Abstract
Proteasome function insufficiency and inadequate protein quality control are strongly implicated in a large subset of cardiovascular disease and may play an important role in their pathogenesis. Protein degradation by the ubiquitin proteasome system can be physiologically regulated. Cardiac muscarinic 2 (M2) receptors were pharmacologically interrogated in intact mice and cultured neonatal rat ventricular myocytes (NRVMs). Proteasome-mediated proteolysis was measured with a surrogate misfolded protein, proteasome peptidase assay, and by characterizing key proteasome subunits. Successful M2 receptor manipulation in cardiomyocytes was determined by measuring an endogenous protein substrate, and in mice, the cardiovascular physiological response. M2 receptor stimulation was associated with increased proteasome-mediated proteolysis and enhanced peptidase activities, while M2 receptor inhibition yielded opposing results. Additionally, M2 receptor manipulation did not alter abundance of the key proteasome subunits, Rpt6 and β5, but significantly shifted their isoelectric points. Inhibition of protein kinase G abrogated the stimulatory effects on proteasome-mediated proteolysis from M2 receptor activation. We conclude that M2 receptor stimulation enhances, whereas M2 receptor inhibition reduces, proteasome-mediated proteolysis likely through posttranslational modifications. Protein kinase G appears to be the mediator of the M2 receptors actions.
Keywords: Muscarinic 2 receptor, proteasome, protein kinase G, cardiomyocyte
1. Introduction
Through targeted proteolysis, the ubiquitin-proteasome system (UPS) regulates a variety of cellular processes, including cell cycle [1], antigen presentation [2], signal transduction [3, 4], DNA repair[5], and protein quality control (PQC) [6, 7]. UPS-mediated proteolysis generally occurs through two steps: ubiquitination and subsequent degradation of the ubiquitinated proteins by the 26S proteasome [6]. Ubiquitination attaches covalently a ubiquitin or a chain of ubiquitin to the target protein molecule via a series of enzymatic reactions involving an ubiquitin activating enzyme (E1), ubiquitin conjugating enzyme (E2), and ubiquitin ligases (E3) [6, 8]. The 26S proteasome is a large catalytic multi-subunit protease complex, whose activity may be regulated by physiological states [9]. The UPS is responsible for the degradation of about 30% of newly synthesized polypeptides that do not properly mature to native proteins in addition to degrading 80-90% of intracellular proteins [5, 8]. It is well-known that the ubiquitination of a specific protein is strictly regulated; increasing lines of evidence indicate that proteasome function is also highly regulated in the cell [10]. We have recently demonstrated that protein kinase G (PKG) positively regulates proteasome activities and thereby facilitates the degradation of misfolded proteins in the cell [11].
Terminally misfolded proteins are primarily degraded by the UPS, however as seen in proteinopathy and ischemia-reperfusion injury there is an excessive production of misfolded proteins which can overwhelm the UPS, especially the proteasome [12-14]. The end result is proteasome functional insufficiency (PFI) and PQC inadequacy [15]. The majority of human failing hearts from various etiologies have an increased amount of ubiquitinated proteins and show signs of abnormal protein aggregations, indicative of PFI and PQC inadequacy [16-19]. These observations suggest that PFI may play an important role in the pathogenesis of cardiovascular disease during progression to congestive heart failure (CHF) in humans [7]. Animal models recapitulating PFI have suggested this to be true [20, 21]. Importantly, the improvement/attenuation of PFI was found to improve cardiovascular function, reduce cardiac remodeling, and lifespan extension [22, 23]. Enhancement of proteasome-mediated proteolysis may be a novel therapeutic strategy; however, a lack of understanding of the regulatory mechanisms represents a critical barrier.
The parasympathetic nervous system (PNS) utilizes the vagus nerve to carry the signal to the heart where acetylcholine is released to stimulate cardiac muscarinic (M) receptors. The human body expresses five subtypes of the M receptor (M1 through M5), four of which (M1 ~ M4) are expressed in cardiac tissue [24]. PNS control of the heart is primarily mediated by the M2 receptor [24, 25]. M2 receptors are located more abundantly in the atria than the ventricles, primarily located at the SA and AV nodes in the atria [24, 26]. While the ability of M2 receptors to elicit control over ventricles is controversial, a growing body of evidence suggests this is the case [27-30]. Cardiac stimulation from the PNS is associated with reduced chronotropy and inotropy, but regulation of cardiac protein degradation by the PNS has not been examined.
An imbalance between the sympathetic nervous system and PNS activities are a common hallmark of many cardiovascular diseases, with depressed PNS activity associated with hypertension [31, 32], diabetes [33], obesity [34], and CHF [35]. In CHF patients, reduced PNS activity is associated with a poorer outcome [36], whereas PNS augmentation through pharmacological activation [37, 38] or vagal stimulation [39, 40] is associated with improved cardiac function and reduced mortality. During chronic adrenergic stress, the stimulation of M2 receptors was found to maintain ventricular contractility and inhibit adverse cardiac remodeling [27]. Whether improved PQC is an underlying mechanism during beneficial M2 receptor stimulation has not been tested. In the present study, we examined the effect of pharmacological modulation of M receptors, particularly the M2 receptor, on myocardial UPS function and explored the underlying mechanism. Our findings suggest that stimulation of M receptors enhances myocardial proteasome activities and UPS-mediated protein degradation via a PKG-dependent manner.
2. Materials and Methods
2.1 Animal models
The protocol for the care and use of animals in this study was approved by the University of South Dakota Institutional Animal Care and Use Committee. GFPu is an enhanced green fluorescence protein (GFP) modified by carboxyl fusion of degron CL1 [41]. GFPdgn is a slightly shorter version of GFPu. Both GFPu and GFPdgn are proven surrogate substrates of the UPS in cardiomyocytes [42, 43]. A UPS “reporter” transgenic (tg) mouse model expressing GFPdgn was created and validated as previously described [42]. GFPdgn mice were maintained in the FVB/N inbred background [42]. Genotypes were determined using PCR analysis of tail DNA. Mice were treated with pilocarpine (1 mg/kg), M2 receptor antagonist methoctramine (1 mg/kg) (Sigma-Aldrich, St. Louis, MO), or volume corrected saline control every four hours intraperitoneally (i.p.). Pilocarpine (Pilo) is a non-selective agonist of M receptors and methoctramine (Meth) is an antagonist of the M2 receptor [24].
2.2 Radiotelemetry
Mice were surgically implanted subcutaneously with radiotelemetry devices (model TA10EA-F20, Data Sciences Incorporated, St. Paul, MN) to monitor conscious heart rate and to record electrocardiograms (ECGs) before and during muscarinic receptor modulation. Mice were allowed to recover for approximately 1 week before treatment manipulations began. To ensure the accuracy of the radiotelemetry devices, the mice were placed on receiving pads and allowed to roam freely with no treatment while heart rate and ECGs were recorded every minute for ten seconds. After 20 hours with little or no change in heart rate or ECG, muscarinic receptor manipulation began and persisted over a period of 24 hours during which heart rates and ECG’s were recorded.
2.3 Primary cell culture of neonatal rat ventricular myocytes (NRVMs)
Cellutron Neomyocytes Isolation System (Cellutron Life Technology, Baltimore, MD, Cat. No. nc-6031) was used to isolate NRVMs by following the manufacturer’s instructions; and the cell culture was performed as previously described [44].
2.4 Recombinant adenoviruses infection of NRVMs
NRVMs in cultures were infected with replication-deficient recombinant adenoviruses harboring the expression cassette for GFPu (Ad-GFPu) [43], or red fluorescent protein (RFP) that does not contain a degron sequence (Ad-RFP), or Ad-GFPu alone, as previously described [43]. In Ad-GFPu and Ad-RFP, the regulatory elements for GFPu and RFP expression are exactly the same [45].
2.5 Protein extraction and western blot analysis
Proteins were extracted from atrial and ventricular myocardium tissue or cultured NRVMs. Bicinchoninic acid (BCA) reagents (Pierce biotechnology, Rockford, IL) were used to determine protein concentrations. Protein fractionation by SDS-PAGE, electrical transferring the fractionated proteins onto PVDF membranes, immune-detection of the proteins of interest, and densitometry were performed as previously described [46]. The following primary antibodies were used: total VASP (vasodilator-stimulated phosphoprotein), Ser239-phosphorylated-VASP, PKG (Cell Signaling), RPT6 (Biomol), β-tubulin (University of Iowa), α-actinin and ubiquitinated proteins (Sigma), and GFP, RFP, and PSMB5 (customized antibodies). The corresponding horseradish peroxidase-conjugated goat anti-mouse or goat anti-rabbit secondary antibodies (Santa Cruz Biotechnology) were used respectively.
2.6 Reverse transcription- polymerase chain reaction (RT-PCR)
The Tri-Reagent (Molecular Research Center, Inc., Cincinnati, OH) was used to isolate total RNA from ventricular myocardial tissue, following the manufacturer’s protocol. To determine RNA concentration Agilent RNA 6000 Nano assay (Agilent technologies, Inc. Germany) was used following the manufacturer’s protocol. The cDNA was generated using SuperScript III First-Strand Synthesis kit (Invitrogen) and carried out according to the manufacturer’s instructions. GFPdgn mRNA levels were quantitatively compared using RT-PCR at the minimum number of cycles. Primers for GFPdgn: forward 5’- GGGCACAAGCTGGAGTACAACT -3’ and reverse 5’- ATGTTGTGGCGGATCTTGAAG -3’. Primers corresponding to GAPDH were included to probe GAPDH in duplex as a control [11].
2.8 Cycloheximide (CHX) chase assay
CHX chase assays were performed as described [23]. NRVMs were subjected to pharmacological treatment incubated in serum-free DMEM containing 100μM CHX (Sigma-Aldrich) to block further protein synthesis. Collection of cells occurred at different consecutive time points after CHX administration. GFPu protein levels were analyzed from whole-cell lysates by western blot analyses.
2.9 Fluorescence confocal microscopy
Ventricular myocardium from GFPdgn tg mice was fixed with 3.8% paraformaldehyde and processed for obtaining 6μm cryosections, as previously described [46]. To discern cardiomyocytes from other cardiac contents, the myocardial sections were stained with Alexa Fluor 568-conjugated phalloidin (Invitrogen) to visualize F-actin. GFPdgn direct fluorescence (green) and the stained F-actin (red) were visualized and imaged using a multi-laser fluorescence confocal microscope (Olympus Fluoview 500, Center Valley, PA).
2.10 Two dimensional (2D) western blot analysis
Two-dimensional gel protein electrophoresis was performed as described [11]. In brief, proteins were extracted from cultured cardiomyocytes through first lysing the cells in 2D lysing buffer (8 M Urea, 2 M Thiourea, 1% DTT, 2% CHAPS, 1% Carrier Ampholytes, and protease inhibitor cocktail). Equal quantities of extracted proteins were placed into a gel rack with a gel strip (IPG gel strips, 11 cm, pH 3-10; Bio-Rad, Hercules, CA) placed on top to rehydrate the membrane at 20°C. Next the gel rack was placed into the Bio-Rad IEF cell machine for isoelectric focusing. For the 2nd dimension, the gel strips were electrophoresed through SDS-PAGE and then transferred to a PVDF membrane with a Trans-blot apparatus (Bio-Rad, Hercules, CA). The subsequent immuno-detection of RPT6 and PSMB5 was performed as described in the western blot analysis.
2.11 Statistical analysis
All continuous variables are presented as mean±SD. Differences between two groups were evaluated for statistical significance using two-tailed Student’s t-test, unless otherwise noted. When evaluating a difference among 3 or more groups, one-way analysis of variance (ANOVA) or when appropriate, 2-way ANOVA, followed by the Holm-Sidak test for pair-wise comparisons were performed. Degradation rates were determined by regression analysis followed by an unpaired t-test to determine significance of the difference in slope. Protein half-life was calculated as log10 of 50% of the protein density, as described by Kagawa et al. [47]. A paired t-test was used to determine significance between protein half-lives [47]. The P value <0.05 were considered statistically significant.
3. Results
3.1 M2 modulation mitigates cardiac proteasome-mediated proteolysis in mice
To probe the potential regulation of PNS on UPS activity in the heart, GFPdgn tg mice were treated with two consecutive intraperitoneal injections (with an 4-hour interval) of the M receptor agonist, pilocarpine (Pilo, 1mg/kg), M2 receptor antagonist, methoctramine (Meth, 1mg/kg), or saline (vehicle control). The mice were sacrificed two hours after the second injection and atrial and ventricular myocardium was respectively sampled for protein and RNA analyses and immunofluorescence confocal microscopy (Figure 1). The treatment regimens were determined on the basis of the half-life of the drugs and the results of our pilot studies which were designed to optimize the dosing by monitoring the conscious electrocardiogram (ECG) using radiotelemetry (Table 1, Supplementary Figure 1). As expected, stimulation of M receptors by Pilo resulted in a reduced heart rate and an increase in the PR and RR intervals (p<0.05). M2 receptor inhibition by Meth had opposing effects. Neither Pilo nor Meth showed statistically significant effects on QRS and QT intervals (Table 1).
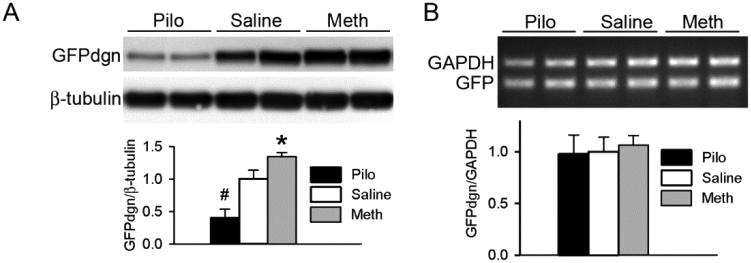
Figure 1. Manipulation of the M2 receptor alters myocardial protein level but not the mRNA level of a UPS surrogate substrate protein in mice.
Ventricular myocardium was sampled from GFPdgn transgenic mice which had been treated with pilocarpine (Pilo, 1 mg/kg), methoctramine (Meth, 1 mg/kg), or vehicle control (Saline) for 6 hours, for protein and total RNA extraction. A, Representative images (upper panel) and pooled densitometry data (lower panel) of western blot analyses for GFPdgn. B, RT-PCR analysis of the mRNA levels of GFPdgn. Representative images (upper panel) and a summary of pooled densitometry data (lower panel) are shown. *p<0.05, #p<0.01 vs. Saline; n=6 mice/group.
Table 1.
Changes in cardiac electrophysiological intervals in mice subject to M receptor manipulation
| Treatment | N | Timing | Heart rate (beats/min) |
RR (ms) | PR (ms) | QRS (ms) | QT (ms) |
|---|---|---|---|---|---|---|---|
| Pilocarpine | 5 | Before | 673.8±15.21 | 89.04±2.13 | 29.58±0.26 | 8.64±0.46 | 19.63±1.17 |
| 5 | After | 552.7±12.84*# | 108.55±2.96*# | 31.85±0.55*# | 9.49±0.53 | 19.88±1.18 | |
| Saline | 5 | Before | 687.6±13.85 | 87.26±2.02 | 30.31±0.44 | 7.43±0.32 | 19.89±1.19 |
| 5 | After | 684.5±11.98 | 87.66±1.92 | 29.71±0.97 | 7.53±0.60 | 19.52±0.92 | |
| Methoctramine | 5 | Before | 672.0±14.67 | 89.28±2.09 | 29.54±0.65 | 8.61±0.29 | 19.40±0.59 |
| 5 | After | 743.6±17.45*# | 80.69±1.81*# | 28.67±0.40*# | 7.89±0.31 | 19.08±0.66 |
ECG tracings were analyzed from mice treated with muscarinic modulators for 24 hours for modifications of electrical movement during muscarinic receptor manipulation. RR, PR, QRS, and QT indicate the R-R interval, the P-R interval, the QRS complex, and the QT interval, respectively.
: p<0.05 vs. respective saline after treatment;
: p<0.05 vs. the same group before treatment.
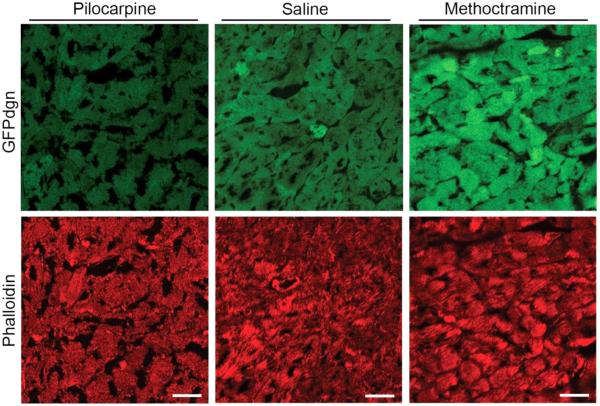
Pilo significantly reduced, whereas Meth significantly increased, the steady state protein levels of GFPdgn in ventricular myocardium (Figure 1A). Neither Pilo nor Meth altered the myocardial steady state mRNA levels of GFPdgn (Figure 1B), indicating that the GFPdgn protein level changes were caused by a post-transcriptional mechanism. The presence of the GFPdgn protein level changes in the cardiomyocyte compartment was confirmed via confocal microscopic examination of GFPdgn direct fluorescence in myocardium (Figure 2). These results suggest that M stimulation enhances, whereas antagonizing the M2 inhibits, the degradation of GFPdgn, a surrogate misfolded protein substrate of the UPS.
Figure 2. Representative confocal fluorescence micrographs of ventricular myocardium from GFPdgn mice.
GFPdgn mice were treated as described in Figure 1. Ventricular myocardial tissue samples were fixed and processed to obtain cryosections. The cryosections were subsequently stained with Fluor 568-conjugated phalloidin to visualize F-actin which is most abundant in cardiomyocytes. The direct green fluorescence of GFPdgn and the indirect red fluorescence of F-actin were then imaged via confocal microscopy. Scale bar = 30μm.
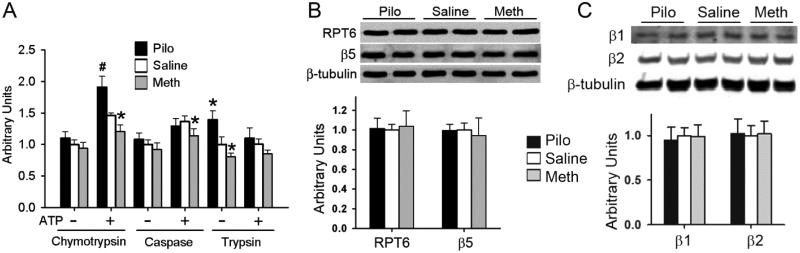
To explore the potential mechanism by which M2 receptors regulate UPS function, we then assessed the effect of M2 modulation on myocardial proteasome peptidase activities and the abundance of representative proteasome subunits. In the absence of ATP, the assays assess the activity of proteasome peptidases in the 20S proteasome. In absence of ATP, the assay did not detect differences in myocardial chymotrypsin-like and caspase-like activities among Pilo, Meth, and Saline treated groups but the assay showed that trypsin-like activity was higher in the Pilo group and lower in the Meth group, compared to the Saline group (Figure 3A). These results suggest stimulation of M2 receptor increases the trypsin-like activity of myocardial 20S proteasomes. In the presence of ATP, the assays are believed to assess 26S proteasome activities. In the presence of ATP, M receptor stimulation by Pilo elicited remarkable increases in chymotrypsin-like activity but showed no effect on caspase-like and trypsin-like activities (Figure 3A). Conversely, M2 blockade by Meth decreased chymotrypsin-like and caspase-like activities (Figure 3A); indicating that M2 receptor may play an important role in positively regulating chymotrypsin-like activity of the myocardial 26S proteasome. Neither Pilo nor Meth treatment caused a significant change in the abundance of representative subunit of the 19S (Rpt6) or the 20S (β5, β2, β1) proteasomes (Figure 3B, 3C). These results suggest that M2 receptor activation stimulates specific proteasome activities without altering the 19S and the 20S proteasome abundance.
Figure 3. Manipulation of the M2 receptor alters myocardial proteasome peptidase activities but not proteasome abundance in mice.
Myocardial tissues were collected for analyses reported here after mice were treated as described in Figure 1. A, the chymotrypsin-like, caspase-like, and trypsin-like activities in the crude protein extracts from ventricular myocardium were assessed in the presence and absence of ATP using specific fluorogenic substrates. *p<0.05, #p<0.01 vs. Saline; n=6 mice/group. B and C, the key proteasome subunits RPT6 of the 19S cap and β5, β2, and β1 subunits of the 20S core were assessed by western blot analyses. β-Tubulin was probed as the loading control. Pooled densitometric data are shown in the lower panels. No statistically significant differences were detected for RPT6,β5, β2, and β1 subunit protein levels between any treatments.
3.2 Proteasome activity is enhanced by M receptor stimulation in cultured cardiomyocytes
Our in vivo data suggest a significant regulatory role for the M2 receptor in modulating myocardial proteasome activities. To examine whether the M receptor-mediated regulation on the proteasome is cardiomyocyte-autonomous, we tested the effect of M2 stimulation by Pilo on the proteasome in cultured NRVMs.
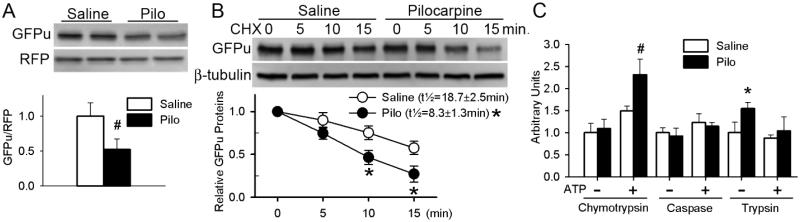
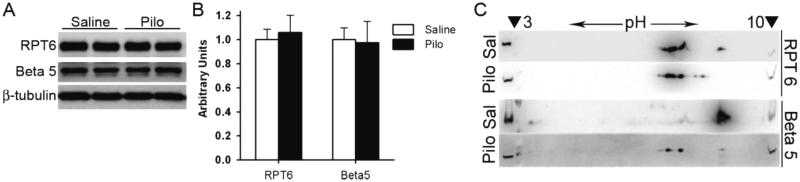
To assess UPS function, GFPu and RFP were overexpressed in cultured NRVMs via an identical adenoviral vector. GFPu is similar to GFPdgn and is a proven UPS surrogate substrate but red fluorescent protein (RFP) is not a substrate. When GFPu and RFP are co-expressed in cultured cells under the control of the identical vector, changes in RFP protein levels provide a reference for potential changes in GFPu protein synthesis [23]. Therefore the GFPu/RFP ratio corrects for protein synthesis and is a more accurate parameter than GFPu alone in assessing UPS function. Pilo induced remarkable decreases in the GFPu/RFP ratio (Figure 4A), suggesting that M2 stimulation destabilizes GFPu. Indeed, cycloheximide (CHX) chase assays confirmed that Pilo markedly shortened GFPu half-life (Figure 4B). These findings from cell cultures confirm that stimulating the M receptors of cardiomyocytes enhances UPS-mediated proteolysis. This enhancement is associated with increased 26S proteasome chymotrypsin-like and 20S proteasome trypsin-like activities (Figure 4C) without an alteration in proteasome abundance (Figure 5A-5B, Supplementary Figure 2). In parallel, Pilo induced an acidic shift of the isoelectric points (pI) of a subpopulation of Rpt6 and β5 subunits (Figure 5C), suggesting that M stimulation enhances proteasome function likely through triggering post-translational modifications (PTMs) capable of decreasing the pI of key proteasome subunits.
Figure 4. Stimulation of M receptors enhances UPS proteolytic function and proteasome activities in cultured cardiomyocytes.
A, Pilocarpine (Pilo, 10μM) or saline control (Saline) was administered to neonatal rat ventricular myocytes in culture (NRVMs) 24h after infection with Ad-GFPu and Ad-RFP. The cells were harvested at 48h of pilocarpine treatment. Total protein extracts were used for western blot analyses of the steady state GFPu and RFP. B, Cycloheximide (CHX) chase assay for GFPu. NRVMs were first infected with Ad-GFPu (10MOI) and 24h later treated with pilocarpine (10μM) or saline. After an additional 24 hours, CHX (100μM) was added to the culture media to inhibit protein synthesis. Cells were harvested at baseline (0 min), 5, 10, and 15 min after CHX addition and the cell lysate was used for immunoblot analysis. Representative images (upper panel) and pooled densitometry data from 4 biological repeats (lower panel) are shown. GFPu half-life (t1/2) in the pilocarpine treated cells was significantly shorter than in the saline control treated cells (8.3±1.3min vs. 18.7±2.5 min; n=4 pairs; p<0.05, paired t-test). C, Proteasome peptidase activity assays. Crude protein extracts from NRVMs that had been treated as in panel A were used for assessing chymotrypsin-like, caspase-like, and trypsin-like activities in the presence (+) and absence (−) of ATP. *p<0.05 vs. Saline, n=12 biological repeats.
Figure 5. Stimulation of M receptors alters the pI of key proteasome subunits in cultured cardiomyocytes.
NRVMs were cultured for 24 hours prior to treatment with the muscarinic receptor agonist, pilocarpine (10μM), or volume corrected vehicle control, saline, for 48 hours. A and B, western blot analyses of the key proteasome subunits, RPT6 and Beta5. Representative images (A) and the summary of pooled densitometry (B) are shown. β-Tubulin was probed as the loading control. N= 6 per treatment. C, Representative images of 2D western blots for Rpt6 and β5 subunits in cultured NRVMs harvested 15 min after pilocarpine (Pilo, 10μM) or volume corrected saline (Sal) treatment. In gel for the 2nd dimension electrophoresis, the isoelectric focusing gel strip (pH 3-10) was placed in the large central well sandwiched by small wells (denoted by arrowheads) that were loaded with the input of the first dimension.
To examine whether macroautophagy is involved in the observed effects, we performed the autophagic flux assay, in which the protein levels of both the lipidated form of microtubule binding protein 1 light chain 3 (LC3-II) and p62 in cultured NRVMs treated with saline or Pilo were measured in the absence or presence of lysosomal inhibition by bafilomycin A1 (BFA),[48] a vacuolar proton-ATPase inhibitor. We found that in the same time frame and treatment protocol that Pilo facilitates the degradation of GFPu, Pilo did not discernibly alter autophagic flux (Supplementary Figure 3).
3.3 PKG mediates M receptor regulation of UPS-mediated proteolysis
The intracellular response of M2 receptor stimulation is thought to be mediated by the G protein βγ subunits and PKG [24, 49]. Our previous studies suggest that PKG is a novel regulator of proteasome-mediated proteolysis [11]. Therefore we investigated whether PKG mediates M2 receptor-regulation of the proteasome.
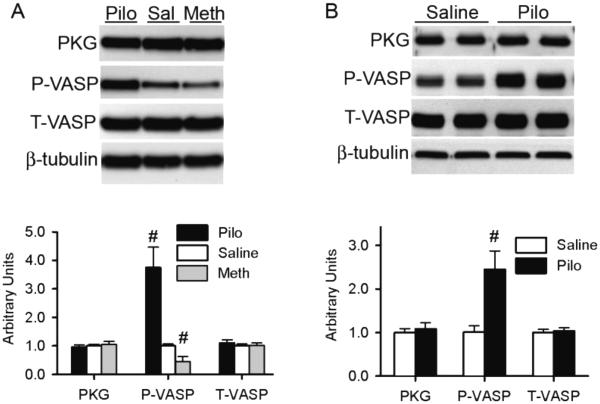
Western blot analyses showed that the myocardial total protein levels of PKG and VASP, a bona fide substrate of PKG [50, 51], were not altered but Ser239-phosphorylated VASP was significantly increased by Pilo and decreased by Meth (Figure 6A), indicating that the M manipulation was successful and induced expected PKG activity changes in the heart. As observed in mice, Pilo treatment markedly increased Ser239-phosphorylated VASP (Figure 6B), indicating that PKG is activated by Pilo in cultured cardiomyocytes.
Figure 6. M2 receptor manipulation alters the phosphorylation of a PKG substrate.
Shown are representative images (upper panels) and pooled densitometry data (lower panels) of western blot analyses for PKG, Ser239-phophorylated VASP (P-VASP), and total VASP (T-VASP). β-Tubulin was probed for loading control. Muscarinic receptors in mice (A) were interrogated as described in Figure 1; those in cultured NRVMs (B) are interrogated as described in Figure 5. N= 6 per treatment.
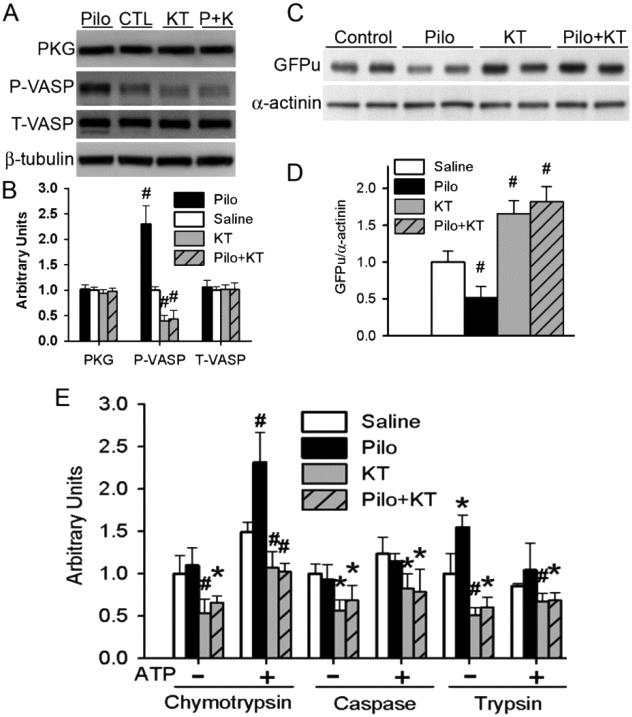
Inasmuch as both our in vivo and in vitro experiments showed PKG activation in parallel to proteasome enhancement by Pilo treatments, we sought to test the role of PKG in triggering proteasome functional changes by M stimulation in cultured cardiomyocytes. To inhibit PKG, we used KT5823, a widely used, cell-permeable, and selective inhibitor of PKG [52]. We first verified that KT5823 suppressed PKG activity of cultured cardiomyocytes and prevented Pilo from increasing PKG activity as evidenced by changes of Ser239-phosphorylated VASP (Figure 7A, 7B). KT5823 accumulated GFPu and inhibited all three proteasome peptidase activities. Pilo-induced decreases in GFPu protein levels and increases in proteasome peptidase activities were all prevented by KT5823 co-treatment (Figure 7C ~ 7E). These results suggest that the regulation of proteasome function by M receptors in cardiomyocytes is mediated by PKG.
Figure 7. Enhancing proteasome function by muscarinic stimulation is PKG-dependent in cardiomyocytes.
Cultured NRVMs were infected with Ad-GFPu (10 MOI) and cultured for additional 24 hours before being treated with pilocarpine (Pilo, 10μM), KT5823 (KT, 1μM), Pilo plus KT (Pilo+KT; P+K), or vehicle control (saline) for 24h. A and B, Representative images (A) and the pooled densitometry data (B) of western blot analyses for the indicated proteins. C and D, Representative images (C) and the pooled densitometry data (D) of western blot analysis for GFPu. α-Actinin was probed as loading control. E, Changes in the proteasomal chymotrypsin-like, caspase-like, and trypsin-like peptidase activities in NRVMs. *p<0.05, #p<0.01 vs. the saline control group; n=6 repeats.
4. Discussion
Decreased PNS control of the heart was observed in clinical and experimental CHF and associated with a poorer outcome of CHF patients [53, 54]. Conversely, vagal stimulation was shown to improve cardiac function and decrease mortality in animal models of CHF [36, 39]; however, the underlying mechanism is incompletely understood. As with PNS dysregulation, a role for PFI has been implicated in at least a subset of heart disease [15, 19]. A growing body of research has been focused on systems that regulate proteasome function, more importantly how proteasome function is modified in different (patho-) physiological states [55-58]. Notably, preserving or enhancing proteasome function was found to be cardioprotective against proteotoxicity and ischemia-reperfusion injury [11, 59, 60]. Previous results from our lab demonstrated that improving proteasome-mediated degradation of misfolded proteins, via genetic and pharmacological approaches, alleviates cardiac pathological conditions with increased production of misfolded/damaged proteins [11, 22, 23]. The present study provides the compelling evidence that: (1) likely through the M2 receptor on cardiomyocytes, the PNS plays an essential role in regulating proteasome-mediated protein degradation in the heart at baseline condition, (2) pharmacological stimulation of M receptors is capable of enhancing proteasome function of the heart, and (3) Proteasomal enhancement by M stimulation is mediated by PKG. These discoveries carry significant clinical implications considering that: (1) the PNS innervates virtually every organ and tissue in the body and (2) the PNS is dysregulated in many pathological conditions.
4.1 M2 stimulation facilitates UPS-mediated protein degradation
Here we show first in mice that M stimulation by Pilo reduces, whereas M2 inhibition by Meth increases, the cardiac protein level of stably expressed GFPdgn (a proven surrogate substrate of the UPS), without affecting the mRNA level of GFPdgn. These findings are corroborated by results from the experiments using cardiomyocyte cultures which showed that pharmacological stimulation of the M receptors decreased the steady state level of GFPu (similar to GFPdgn, a UPS surrogate substrate) and, more importantly, shortened GFPu protein half-life. Although Pilo is a non-selective agonist for M receptors, M2 is the main isoform of M receptors expressed in mammalian cardiomyocytes [24]; hence, it is highly likely that M2 receptors are the primary receptor of cardiomyocytes stimulated by Pilo. This view is supported by the observation that the M2 selective antagonist, Meth, attenuated proteasome GFPdgn clearance. These results indicate that the effect of M2 activation on myocardial UPS function is cardiomyocyte-autonomous and that PNS plays an important role in the regulation of UPS proteolytic function in the heart.
Interestingly, it was recently reported that nicotinic acetylcholine receptor stimulation blunts the increases of renal proteasome activities associated with acute experimental inflammation [61]. These findings bring up an intriguing possibility that PNS may differentially regulate proteasome function in a cell, depending on type of cholinergic receptors expressed on the cell.
4.2 Potential mechanisms of M2 enhancement of cardiac UPS function
Protein degradation by the UPS consists of two main steps: ubiquitination of the target protein molecule and subsequent degradation of the ubiquitinated protein by the 26S proteasome. Although it is unclear at this time whether M stimulation alters the ubiquitination of specific proteins, our data reveal that M2 stimulation induced enhancement of the degradation of a UPS surrogate substrate is associated with increased proteasomal peptidase activities in mouse hearts and cultured cardiomyocytes, suggesting that enhanced proteasome activities may be responsible for the enhanced UPS function. This notion is consistent with our observation that the steady state protein levels of bona fide native UPS substrates (eg, β-catenin, GATA4) were not reduced, but surrogate misfolded proteins (GFPdgn and GFPu) were destabilized, by M stimulation in vivo and in vitro. GFPdgn and GFPu carry degron CL1 which is characterized by surface exposure of a stretch of hydrophobic amino acid residues, a signature conformation of protein misfolding [8]. Recent studies have demonstrated that the degradation of a native protein is not increased by simply enhancing proteasome function without affecting ubiquitination [11, 22, 62]. This is because the rate limiting step of UPS-mediated degradation of native proteins (ie, regulatory degradation) resides in the ubiquitination step, and in general the proteasome is incapable of degrading a native protein that has not been polyubiquitinated. However, the rate-limiting step of the degradation of misfolded proteins by the UPS in the cell resides likely in the proteasomal degradation step as evidenced by misfolded proteins that are accumulated in the cell under proteotoxic stress are usually ubiquitinated and measures to enhance proteasome function can facilitate their removal [11, 13, 22, 62, 63].
The primary determinants of proteasome peptidase activities are proteasome abundance and posttranslational modifications to the proteasome [10]. The M2 regulation of proteasome activities is not due to altered proteasome abundance, as no changes were detected from key proteasome subunits in cultured cardiomyocytes or intact mice. We have further unveiled that M2 activation shifts the pI of key subunits of the 19S and 20S proteasomes toward the acidic side, a change that is usually caused by increased phosphorylation and capable of increasing proteasome peptidase activities [64]. These findings suggest that PNS innervation via the M2 receptor plays an important physiological role in supporting proteasome function in the heart and pharmacological stimulation of M receptors can lead to posttranslational modifications to the proteasome and increase proteasome activity in the heart.
PKG is a known main downstream kinase to mediate M2 intracellular signaling in cardiomyocytes [24, 49]; and notably, the effects of M2 manipulation on cardiac UPS function are consistent with our previous results of PKG manipulation [11], suggesting that PKG may be the mediator of the muscarinic response. Indeed, we show this is likely the case. First, the phosphorylation of a PKG substrate (VASP) is increased after M stimulation in mice and cells, which was reversed in cultured cardiomyocytes by PKG inhibition; conversely, M2 inhibition reduces myocardial VASP phosphorylation in mice. Second, in cultured cardiomyocytes, M2 stimulation (this study) and PKG activation (previous study) induced the same pattern of changes in proteasome peptidase activities: both increased the 26S proteasome chymotrypsin-like activity and the 20S trypsin-like activity but showed no discernible effect on the caspase-like activity [11]. Third, both M2 stimulation and PKG activation shifted similarly the pI of a subpopulation of PSMB5 and Rpt6 toward the acidic side [11]. Lastly, the M2-mediated enhancement of UPS-meditated protein degradation is reversed by PKG inhibition in cardiomyocytes. These results indicate the importance of PKG as a protein kinase in regulating cardiomyocyte PQC. It will be important to test if PKG can directly phosphorylate proteasome subunits and, if so, whether the phosphorylation is responsible for increased proteasomal degradation of misfolded proteins.
4.3 Significance and clinical implication
Here we show that administration of an M2 antagonist (Meth) remarkably impairs UPS-mediated protein degradation of a surrogate misfolded protein, providing the first evidence that PNS innervation via the M2-PKG cascade positively regulates proteasome function in the heart and thereby facilitates the degradation of misfolded proteins in cardiomyocytes. This is a significant discovery because it suggests that reduced PNS activity may contribute to the development of myocardial PFI under pathological conditions. Both myocardial PFI and reduced PNS activity are observed in many forms of cardiovascular disease [7, 19, 36, 54].
Previous studies have indicated the accumulation of misfolded proteins and inadequate proteasome function in cardiac disease from various etiologies [7, 13, 16, 19]. It has also been shown that enhancement of the proteasome can protect the heart from proteotoxic stress [11, 22, 62]. The present study has also demonstrated that pharmacological stimulation of the M receptors can enhance myocardial proteasome function. This is potentially significant because pharmacological agents capable of enhancing proteasome function and facilitating the removal of misfolded proteins in cardiomyocytes are limited to our previous finding of sildenafil [11]. Therefore, M2 agonists are attractive drug candidates to treat proteinopathies and a possible supplement to current CHF treatment regimens. Moreover, enhancement of PNS/M2/PKG activity has been demonstrated to be protective during many diseases [27, 39, 40, 65]. Findings of the present study suggest a novel underlying mechanism that is at least partly responsible through increased UPS-mediated degradation of misfolded proteins. It should be pointed out that while our findings are provocative, a limitation is that this work examined primarily the short term effects of PNS manipulation on proteasome function. In future work it will be interesting and important to examine the long term consequences of PNS manipulation of proteasome function on clearance of misfolded proteins and cardiac pathologies.
Supplementary Material
Highlights.
Muscarinic stimulation facilitates cardiac proteasomal degradation
Muscarinic 2 receptor blockade suppresses cardiac proteasomal degradation
Muscarinic stimulation increases proteasome peptidase activity in cardiomyocytes
Muscarinic stimulation of the proteasome is mediated by protein kinase G
5. ACKNOWLEDGEMENTS
We thank the Imaging Core of the Division of Basic Biomedical Sciences for assistance with confocal microscopy and Ms. Andrea Jahn for maintaining mouse colonies and genotyping. Dr. X. Wang was a recipient of the Established Investigator Award of the American Heart Association.
6. Sources of Funding
This work was supported in part by NIH grants R01HL085629 and R01072166, American Heart Association grants to 0740025N (to X.W.) and 11PRE5730009 (to M.J.R). The Imaging Core was supported by an NIH grant 5P20RR015567.
Footnotes
7. Disclosure: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
8. References
- 1.Castro A, Bernis C, Vigneron S, Labbe JC, Lorca T. The anaphase-promoting complex: a key factor in the regulation of cell cycle. Oncogene. 2005;24:314–25. doi: 10.1038/sj.onc.1207973. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg AL, Cascio P, Saric T, Rock KL. The importance of the proteasome and subsequent proteolytic steps in the generation of antigenic peptides. Mol Immunol. 2002;39:147–64. doi: 10.1016/s0161-5890(02)00098-6. [DOI] [PubMed] [Google Scholar]
- 3.Ciechanover A, Orian A, Schwartz AL. Ubiquitin-mediated proteolysis: biological regulation via destruction. Bioessays. 2000;22:442–51. doi: 10.1002/(SICI)1521-1878(200005)22:5<442::AID-BIES6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 4.Haglund K, Di Fiore PP, Dikic I. Distinct monoubiquitin signals in receptor endocytosis. Trends Biochem Sci. 2003;28:598–603. doi: 10.1016/j.tibs.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Robbins J. Heart failure and protein quality control. Circ Res. 2006;99:1315–28. doi: 10.1161/01.RES.0000252342.61447.a2. [DOI] [PubMed] [Google Scholar]
- 7.Su H, Wang X. The ubiquitin-proteasome system in cardiac proteinopathy: a quality control perspective. Cardiovasc Res. 2010;85:253–62. doi: 10.1093/cvr/cvp287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Su H, Ranek MJ. Protein quality control and degradation in cardiomyocytes. J Mol Cell Cardiol. 2008;45:11–27. doi: 10.1016/j.yjmcc.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powell SR. The cardiac 26S proteasome: regulating the regulator. Circ Res. 2006;99:342–5. doi: 10.1161/01.RES.0000239412.40685.61. [DOI] [PubMed] [Google Scholar]
- 10.Cui Z, Scruggs SB, Gilda JE, Gomes AV. Regulation of cardiac proteasomes by ubiquitination, sumoylation, and beyond. J Mol Cell Cardiol. 2013 doi: 10.1016/j.yjmcc.2013.10.008. doi: 10.1016/j.yjmcc.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ranek MJ, Terpstra EJM, Li J, Kass DA, Wang X. Protein kinase G positively regulates proteasome-mediated degradation of misfolded proteins. Circulation. 2013;128:365–76. doi: 10.1161/CIRCULATIONAHA.113.001971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bence NF, Sampat RM, Kopito RR. Impairment of the ubiquitin-proteasome system by protein aggregation. Science. 2001;292:1552–5. doi: 10.1126/science.292.5521.1552. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Tang M, Mestril R, Wang X. Aberrant protein aggregation is essential for a mutant desmin to impair the proteolytic function of the ubiquitin-proteasome system in cardiomyocytes. J Mol Cell Cardiol. 2006;40:451–4. doi: 10.1016/j.yjmcc.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Chen Q, Huang W, Horak KM, Zheng H, Mestril R, et al. Impairment of the ubiquitin-proteasome system in desminopathy mouse hearts. FASEB J. 2006;20:362–4. doi: 10.1096/fj.05-4869fje. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Li J, Zheng H, Su H, Powell SR. Proteasome functional insufficiency in cardiac pathogenesis. Am J Physiol Heart Circ Physiol. 2011;301:H2207–19. doi: 10.1152/ajpheart.00714.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weekes J, Morrison K, Mullen A, Wait R, Barton P, Dunn MJ. Hyperubiquitination of proteins in dilated cardiomyopathy. Proteomics. 2003;3:208–16. doi: 10.1002/pmic.200390029. [DOI] [PubMed] [Google Scholar]
- 17.Kostin S, Pool L, Elsasser A, Hein S, Drexler HC, Arnon E, et al. Myocytes die by multiple mechanisms in failing human hearts. Circ Res. 2003;92:715–24. doi: 10.1161/01.RES.0000067471.95890.5C. [DOI] [PubMed] [Google Scholar]
- 18.Gianni D, Li A, Tesco G, McKay KM, Moore J, Raygor K, et al. Protein aggregates and novel presenilin gene variants in idiopathic dilated cardiomyopathy. Circulation. 2010;121:1216–26. doi: 10.1161/CIRCULATIONAHA.109.879510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Predmore JM, Wang P, Davis F, Bartolone S, Westfall MV, Dyke DB, et al. Ubiquitin proteasome dysfunction in human hypertrophic and dilated cardiomyopathies. Circulation. 2010;121:997–1004. doi: 10.1161/CIRCULATIONAHA.109.904557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Osinska H, Klevitsky R, Gerdes AM, Nieman M, Lorenz J, et al. Expression of R120G-alphaB-crystallin causes aberrant desmin and alphaB-crystallin aggregation and cardiomyopathy in mice. Circ Res. 2001;89:84–91. doi: 10.1161/hh1301.092688. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Osinska H, Dorn GW, Nieman M, Lorenz JN, Gerdes AM, et al. Mouse model of desmin-related cardiomyopathy. Circulation. (2nd) 2001;103:2402–7. doi: 10.1161/01.cir.103.19.2402. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Horak KM, Su H, Sanbe A, Robbins J, Wang X. Enhancement of proteasomal function protects against cardiac proteinopathy and ischemia/reperfusion injury in mice. J Clin Invest. 2011;121:3689–700. doi: 10.1172/JCI45709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Powell SR, Wang X. Enhancement of proteasome function by PA28α overexpression protects against oxidative stress. Faseb J. 2011;25:883–93. doi: 10.1096/fj.10-160895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhein S, van Koppen CJ, Brodde OE. Muscarinic receptors in the mammalian heart. Pharmacol Res. 2001;44:161–82. doi: 10.1006/phrs.2001.0835. [DOI] [PubMed] [Google Scholar]
- 25.Caulfield MP. Muscarinic receptors--characterization, coupling and function. Pharmacol Ther. 1993;58:319–79. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- 26.Kawano H, Okada R, Yano K. Histological study on the distribution of autonomic nerves in the human heart. Heart Vessels. 2003;18:32–9. doi: 10.1007/s003800300005. [DOI] [PubMed] [Google Scholar]
- 27.LaCroix C, Freeling J, Giles A, Wess J, Li YF. Deficiency of M2 muscarinic acetylcholine receptors increases susceptibility of ventricular function to chronic adrenergic stress. Am J Physiol Heart Circ Physiol. 2008;294:H810–20. doi: 10.1152/ajpheart.00724.2007. [DOI] [PubMed] [Google Scholar]
- 28.Hare JM, Keaney JF, Jr., Balligand JL, Loscalzo J, Smith TW, Colucci WS. Role of nitric oxide in parasympathetic modulation of beta-adrenergic myocardial contractility in normal dogs. J Clin Invest. 1995;95:360–6. doi: 10.1172/JCI117664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henning RJ, Khalil IR, Levy MN. Vagal stimulation attenuates sympathetic enhancement of left ventricular function. Am J Physiol. 1990;258:H1470–5. doi: 10.1152/ajpheart.1990.258.5.H1470. [DOI] [PubMed] [Google Scholar]
- 30.Nagata K, Ye C, Jain M, Milstone DS, Liao R, Mortensen RM. Galpha(i2) but not Galpha(i3) is required for muscarinic inhibition of contractility and calcium currents in adult cardiomyocytes. Circ Res. 2000;87:903–9. doi: 10.1161/01.res.87.10.903. [DOI] [PubMed] [Google Scholar]
- 31.Dabrowska B, Dabrowski A, Skrobowski A. Parasympathetic withdrawal precedes spontaneous blood pressure elevations in women with primary hypertension. Cardiology. 1996;87:119–24. doi: 10.1159/000177073. [DOI] [PubMed] [Google Scholar]
- 32.Langewitz W, Ruddel H, Schachinger H. Reduced parasympathetic cardiac control in patients with hypertension at rest and under mental stress. Am Heart J. 1994;127:122–8. doi: 10.1016/0002-8703(94)90517-7. [DOI] [PubMed] [Google Scholar]
- 33.Dall'ago P, D'Agord Schaan B, da Silva VO, Werner J, da Silva Soares PP, de Angelis K, et al. Parasympathetic dysfunction is associated with baroreflex and chemoreflex impairment in streptozotocin-induced diabetes in rats. Auton Neurosci. 2007;131:28–35. doi: 10.1016/j.autneu.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Bunag RD, Krizsan D, Itoh H. Diminished cardiovascular responsiveness to vagal stimulation in obese rats. Am J Physiol. 1990;259:R842–8. doi: 10.1152/ajpregu.1990.259.4.R842. [DOI] [PubMed] [Google Scholar]
- 35.Girgis I, Chakko S, de Marchena E, Jara C, Diaz P, Castellanos A, et al. Effect of clonidine on heart rate variability in congestive heart failure. Am J Cardiol. 1998;82:335–7. doi: 10.1016/s0002-9149(98)00329-4. [DOI] [PubMed] [Google Scholar]
- 36.Olshansky B, Sabbah HN, Hauptman PJ, Colucci WS. Parasympathetic nervous system and heart failure: pathophysiology and potential implications for therapy. Circulation. 2008;118:863–71. doi: 10.1161/CIRCULATIONAHA.107.760405. [DOI] [PubMed] [Google Scholar]
- 37.La Rovere MT, Bigger JT, Jr., Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–84. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 38.Pedretti RF, Prete G, Foreman RD, Adamson PB, Vanoli E. Autonomic modulation during acute myocardial ischemia by low-dose pirenzepine in conscious dogs with a healed myocardial infarction: a comparison with beta-adrenergic blockade. J Cardiovasc Pharmacol. 2003;41:671–7. doi: 10.1097/00005344-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation. 2004;109:120–4. doi: 10.1161/01.CIR.0000105721.71640.DA. [DOI] [PubMed] [Google Scholar]
- 40.Zamotrinsky AV, Kondratiev B, de Jong JW. Vagal neurostimulation in patients with coronary artery disease. Auton Neurosci. 2001;88:109–16. doi: 10.1016/S1566-0702(01)00227-2. [DOI] [PubMed] [Google Scholar]
- 41.Gilon T, Chomsky O, Kulka RG. Degradation signals for ubiquitin system proteolysis in Saccharomyces cerevisiae. EMBO J. 1998;17:2759–66. doi: 10.1093/emboj/17.10.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumarapeli AR, Horak KM, Glasford JW, Li J, Chen Q, Liu J, et al. A novel transgenic mouse model reveals deregulation of the ubiquitin-proteasome system in the heart by doxorubicin. FASEB J. 2005;19:2051–3. doi: 10.1096/fj.05-3973fje. [DOI] [PubMed] [Google Scholar]
- 43.Dong X, Liu J, Zheng H, Glasford JW, Huang W, Chen QH, et al. In situ dynamically monitoring the proteolytic function of the ubiquitin-proteasome system in cultured cardiac myocytes. Am J Physiol Heart Circ Physiol. 2004;287:H1417–25. doi: 10.1152/ajpheart.01233.2003. [DOI] [PubMed] [Google Scholar]
- 44.Kumarapeli AR, Su H, Huang W, Tang M, Zheng H, Horak KM, et al. Alpha B-crystallin suppresses pressure overload cardiac hypertrophy. Circ Res. 2008;103:1473–82. doi: 10.1161/CIRCRESAHA.108.180117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tydlacka S, Wang CE, Wang X, Li S, Li XJ. Differential activities of the ubiquitin-proteasome system in neurons versus glia may account for the preferential accumulation of misfolded proteins in neurons. J Neurosci. 2008;28:13285–95. doi: 10.1523/JNEUROSCI.4393-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su H, Li J, Menon S, Liu J, Kumarapeli AR, Wei N, et al. Perturbation of cullin deneddylation via conditional Csn8 ablation impairs the ubiquitin-proteasome system and causes cardiomyocyte necrosis and dilated cardiomyopathy in mice. Circ Res. 2011;108:40–50. doi: 10.1161/CIRCRESAHA.110.230607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kagawa T, Watanabe N, Mochizuki K, Numari A, Ikeno Y, Itoh J, et al. Phenotypic differences in PFIC2 and BRIC2 correlate with protein stability of mutant Bsep and impaired taurocholate secretion in MDCK II cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G58–67. doi: 10.1152/ajpgi.00367.2007. [DOI] [PubMed] [Google Scholar]
- 48.Su H, Li F, Ranek MJ, Wei N, Wang X. COP9 signalosome regulates autophagosome maturation. Circulation. 2011;124:2117–28. doi: 10.1161/CIRCULATIONAHA.111.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brodde OE, Bruck H, Leineweber K, Seyfarth T. Presence, distribution and physiological function of adrenergic and muscarinic receptor subtypes in the human heart. Basic Res Cardiol. 2001;96:528–38. doi: 10.1007/s003950170003. [DOI] [PubMed] [Google Scholar]
- 50.Deguchi A, Soh JW, Li H, Pamukcu R, Thompson WJ, Weinstein IB. Vasodilator-stimulated phosphoprotein (VASP) phosphorylation provides a biomarker for the action of exisulind and related agents that activate protein kinase G. Mol Cancer Ther. 2002;1:803–9. [PubMed] [Google Scholar]
- 51.Moens AL, Takimoto E, Tocchetti CG, Chakir K, Bedja D, Cormaci G, et al. Reversal of cardiac hypertrophy and fibrosis from pressure overload by tetrahydrobiopterin: efficacy of recoupling nitric oxide synthase as a therapeutic strategy. Circulation. 2008;117:2626–36. doi: 10.1161/CIRCULATIONAHA.107.737031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hidaka H, Kobayashi R. Pharmacology of protein kinase inhibitors. Annu Rev Pharmacol Toxicol. 1992;32:377–97. doi: 10.1146/annurev.pa.32.040192.002113. [DOI] [PubMed] [Google Scholar]
- 53.Porter TR, Eckberg DL, Fritsch JM, Rea RF, Beightol LA, Schmedtje JF, Jr., et al. Autonomic pathophysiology in heart failure patients. Sympathetic-cholinergic interrelations. J Clin Invest. 1990;85:1362–71. doi: 10.1172/JCI114580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Azevedo ER, Parker JD. Parasympathetic control of cardiac sympathetic activity: normal ventricular function versus congestive heart failure. Circulation. 1999;100:274–9. doi: 10.1161/01.cir.100.3.274. [DOI] [PubMed] [Google Scholar]
- 55.Scruggs SB, Zong NC, Wang D, Stefani E, Ping P. Post-translational Modification of Cardiac Proteasomes: Functional Delineation Enabled by Proteomics. Am J Physiol Heart Circ Physiol. 2012 doi: 10.1152/ajpheart.00189.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Asai M, Tsukamoto O, Minamino T, Asanuma H, Fujita M, Asano Y, et al. PKA rapidly enhances proteasome assembly and activity in in vivo canine hearts. J Mol Cell Cardiol. 2009;46:452–62. doi: 10.1016/j.yjmcc.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 57.Lu H, Zong C, Wang Y, Young GW, Deng N, Souda P, et al. Revealing the dynamics of the 20 S proteasome phosphoproteome: a combined CID and electron transfer dissociation approach. Mol Cell Proteomics. 2008;7:2073–89. doi: 10.1074/mcp.M800064-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang F, Hu Y, Huang P, Toleman CA, Paterson AJ, Kudlow JE. Proteasome function is regulated by cyclic AMP-dependent protein kinase through phosphorylation of Rpt6. J Biol Chem. 2007;282:22460–71. doi: 10.1074/jbc.M702439200. [DOI] [PubMed] [Google Scholar]
- 59.Divald A, Kivity S, Wang P, Hochhauser E, Roberts B, Teichberg S, et al. Myocardial ischemic preconditioning preserves postischemic function of the 26S proteasome through diminished oxidative damage to 19S regulatory particle subunits. Circ Res. 2010;106:1829–38. doi: 10.1161/CIRCRESAHA.110.219485. [DOI] [PubMed] [Google Scholar]
- 60.Churchill EN, Ferreira JC, Brum PC, Szweda LI, Mochly-Rosen D. Ischaemic preconditioning improves proteasomal activity and increases the degradation of deltaPKC during reperfusion. Cardiovasc Res. 2010;85:385–94. doi: 10.1093/cvr/cvp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chatterjee PK, Yeboah MM, Dowling O, Xue X, Powell SR, Al-Abed Y, et al. Nicotinic acetylcholine receptor agonists attenuate septic acute kidney injury in mice by suppressing inflammation and proteasome activity. PLoS One. 2012;7:e35361. doi: 10.1371/journal.pone.0035361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li J, Powell SR, Wang X. Enhancement of proteasome function by PA28α overexpression protects against oxidative stress. Faseb J. 2011;25:883–93. doi: 10.1096/fj.10-160895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen Q, Liu JB, Horak KM, Zheng H, Kumarapeli AR, Li J, et al. Intrasarcoplasmic amyloidosis impairs proteolytic function of proteasomes in cardiomyocytes by compromising substrate uptake. Circ Res. 2005;97:1018–26. doi: 10.1161/01.RES.0000189262.92896.0b. [DOI] [PubMed] [Google Scholar]
- 64.Zong C, Gomes AV, Drews O, Li X, Young GW, Berhane B, et al. Regulation of murine cardiac 20S proteasomes: role of associating partners. Circ Res. 2006;99:372–80. doi: 10.1161/01.RES.0000237389.40000.02. [DOI] [PubMed] [Google Scholar]
- 65.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–22. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.