ERM-binding phosphoprotein 50), the stability of CFTR was shown to depend on interactions of a hydrophobic motif with the intermediate filament protein keratin 18, or by interaction of an N-terminal sequence with the actin-binding protein filamin-A [67], [68], [69], [70]. An alternative way to stabilise Crb apically could be via homophilic cis-interactions mediated by the extracellular domains of Crb proteins, which is still present in the protein encoded by the mutant crb8F105 allele. Homophilic interactions have recently been suggested as a mechanism for Crb stabilisation in the embryonic epidermis and the follicle epithelium of Drosophila [71], [72] and in the zebrafish retina, where they mediate the formation of the cone mosaic [73]. Alternatively, another, yet unknown protein specifically expressed in the BCs could stabilise Crb by heterophilic interactions of the extracellular domains.
The BCs of the large intestine not only differ from the PCs by a different mechanism for Crb stabilisation, but also by a much higher level of Crb protein on the apical surface, which is associated with a higher transcript level [28]. As shown here, the high level of Crb expression has an influence on the length of the microvilli in these cells, but not on the formation of the microvilli per se. This is in line with results obtained from overexpression in other epithelial cells, which can induce enlarged or ectopic apical surfaces [14], [17]. Experimentally induced overexpression of Crb in dorsal PCs results in ectopic apical proteins. Due to the disorganisation of the epithelium microvilli length could not be measured. An interesting speculation to explain Crb accumulation in the BCs relies on a reverse scenario, in which microvilli are required for apical retention of membrane proteins. This mechanism has been recently derived from studies in mice lacking the three actin-bundling proteins villin, espin and plastin-1. Enterocytes of triple knock-out mice do form microvilli, which lack, however, the typical actin filament bundles. Strikingly, apical transmembrane proteins and enzymes are poorly retained [74]. Assuming a similar mechanism in the Drosophila hindgut, the stronger accumulation of Crb in BCs could be a consequence of longer microvilli. Similar as in crb, microvilli length of BCs is also reduced in embryos mutant for slit or robo, but increased in robo2 and robo/robo2 double mutants. However, no difference in Crb staining was found in the BCs of these mutants compared to that of wild-type [60]. This indicates that there is no obvious dependence of Crb abundance on the length of the microvilli of BCs.
Alternatively, a different physiological state of BCs and PCs could be responsible for the different behaviour of Crb. Epithelial cells of the proximal renal tubule of acidotic rats, for example, adapted to this change by altering the protein composition in the microvilli and the apical cortex. The change included transmembrane proteins such as transporters, but also scaffolding proteins or proteins involved in trafficking [75]. Finally, microvilli in BCs could define a distinct lipid microdomain responsible for plasma membrane domain-specific retention. For example, segregation of different raft-associated gangliosides into microvilli or the smooth portion of the apical membrane of MDCK cells correlate with the differential segregation of the pentaspan protein prominin-1 in microvilli [76], [77]. Interestingly, the single-span transmembrane protein Stranded-at-Second is reduced on the apical microvilli of BCs in comparison to its level in the PCs (see Fig. 3), supporting the idea that the expanded apical membrane of the BCs may regulate differential retention of only a subset of proteins. Whether any of the discussed mechanisms is used by the BCs to enrich Crb on the apical surface and which function the BCs have in the hindgut requires further investigation.
Acknowledgments
We thank D. Bilder, E. Organ and D. Cavener, A. Wodarz, J. Knoblich and the Developmental Studies Hybridoma Bank (DSHB) for antibodies and the Bloomington Drosophila stock centre for flies. We are grateful to Michaela Yuan for acquiring electron micrographs and the fly and light and electron microscopy facilities of MPI-CBG for continuous support.
Funding Statement
This work was supported by the Max-Planck Society (MPG). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Apodaca G, Gallo LI, Bryant DM (2012) Role of membrane traffic in the generation of epithelial cell asymmetry. Nat Cell Biol 14: 1235–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cao X, Surma MA, Simons K (2012) Polarized sorting and trafficking in epithelial cells. Cell Res 22: 793–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Golachowska MR, Hoekstra D, van ISC (2010) Recycling endosomes in apical plasma membrane domain formation and epithelial cell polarity. Trends Cell Biol 20: 618–626. [DOI] [PubMed] [Google Scholar]
- 4. Weisz OA, Rodriguez-Boulan E (2009) Apical trafficking in epithelial cells: signals, clusters and motors. J Cell Sci 122: 4253–4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mellman I, Nelson WJ (2008) Coordinated protein sorting, targeting and distribution in polarized cells. Nat Rev Mol Cell Biol 9: 833–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bulgakova NA, Knust E (2009) The Crumbs complex. J Cell Sci 122: 2587–2596. [DOI] [PubMed] [Google Scholar]
- 7. Tepass U (2012) The apical polarity protein network in Drosophila epithelial cells: regulation of polarity, junctions, morphogenesis, cell growth, and survival. Annu Rev Cell Dev Biol 28: 655–685. [DOI] [PubMed] [Google Scholar]
- 8. Tepass U, Knust E (1990) Phenotypic and developmental analysis of mutations at the crumbs locus, a gene required for the development of epithelia in Drosophila melanogaster . Roux's Arch Dev Biol 199: 189–206. [DOI] [PubMed] [Google Scholar]
- 9. Tepass U, Knust E (1993) crumbs and stardust act in a genetic pathway that controls the organization of epithelia in Drosophila melanogaster . Dev Biol 159: 311–326. [DOI] [PubMed] [Google Scholar]
- 10. Grawe F, Wodarz A, Lee B, Knust E, Skaer H (1996) The Drosophila genes crumbs and stardust are involved in the biogenesis of adherens junctions. Development 122: 951–959. [DOI] [PubMed] [Google Scholar]
- 11. Tepass U (1996) Crumbs, a component of the apical membrane, is required for zonula adherens formation in primary epithelia of Drosophila . Dev Biol 177: 217–225. [DOI] [PubMed] [Google Scholar]
- 12. Whiteman EL, Fan S, Harder JL, Walton KD, Liu CJ, et al. (2014) Crumbs3 is Essential for Proper Epithelial Development and Viability. Mol Cell Biol 34: 43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xiao Z, Patrakka J, Nukui M, Chi L, Niu D, et al. (2011) Deficiency in Crumbs homolog 2 (Crb2) affects gastrulation and results in embryonic lethality in mice. Dev Dyn 240: 2646–2656. [DOI] [PubMed] [Google Scholar]
- 14. Wodarz A, Hinz U, Engelbert M, Knust E (1995) Expression of Crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila . Cell 82: 67–76. [DOI] [PubMed] [Google Scholar]
- 15. Pellikka M, Tanentzapf G, Pinto M, Smith C, McGlade CJ, et al. (2002) Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature 416: 143–149. [DOI] [PubMed] [Google Scholar]
- 16. Richard M, Muschalik N, Grawe F, Özüyaman S, Knust E (2009) A role for the extracellular domain of Crumbs in morphogenesis of Drosophila photoreceptor cells. Eur J Cell Biol 88: 765–777. [DOI] [PubMed] [Google Scholar]
- 17. Muschalik N, Knust E (2011) Increased levels of the cytoplasmic domain of Crumbs repolarise developing Drosophila photoreceptors. J Cell Sci 124: 3715–3725. [DOI] [PubMed] [Google Scholar]
- 18. Blankenship JT, Fuller MT, Zallen JA (2007) The Drosophila homolog of the Exo84 exocyst subunit promotes apical epithelial identity. J Cell Sci 120: 3099–3110. [DOI] [PubMed] [Google Scholar]
- 19. Pocha SM, Wassmer T, Niehage C, Hoflack B, Knust E (2011) Retromer controls epithelial cell polarity by trafficking the apical determinant Crumbs. Curr Biol 21: 1111–1117. [DOI] [PubMed] [Google Scholar]
- 20. Zhou B, Wu Y, Lin X (2011) Retromer regulates apical–basal polarity through recycling Crumbs. Dev Biol 360: 87–95. [DOI] [PubMed] [Google Scholar]
- 21. Harris KP, Tepass U (2008) Cdc42 and Par proteins stabilize dynamic adherens junctions in the Drosophila neuroectoderm through regulation of apical endocytosis. J Cell Biol 183: 1129–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roeth JF, Sawyer JK, Wilner DA, Peifer M (2009) Rab11 Helps Maintain Apical Crumbs and Adherens Junctions in the Drosophila Embryonic Ectoderm. PLoS ONE 4: e7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bachmann A, Schneider M, Grawe F, Theilenberg E, Knust E (2001) Drosophila Stardust is a partner of Crumbs in the control of epithelial cell polarity. Nature 414: 638–643. [DOI] [PubMed] [Google Scholar]
- 24. Hong Y, Stronach B, Perrimon N, Jan LY, Jan YN (2001) Drosophila Stardust interacts with Crumbs to control polarity of epithelia but not neuroblasts. Nature 414: 634–638. [DOI] [PubMed] [Google Scholar]
- 25. Lengyel JA, Iwaki DD (2002) It takes guts: the Drosophila hindgut as a model system for organogenesis. Dev Biol 243: 1–19. [DOI] [PubMed] [Google Scholar]
- 26. Murakami R, Shiotsuki Y (2001) Ultrastructure of the hindgut of Drosophila larvae, with special reference to the domains identified by specific gene expression patterns. J Morph 248: 144–150. [DOI] [PubMed] [Google Scholar]
- 27. Fuss B, Hoch M (2002) Notch signaling controls cell fate specification along the dorsoventral axis of the Drosophila gut. Curr Biol 12: 171–179. [DOI] [PubMed] [Google Scholar]
- 28. Tepass U, Theres C, Knust E (1990) crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell 61: 787–799. [DOI] [PubMed] [Google Scholar]
- 29. Iwaki DD, Lengyel JA (2002) A Delta-Notch signaling border regulated by Engrailed/Invected repression specifies boundary cells in the Drosophila hindgut. Mech Dev 114: 71–84. [DOI] [PubMed] [Google Scholar]
- 30. Jürgens G, Wieschaus E, Nüsslein-Volhard C, Kluding H (1984) Mutations affecting the pattern of the larval cuticle of Drosophila melanogaster. II. Zygotic loci on the third chromosome. Roux's Arch Dev Biol 193: 283–295. [DOI] [PubMed] [Google Scholar]
- 31. Huang J, Zhou W, Dong W, Watson AM, Hong Y (2009) Directed, efficient, and versatile modifications of the Drosophila genome by genomic engineering. Proc Natl Acad Sci 106: 8284–8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wodarz A, Grawe F, Knust E (1993) Crumbs is involved in the control of apical protein targeting during Drosophila epithelial development. Mech Dev 44: 175–187. [DOI] [PubMed] [Google Scholar]
- 33. Klose S, Flores-Benitez D, Riedel F, Knust E (2013) Fosmid-based structure-function analysis reveals functionally distinct domains in the cytoplasmic domain of Drosophila Crumbs. G3 3: 153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Han K, Manley JL (1993) Functional domains of the Drosophila Engrailed protein. Embo J 12: 2723–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wodarz A, Ramrath A, Kuchinke U, Knust E (1999) Bazooka provides an apical cue for Inscuteable localization in Drosophila neuroblasts. Nature 402: 544–547. [DOI] [PubMed] [Google Scholar]
- 36. Richard M, Grawe F, Knust E (2006) DPATJ plays a role in retinal morphogenesis and protects against light-dependent degeneration of photoreceptor cells in the Drosophila eye. Dev Dyn 235: 895–907. [DOI] [PubMed] [Google Scholar]
- 37. Betschinger J, Mechtler K, Knoblich JA (2003) The Par complex directs asymmetric cell division by phosphorylating the cytoskeleton protein Lgl. Nature 422: 326–330. [DOI] [PubMed] [Google Scholar]
- 38. Bachmann A, Timmer M, Sierralta J, Pietrini G, Gundelfinger ED, et al. (2004) Cell type-specific recruitment of Drosophila Lin-7 to distinct MAGUK-based protein complexes defines novel roles for Sdt and Dlg-S97. J Cell Sci 117: 1899–1909. [DOI] [PubMed] [Google Scholar]
- 39. Berger S, Bulgakova NA, Grawe F, Johnson K, Knust E (2007) Unravelling the genetic complexity of Drosophila stardust during photoreceptor morphogenesis and prevention of light-induced degeneration. Genetics 176: 2189–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, et al. (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bolte S, Cordelieres FP (2006) A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 224: 213–232. [DOI] [PubMed] [Google Scholar]
- 42. Tepass U, Hartenstein V (1994) The development of cellular junctions in the Drosophila embryo. Dev Biol 161: 563–596. [DOI] [PubMed] [Google Scholar]
- 43. Klebes A, Knust E (2000) A conserved motif in Crumbs is required for E-cadherin localisation and zonula adherens formation in Drosophila . Curr Biol 10: 76–85. [DOI] [PubMed] [Google Scholar]
- 44. Horne-Badovinac S, Bilder D (2008) Dynein regulates epithelial polarity and the apical localization of stardust A mRNA. PLoS Genet 4: e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kuchinke U, Grawe F, Knust E (1998) Control of spindle orientation in Drosophila by the Par-3-related PDZ-domain protein Bazooka. Curr Biol 8: 1357–1365. [DOI] [PubMed] [Google Scholar]
- 46. Petronczki M, Knoblich JA (2001) DmPAR-6 directs epithelial polarity and asymmetric cell division of neuroblasts in Drosophila . Nat Cell Biol 3: 43–49. [DOI] [PubMed] [Google Scholar]
- 47. Wodarz A, Ramrath A, Grimm A, Knust E (2000) Drosophila atypical protein kinase C associates with Bazooka and controls polarity of epithelia and neuroblasts. J Cell Biol 150: 1361–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Krahn MP, Buckers J, Kastrup L, Wodarz A (2010) Formation of a Bazooka-Stardust complex is essential for plasma membrane polarity in epithelia. J Cell Biol 190: 751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen J, Zhang M (2013) The Par3/Par6/aPKC complex and epithelial cell polarity. Exp Cell Res 319: 1357–1364. [DOI] [PubMed] [Google Scholar]
- 50. Kempkens Ö, Médina E, Fernandez-Ballester G, Özüyaman S, Le Bivic A, et al. (2006) Computer modelling in combination with in vitro studies reveals similar binding affinities of Drosophila Crumbs for the PDZ domains of Stardust and DmPar-6. Eur J Cell Biol 85: 753–767. [DOI] [PubMed] [Google Scholar]
- 51. Lemmers C, Michel D, Lane-Guermonprez L, Delgrossi M-H, Médina E, et al. (2004) CRB3 binds directly to Par6 and regulates the morphogenesis of the tight junctions in mammalian epithelial cells. Mol Biol Cell 15: 1324–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bilder D, Schober M, Perrimon N (2003) Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat Cell Biol 5: 53–58. [DOI] [PubMed] [Google Scholar]
- 53. Bilder D, Perrimon N (2000) Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature 403: 676–680. [DOI] [PubMed] [Google Scholar]
- 54. Tanentzapf G, Smith C, McGlade J, Tepass U (2000) Apical, lateral, and basal polarization cues contribute to the development of the follicular epithelium during Drosophila oogenesis. J Cell Biol 151: 891–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ellenbroek SI, Iden S, Collard JG (2012) Cell polarity proteins and cancer. Semin Cancer Biol 22: 208–215. [DOI] [PubMed] [Google Scholar]
- 56. Elsum I, Yates L, Humbert PO, Richardson HE (2012) The Scribble-Dlg-Lgl polarity module in development and cancer: from flies to man. Essays Biochem 53: 141–168. [DOI] [PubMed] [Google Scholar]
- 57. Wieschaus E, Nüsslein-Volhard C, Jürgens G (1984) Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. III. Zygotic loci on the X chromosome and fourth chromosome. Roux's Arch Dev Biol 193: 296–307. [DOI] [PubMed] [Google Scholar]
- 58. Laprise P, Beronja S, Silva-Gagliardi NF, Pellikka M, Jensen AM, et al. (2006) The FERM protein Yurt is a negative regulatory component of the Crumbs complex that controls epithelial polarity and apical membrane size. Dev Cell 11: 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ling C, Zheng Y, Yin F, Yu J, Huang J, et al. (2010) The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proc Natl Acad Sci 107: 10532–10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Soplop NH, Cheng Y-S, Kramer SG (2012) Roundabout is Required in the Visceral Mesoderm for Proper Microvillus Length in the Hindgut Epithelium. Dev Dyn 241: 759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bulgakova NA, Kempkens Ö, Knust E (2008) Multiple domains of Drosophila Stardust differentially mediate localisation of the Crumbs/Stardust complex during photoreceptor development. J Cell Sci 121: 2018–2026. [DOI] [PubMed] [Google Scholar]
- 62. Roh MH, Makarova O, Liu CJ, Shin K, Lee S, et al. (2002) The Maguk protein, Pals1, functions as an adapter linking mammalian homologues of Crumbs and Discs Lost. J Cell Biol 157: 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Makarova O, Roh MH, Liu C-J, Laurinec S, Margolis B (2003) Mammalian Crumbs3 is a small transmembrane protein linked to protein associated with Lin-7 (Pals1). Gene 302: 21–29. [DOI] [PubMed] [Google Scholar]
- 64. Campbell K, Knust E, Skaer H (2009) Crumbs stabilises epithelial polarity during tissue remodelling. J Cell Sci 122: 2604–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hamada K, Shimizu T, Yonemura S, Tsukita S, Hakoshima T (2003) Structural basis of adhesion-molecule recognition by ERM proteins revealed by the crystal structure of the radixin-ICAM-2 complex. Embo J 22: 502–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fan S, Fogg VC, Wang Q, Chen XW, Liu CJ, et al. (2007) A novel Crumbs3 isoform regulates cell division and ciliogenesis via importin beta interactions. J Cell Biol 178: 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Thelin WR, Chen Y, Gentzsch M, Kreda SM, Sallee JL, et al. (2007) Direct interaction with filamins modulates the stability and plasma membrane expression of CFTR. J Clin Invest 117: 364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Duan Y, Sun Y, Zhang F, Zhang WK, Wang D, et al. (2012) Keratin K18 increases cystic fibrosis transmembrane conductance regulator (CFTR) surface expression by binding to its C-terminal hydrophobic patch. J Biol Chem 287: 40547–44059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Short DB, Trotter KW, Reczek D, Kreda SM, Bretscher A, et al. (1998) An apical PDZ protein anchors the cystic fibrosis transmembrane conductance regulator to the cytoskeleton. J Biol Chem 273: 19797–19801. [DOI] [PubMed] [Google Scholar]
- 70. Swiatecka-Urban A, Duhaime M, Coutermarsh B, Karlson KH, Collawn J, et al. (2002) PDZ domain interaction controls the endocytic recycling of the cystic fibrosis transmembrane conductance regulator. J Biol Chem 277: 40099–40105. [DOI] [PubMed] [Google Scholar]
- 71. Fletcher GC, Lucas EP, Brain R, Tournier A, Thompson BJ (2012) Positive Feedback and Mutual Antagonism Combine to Polarize Crumbs in the Drosophila Follicle Cell Epithelium. Curr Biol 22: 1–7. [DOI] [PubMed] [Google Scholar]
- 72. Letizia A, Ricardo S, Moussian B, Martín N, Llimargas M (2013) A functional role of the extracellular domain of Crumbs in cell architecture and apicobasal polarity. J Cell Sci 126: 2157–2163. [DOI] [PubMed] [Google Scholar]
- 73. Zou J, Wang X, Wei X (2012) Crb Apical Polarity Proteins Maintain Zebrafish Retinal Cone Mosaics via Intercellular Binding of Their Extracellular Domains. Dev Cell 22: 1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Revenu C, Ubelmann F, Hurbain I, El-Marjou F, Dingli F, et al. (2012) A new role for the architecture of microvillar actin bundles in apical retention of membrane proteins. Mol Biol Cell 23: 324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Walmsley SJ, Freund DM, Curthoys NP (2012) Proteomic profiling of the effect of metabolic acidosis on the apical membrane of the proximal convoluted tubule. Am J Physiol Renal Physiol 302: F1465–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Janich P, Corbeil D (2007) GM1 and GM3 gangliosides highlight distinct lipid microdomains within the apical domain of epithelial cells. FEBS Lett 581: 1783–1787. [DOI] [PubMed] [Google Scholar]
- 77. Röper K, Corbeil D, Huttner WB (2000) Retention of prominin in microvilli reveals distinct cholesterol-based lipid micro-domains in the apical plasma membrane. Nat Cell Biol 2: 582–592. [DOI] [PubMed] [Google Scholar]