Abstract
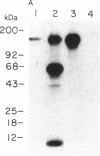
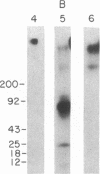
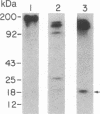
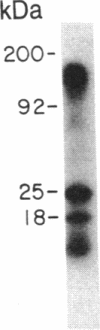
A monoclonal antibody (C6.7) has been generated against the calcium-replete form of human platelet thrombospondin (TSP). C6.7 is specific for TSP as determined by both competitive radioimmunoassay and immunoprecipitation. This antibody inhibits both thrombin- and A23187-induced aggregation of gel-filtered platelets in a concentration-dependent manner without affecting the secretion of serotonin. The epitope on TSP recognized by C6.7 has been localized to an 18-kDa fragment that is present in mild chymotryptic digests of TSP. This fragment is disulfide-linked to a 120- to 140-kDa fragment in unreduced digests, and both reduction and denaturation are required to separate the 18-kDa peptide from the larger fragments. A 25-kDa heparin binding domain is also present in the chymotryptic digest. However, the 18-kDa peptide is distinct from the heparin binding domain. The amino acid sequence at the NH2 terminus of the 18-kDa fragment is Asp-Thr-Asn-Pro-Thr-Arg-Ala-Gln-Gly-Tyr-.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agam G., Gartner T. K., Livne A. Inhibition of platelet aggregation and endogenous lectin activity by oligoamines. Thromb Res. 1984 Feb 1;33(3):245–257. doi: 10.1016/0049-3848(84)90160-9. [DOI] [PubMed] [Google Scholar]
- Baenziger N. L., Brodie G. N., Majerus P. W. A thrombin-sensitive protein of human platelet membranes. Proc Natl Acad Sci U S A. 1971 Jan;68(1):240–243. doi: 10.1073/pnas.68.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baenziger N. L., Brodie G. N., Majerus P. W. Isolation and properties of a thrombin-sensitive protein of human platelets. J Biol Chem. 1972 May 10;247(9):2723–2731. [PubMed] [Google Scholar]
- Booth W. J., Berndt M. C., Castaldi P. A. An altered platelet granule glycoprotein in patients with essential thrombocythemia. J Clin Invest. 1984 Feb;73(2):291–297. doi: 10.1172/JCI111213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth W. J., Furby F. H., Berndt M. C., Castaldi P. A. Factor VIII/von Willebrand factor has potent lectin activity. Biochem Biophys Res Commun. 1984 Jan 30;118(2):495–501. doi: 10.1016/0006-291x(84)91330-5. [DOI] [PubMed] [Google Scholar]
- Coligan J. E., Slayter H. S. Structure of thrombospondin. J Biol Chem. 1984 Mar 25;259(6):3944–3948. [PubMed] [Google Scholar]
- Dixit V. M., Grant G. A., Frazier W. A., Santoro S. A. Isolation of the fibrinogen-binding region of platelet thrombospondin. Biochem Biophys Res Commun. 1984 Mar 30;119(3):1075–1081. doi: 10.1016/0006-291x(84)90884-2. [DOI] [PubMed] [Google Scholar]
- Dixit V. M., Grant G. A., Santoro S. A., Frazier W. A. Isolation and characterization of a heparin-binding domain from the amino terminus of platelet thrombospondin. J Biol Chem. 1984 Aug 25;259(16):10100–10105. [PubMed] [Google Scholar]
- Fujimura K., Phillips D. R. Calcium cation regulation of glycoprotein IIb-IIIa complex formation in platelet plasma membranes. J Biol Chem. 1983 Sep 10;258(17):10247–10252. [PubMed] [Google Scholar]
- Gartner T. K., Gerrard J. M., White J. G., Williams D. C. Fibrinogen is the receptor for the endogenous lectin of human platelets. Nature. 1981 Feb 19;289(5799):688–690. doi: 10.1038/289688a0. [DOI] [PubMed] [Google Scholar]
- Gartner T. K., Gerrard J. M., White J. G., Williams D. C. The endogenous lectin of human platelets is an alpha-granule component. Blood. 1981 Jul;58(1):153–157. [PubMed] [Google Scholar]
- Gartner T. K., Walz D. A., Aiken M., Starr-Spires L., Ogilvie M. L. Antibodies against a 23Kd heparin binding fragment of thrombospondin inhibit platelet aggregation. Biochem Biophys Res Commun. 1984 Oct 15;124(1):290–295. doi: 10.1016/0006-291x(84)90950-1. [DOI] [PubMed] [Google Scholar]
- Gartner T. K., Williams D. C., Phillips D. R. Platelet plasma membrane lectin activity. Biochem Biophys Res Commun. 1977 Nov 21;79(2):592–599. doi: 10.1016/0006-291x(77)90198-x. [DOI] [PubMed] [Google Scholar]
- Gerrard J. M., Phillips D. R., Rao G. H., Plow E. F., Walz D. A., Ross R., Harker L. A., White J. G. Biochemical studies of two patients with the gray platelet syndrome. Selective deficiency of platelet alpha granules. J Clin Invest. 1980 Jul;66(1):102–109. doi: 10.1172/JCI109823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg M. H., Painter R. G., Forsyth J., Birdwell C., Plow E. F. Thrombin increases expression of fibronectin antigen on the platelet surface. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1049–1053. doi: 10.1073/pnas.77.2.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverstick D. M., Dixit V. M., Grant G. A., Frazier W. A., Santoro S. A. Localization of the hemagglutinating activity of platelet thrombospondin to a 140 000-dalton thermolytic fragment. Biochemistry. 1984 Nov 6;23(23):5597–5603. doi: 10.1021/bi00318a033. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Leung L. L., Nachman R. L., Levin R. I., Mosher D. F. Thrombospondin is the endogenous lectin of human platelets. Nature. 1982 Jan 21;295(5846):246–248. doi: 10.1038/295246a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lahav J., Schwartz M. A., Hynes R. O. Analysis of platelet adhesion with a radioactive chemical crosslinking reagent: interaction of thrombospondin with fibronectin and collagen. Cell. 1982 Nov;31(1):253–262. doi: 10.1016/0092-8674(82)90425-1. [DOI] [PubMed] [Google Scholar]
- Lawler J. W., Slayter H. S., Coligan J. E. Isolation and characterization of a high molecular weight glycoprotein from human blood platelets. J Biol Chem. 1978 Dec 10;253(23):8609–8616. [PubMed] [Google Scholar]
- Lawler J. W., Slayter H. S. The release of heparin binding peptides from platelet thrombospondin by proteolytic action of thrombin, plasmin and trypsin. Thromb Res. 1981 May 1;22(3):267–279. doi: 10.1016/0049-3848(81)90119-5. [DOI] [PubMed] [Google Scholar]
- Lawler J., Chao F. C., Cohen C. M. Evidence for calcium-sensitive structure in platelet thrombospondin. Isolation and partial characterization of thrombospondin in the presence of calcium. J Biol Chem. 1982 Oct 25;257(20):12257–12265. [PubMed] [Google Scholar]
- Lawler J., Simons E. R. Cooperative binding of calcium to thrombospondin. The effect of calcium on the circular dichroism and limited tryptic digestion of thrombospondin. J Biol Chem. 1983 Oct 25;258(20):12098–12101. [PubMed] [Google Scholar]
- Leung L. L., Nachman R. L. Complex formation of platelet thrombospondin with fibrinogen. J Clin Invest. 1982 Sep;70(3):542–549. doi: 10.1172/JCI110646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung L. L. Role of thrombospondin in platelet aggregation. J Clin Invest. 1984 Nov;74(5):1764–1772. doi: 10.1172/JCI111595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margossian S. S., Lawler J. W., Slayter H. S. Physical characterization of platelet thrombospondin. J Biol Chem. 1981 Jul 25;256(14):7495–7500. [PubMed] [Google Scholar]
- McDonald J. A., Kelley D. G. Degradation of fibronectin by human leukocyte elastase. Release of biologically active fragments. J Biol Chem. 1980 Sep 25;255(18):8848–8858. [PubMed] [Google Scholar]
- McEver R. P., Bennett E. M., Martin M. N. Identification of two structurally and functionally distinct sites on human platelet membrane glycoprotein IIb-IIIa using monoclonal antibodies. J Biol Chem. 1983 Apr 25;258(8):5269–5275. [PubMed] [Google Scholar]
- Mosher D. F., Doyle M. J., Jaffe E. A. Synthesis and secretion of thrombospondin by cultured human endothelial cells. J Cell Biol. 1982 May;93(2):343–348. doi: 10.1083/jcb.93.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby S. M., Raugi G. J., Bornstein P. Interactions of thrombospondin with extracellular matrix proteins: selective binding to type V collagen. J Cell Biol. 1984 Feb;98(2):646–652. doi: 10.1083/jcb.98.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachman R. L., Leung L. L. Complex formation of platelet membrane glycoproteins IIb and IIIa with fibrinogen. J Clin Invest. 1982 Feb;69(2):263–269. doi: 10.1172/JCI110448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. R., Jennings L. K., Prasanna H. R. Ca2+-mediated association of glycoprotein G (thrombinsensitive protein, thrombospondin) with human platelets. J Biol Chem. 1980 Dec 25;255(24):11629–11632. [PubMed] [Google Scholar]
- Plow E. F., Ginsberg M. H. Specific and saturable binding of plasma fibronectin to thrombin-stimulated human platelets. J Biol Chem. 1981 Sep 25;256(18):9477–9482. [PubMed] [Google Scholar]
- Raugi G. J., Mumby S. M., Abbott-Brown D., Bornstein P. Thrombospondin: synthesis and secretion by cells in culture. J Cell Biol. 1982 Oct;95(1):351–354. doi: 10.1083/jcb.95.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglio S. D., Slayter H. S. Use of a radioimmunoassay to quantify thrombospondin. Blood. 1982 Jan;59(1):162–166. [PubMed] [Google Scholar]
- Santoro S. A., Cowan J. F. Adsorption of von Willebrand factor by fibrillar collagen--implications concerning the adhesion of platelets to collagen. Coll Relat Res. 1982 Jan;2(1):31–43. doi: 10.1016/s0174-173x(82)80039-3. [DOI] [PubMed] [Google Scholar]
- Santoro S. A. Inhibition of platelet aggregation by fibronectin. Biochem Biophys Res Commun. 1983 Oct 14;116(1):135–140. doi: 10.1016/0006-291x(83)90391-1. [DOI] [PubMed] [Google Scholar]
- Tollefsen D. M., Majerus P. W. Inhibition of human platelet aggregation by monovalent antifibrinogen antibody fragments. J Clin Invest. 1975 Jun;55(6):1259–1268. doi: 10.1172/JCI108045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. The major cell surface glycoprotein of chick embryo fibroblasts is an agglutinin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3158–3162. doi: 10.1073/pnas.72.8.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]