Abstract
Plant clonality, the ability of a plant species to reproduce itself vegetatively through ramets (shoot-root units), occurs in many plant species and is considered to be more frequent in cold or wet environments. However, a deeper understanding on the clonality-climate relationships along large geographic gradients is still scarce. In this study we revealed the clonality-climate relationships along latitudinal gradient of entire China spanning from tropics to temperate zones using clonality data for 4015 vascular plant species in 545 terrestrial communities. Structural equation modeling (SEM) showed that, in general, the preponderance of clonality increased along the latitudinal gradient towards cold, dry or very wet environments. However, the distribution of clonality in China was significantly but only weakly correlated with latitude and four climatic factors (mean annual temperature, temperature seasonality, mean annual precipitation, precipitation seasonality). Clonality of woody and herbaceous species had opposite responses to climatic variables. More precisely, woody clonality showed higher frequency in wet or climatically stable environments, while herbaceous clonality preferred cold, dry or climatically instable environments. Unexplained variation in clonality may be owed to the influences of other environmental conditions and to different clonal strategies and underlying traits adopted by different growth forms and phylogenetic lineages. Therefore, in-depth research in terms of more detailed clonal growth form, phylogeny and additional environmental variables are encouraged to further understand plant clonality response to climatic and/or edaphic conditions.
Introduction
Plant clonality is characterized by the ability of a plant species to reproduce itself vegetatively through ramets (shoot-root units) that are connected by spacers [1]–[3]. It is one of the key life-history strategies for adapting to environmental challenges in many species [4], [5]. Clonal growth enables plants to spread horizontally for exploiting limited resources in patchy environments, to share resource among ramets, and to store resource [1], [6]–[8]. Therefore, clonality has important implications for species survival and population persistence. Clonal plants dominate in many vegetations [5], [9], [10] and their distribution affects the species composition and structure of communities [11]–[14].
Distribution patterns of clonal species strongly depend on environmental conditions. Clonality is often considered to be important for the persistence of plants and the maintenance of plant communities in unfavorable environments, especially in cold or wet habitats [8], [15], [16]. The predominance of clonality is achieved by species turnover of non-clonal species and their replacement by clonal species along temperature and moisture gradients. Previous comparative studies on the flora of central Europe showed that clonal plants were more frequent at lower mean annual temperature (MAT) [5], [8]. Similarly, there is much evidence that clonality is more common in wet habitats, where water-logged conditions can cause oxygen stress [5], [8], [17], [18]. However, the distribution patterns of plant clonality along climatic gradients have been questioned recently [16], [19]. On the one hand, research on the distribution pattern of clonality along the Northeast China Transect (NECT) revealed that clonal plants were more abundant and important in communities at the western part of the NECT, where MAT and mean annual precipitation (MAP) were relatively low [20]. On the other hand, Klimešová and Doležal [19] did not find evidence that a higher proportion of clonal species appeared in cold environments in an analysis of a comprehensive dataset including arctic, alpine and reference regions. Whilst studies on plant clonality-climate relationships along geographic gradients have been accumulating, comprehensive datasets across large spatial gradients are still scarce.
Since clonal plants are considered to be more adapted to stressful (cold, dry or wet) environments than non-clonal plants, here we tested the hypothesis that clonal plants are more frequent in cold or wet habitats. In China, climate varies strikingly and shows clear latitudinal patterns. At the same time vegetation regions in China are very diverse and are broadly distributed from the cold temperate zones to the tropics. We thus expected that: (1) there were significant relationships between plant clonality and annual temperature and/or precipitation, or the seasonality of each of these, across China. However, as these single climatic variables may covary and interact along latitudinal gradients [21], latitude might best explain clonality as an integrative measure of these covarying gradients. Thus we expected that (2) strong latitudinal pattern of plant clonality would be observed over entire China.
Methods
Data Collection
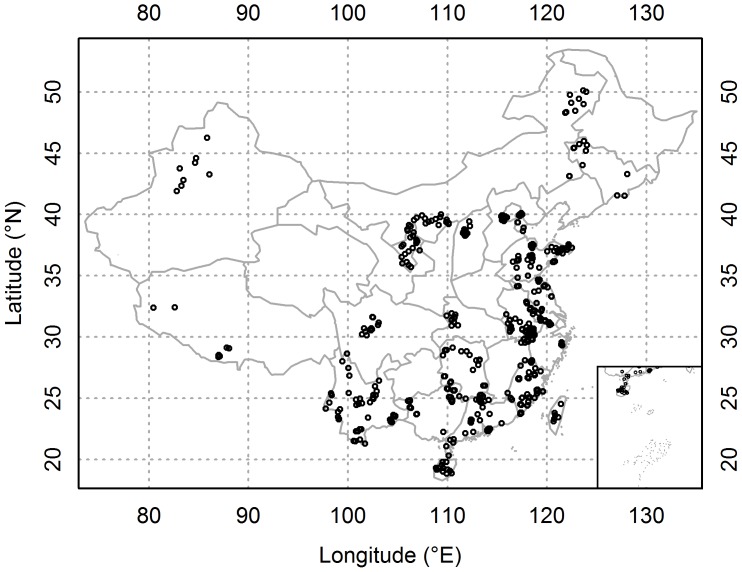
We collected plant species data based on personal communications (see Acknowledgements) and carried out field investigations in the arid and semi-arid areas of China, authorized by the Chinese Academy of Sciences (CAS). A total of 545 communities across the whole of China, ranging from 18°50'24'' to 50°7'48'' N, and from 80°26'24'' to 128°4'12'' E, were sampled (Figure 1). Floristic surveys using the plot method [22] were carried out by the same methodology. In the forest communities, plant species in the tree, shrub, and herb layers were sampled using one 20×20 m plot, four 2×2 m subplots and four 1×1 m subplots, respectively. In the shrub communities, plants in the shrub and herb layers were sampled using four 2×2 m plots and four 1×1 m subplots, respectively. In the herbaceous communities, plants were sampled using four 1 m×1 m plots. Species richness in each community was determined. Vascular plant species were categorized into non-clonal plants and clonal plants based on simple morphological characters described in the ‘Flora Reipublicae Popularis Sinicae’ [23], or by checking herbarium specimens of species if their descriptions on clonal characteristics were unavailable. We also used the CLO-PLA database [24] as a supplement for plant clonality identification: a species that had any of the 17 clonal growth organs in the CLO-PLA database was considered to be a clonal plant. Except for herbaceous species, woody species comprising trees and shrubs were also included in this study. Clonality of woody species was determined according to the spontaneous clonal growth by layering, rhizome production, or root suckering [25], [26]. The proportion of clonal species in a plant community, representing the relative importance of clonality, was determined by dividing the number of clonal species by the total number of species in a plant community. Totally, there were 4015 species (1360 genera, 227 families, 72 orders) in 545 plots, of which 1579 were determined as clonal species (595 genera, 154 families, 59 orders). Mean annual temperature (MAT, °C), mean annual precipitation (MAP, mm), temperature seasonality (TS) and precipitation seasonality (PS) of each site were extracted from the WorldClim dataset [27] based on its geographical coordinate (Table S1). MAT and MAP represent the annual average trends of heat and moisture for a given site, respectively while temperature seasonality and precipitation seasonality represent intra-annual climate instability.
Figure 1. Geographical positions of sampling sites in China.
Data analysis
Examining the bivariate relationships between variables is an important precursor before using structural equation modeling (SEM) because it allows potentially nonlinear relationships to be identified [28]–[30]. We used both linear and quadratic logistic regressions to test the bivariate relationships between clonality and latitude and each of four climatic variables, respectively, using the glm function with quasibinomial family and logit link in R, because the response variables were overdispersed [31]. In these regressions, we weighted the proportion of clonal species with species richness in each community. Similar procedures were used to uncover the relationship between each climatic factor and clonality for either woody species or herbaceous species due to disparate types of clonality of woody and herbaceous growths (see above). We used the chi-squared test and Nagelkerke's R square to carry out the choice of models on linear or quadratic logistic regressions. These analyses were performed in R package 2.15.0 [32].
We used structural equation modeling (SEM) to quantify the networks of interactions among latitude, climatic variables and clonality. SEM is a modern version of path analysis that allows for both the direct and indirect theoretical causal relationships between networks of intercorrelated variables to be tested [29], [33]. Variables were standardized to similar ranges prior to analysis (latitude and MAT were divided by 10, temperature seasonality by 100, MAP and precipitation seasonality by 1000). The SEMs were fitted using M-plus 6.1 with the binary response variable [34]. An initial SEM was specified based on prior theoretical knowledge. In this model, we assumed that latitude affects climatic variables which in turn affect plant clonality, in which climatic variables may interact. The chi-squared test of model fit was used to determine whether the fit between model and data was adequate (P>0.05).
Results
Clonality-climate relationship
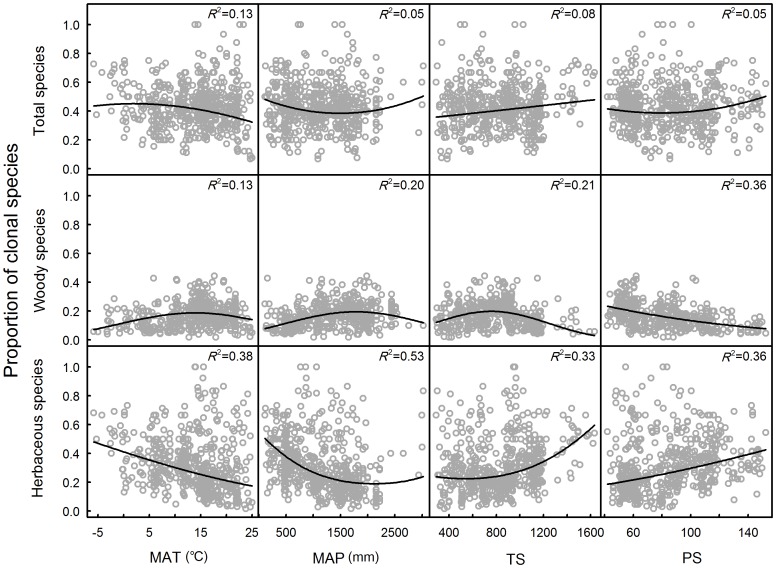
Logistic regressions exhibited an increasing trend of the proportion of clonality in plant communities with temperature seasonality (TS) and precipitation seasonality (PS) while it decreased with MAT and MAP (Figure 2a, b, c, d), in which MAT explained 13% of the total variation of clonality. Higher proportions of clonality were observed in the sites with over 2500-mm MAP, but this pattern was only based on few communities. Similar patterns can be seen in the relationships between herbaceous clonality and all climate factors considered (Figure 2i, j, k, l). Woody clonality showed contrasting patterns with total clonality pattern except for TS (Figure 2e, f, g, h), in which woody clonality increased up to a peak and decreased with TS increase. When the proportions of plant clonality were separated based on two contrasting growth forms, the four climate factors could explain much more variation within herbaceous or woody clonality than that in total clonality in a plant community (Figure 2).
Figure 2. Relationships between proportion of clonal species and climatic variables (MAT: mean annual temperature, MAP: mean annual precipitation, TS: temperature seasonality and PS: precipitation seasonality).
a–d: proportion of all clonal species vs climatic variables; e–h: proportion of woody clonal species vs climatic variables; i–l: proportion of herbaceous clonal species vs climatic variables.
Latitudinal pattern of clonality
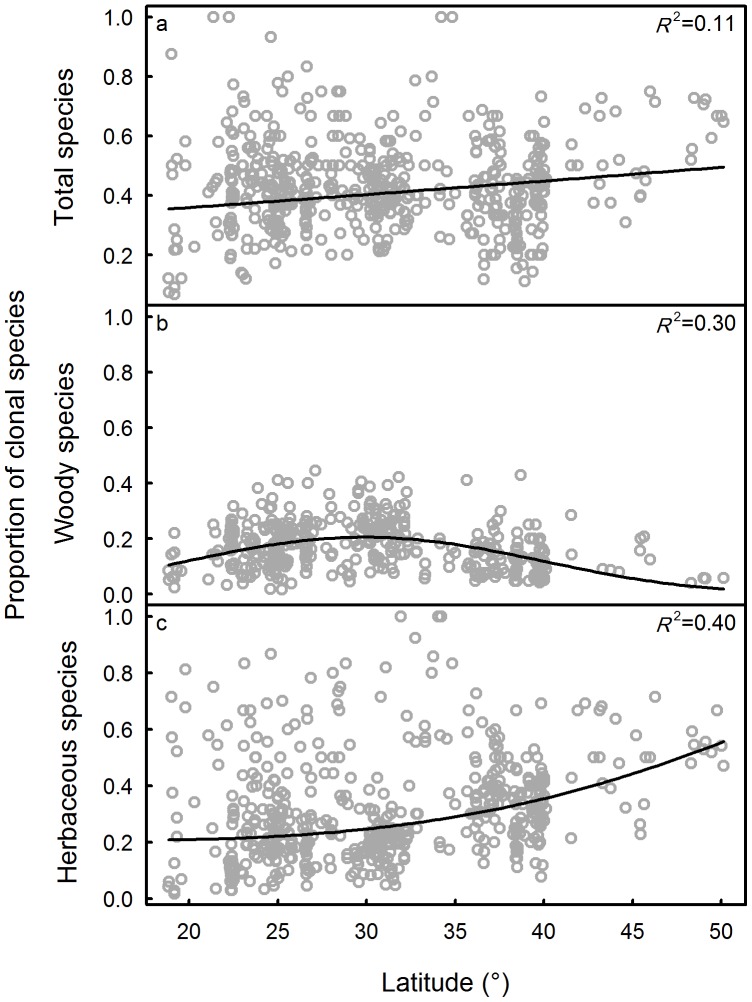
There was a positive linear relationship between clonality as a proportion of all species in a community and latitude, since the quadratic model was not significantly better than the linear model (P = 0.86, Figure 3a). Clonality of woody species increased first and then decreased with increasing latitude (Figure 3b). Clonality of herbaceous species increased more steeply with increasing latitude (Figure 3c).
Figure 3. Relationships between proportion of clonal species and latitude.
a: all species; b: within woody species; c: within herbaceous species.
Structural Equation Modeling
The fits between the modified structural equation models and data were adequate for proportion clonality of all species in a community, woody species and herbaceous species (χ2 1 = 0.04, P = 0.83; χ2 1<0.001, P = 0.99; χ2 1<0.001, P = 0.99; respectively). Climate factors and latitude together explained 1, 4 and 4% of the variation in all species (Figure 4a), woody species (Figure 4b) and herbaceous species (Figure 4c), respectively. Latitude had a direct positive effect on variation in proportion clonality of all species but MAP had a negative effect (Figure. 4a). For woody clonality, latitude and MAP had direct positive effects, while TS and PS had negative effects (Figure 4b); for herbaceous clonality, MAT and MAP had negative effects but PS had a negative effect (Figure 4c).
Figure 4. Structural equation models for variation of clonality.
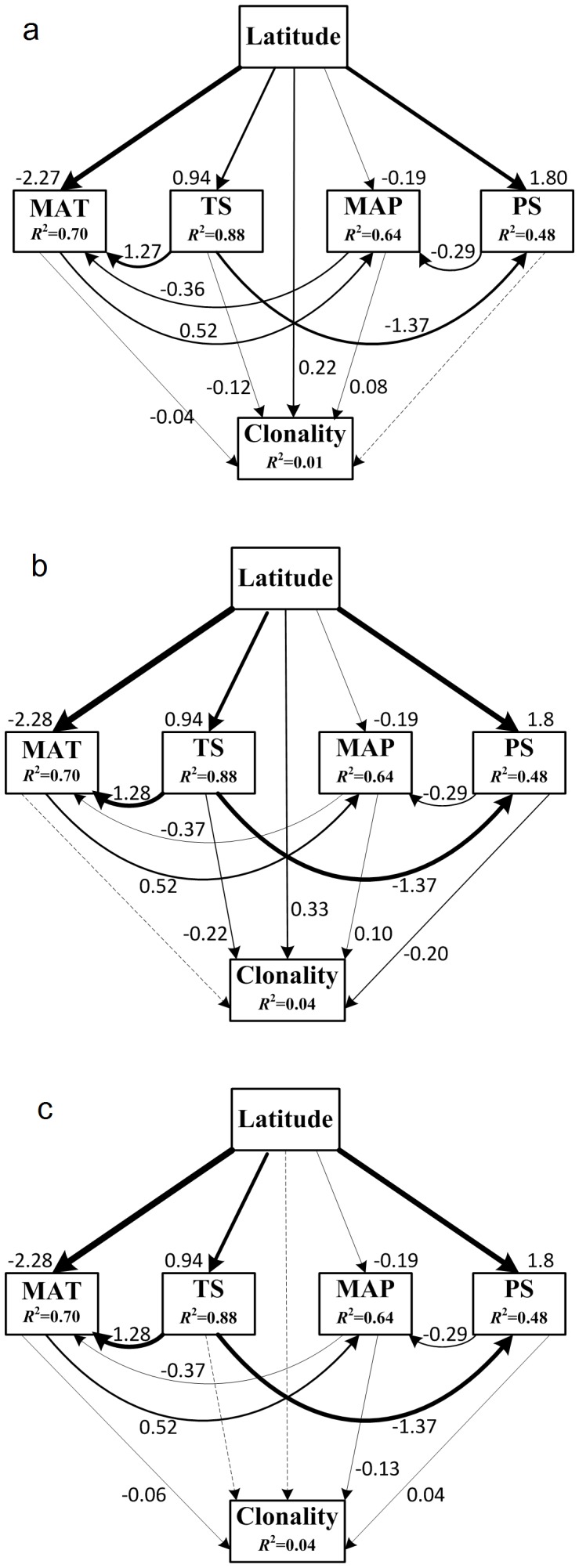
Non-significant paths are indicated by dotted arrows. The thickness of the solid arrows reflects the magnitude of the standardized SEM coefficients. Standardized coefficients are listed beside each significant path, as is the variance explained (R 2) for each endogenous variable. a: all species; b: within woody species; c: within herbaceous species.
Discussion
Latitudinal pattern in clonality
Clonality was expected to increase along the latitudinal gradient. Relatively higher latitude presents more stressful conditions with lower temperature and precipitation and higher seasonality. Clonality was considered be more frequent in these cold or dry conditions. This study provides new, large scale evidence that clonal plants show a latitudinal pattern and increase along a latitudinal gradient across entire China spanning from the tropics to the temperate zones. Latitudinal patterns were better explained by the underlying environmental factors [28], [29]. In previous studies, smaller seeds, shorter plant heights and predominance of herbaceous growth forms at high latitude could be related to low temperature and precipitation [21], [30], [35]. These relationships suggest that the latitudinal gradients of temperature, precipitation and their seasonality in our study may also be the causes of the distribution pattern of plant clonality.
Clonality of woody species mainly decreased with latitude but the trend was reversed in herbaceous species. This suggests that different growth forms may have different adaptive clonal strategies to the complex environmental conditions. As a matter of fact, herbaceous clonal plants dominate the latitudinal pattern of clonality owing to their species number being almost twice as that of woody clonal plants in the communities sampled. Temperature, precipitation and their seasonality therefore are direct forcing drivers of the latitudinal pattern of clonality.
Adaptation of clonality on climate
In the present study, we found that clonality increased with decreasing temperature and with increasing precipitation. Thus, this study provides support for the hypothesis that clonal plants are more frequent in cold or very wet habitats across a huge climatic gradient [5], [8].
However, clonality was significantly but only weakly correlated with the climatic factors. The lack of strong correlation may be due to issues of growth form. We found that clonality of woody species and herbaceous species had opposite response to climatic factors. Woody clonality was more frequent in very wet or climatically stable circumstances, while herbaceous clonality was more frequent in cold, dry or climatically instable conditions. Several other studies have found that different clonal traits underpinned different adaptative strategies with respect to environmental factors [36]–[38]. This suggests that different responses to environmental gradients may actually be due to different advantages brought by different clonal growth forms or organs.
Increasing precipitation and decreasing climatic seasonality represent the favourable climates associated with stronger competition. Clonality gives woody plants more vigour to compete in the increasingly competitive habitats along these gradients [39], [40]. Cold temperatures exert abiotic limitations on sexual reproduction, especially in herbaceous plants, such as limiting the capacity either to flower or to fertilize ovules [41], inhibition of fruit or seed development [42], seed germination [43] and seedling establishment [44]. However, horizontal spreading of clonal genets can enable herbaceous plants to migrate over short distances and to occupy space under conditions that limit seedling recruitment including poor seed production and dispersal [4]. Belowground physiological integration of rhizomes and roots [45], [46] and resource storage in organs such as tubers and bulbs [5], [46], [47], could enable the herbaceous clonal plants to overcome drought periods [48], [49] and provides them with a long lifespan and asexual reproduction insurance.
Conclusions
Our results are consistent with the hypothesis that clonality is more frequent in cold, dry or particularly wet environments. Clonality in China adheres to a latitudinal pattern and increases with increasing latitude. However, the distribution of clonality in China is significantly but only weakly correlated with latitude and climatic factors. This may be due to clonality of woody and herbaceous species adopting opposite response strategies with respect to climatic variables. More precisely, woody clonality shows higher frequency in wet or climatically stable circumstances, while herbaceous clonality prevails in cold, dry or climatically unstable conditions. It is also possible that, across or within growth forms, stronger climatic patterns might be seen within certain phylogenetic lineages and that different patterns for different lineages cancel each other out in the complete species set. Further unexplained variation in clonality may be owed to the influences of other environmental conditions not taken into account here, including edaphic variation. Therefore, in-depth research in terms of more detailed clonal growth forms or phylogenetic lineages and additional more environmental variables are encouraged to further enhance our understanding of plant clonality response to climatic and/or edaphic gradients.
Supporting Information
Coordinates of sites and their species richness and proportion of clonal plants.
(XLS)
Acknowledgments
Thanks to several colleagues providing vegetation plot data, including Jinting Wang and Xianpu Wang for the Qinghai-Tibet plateau and Guangxi province, respectively; to Jiandong Li for Jilin province; Yongchang Song for Zhejiang province; Baoyin Taogetao for steppes Inner Mongolia; to Guiting Tang and Zhiyuan Huang for Tinjin city and Jiangsu province, respectively; to Shaoqing Wang and Jianhui Huang for Hongkong and Beijing, respectively; and to Shizhou Sun for plot data of some other regions. We thank Prof. J.H.C. Cornelissen, Dr. Will Cornwell at VU University Amsterdam, and Dr. Xiangcheng Mi and Dr. Lingfeng Mao at Institute of Botany, Chinese Academy of Sciences (CAS), and Dr. Jushan Liu at Northeast Normal University for their initial statistical advice and discussions. We also thank Prof. J.H.C. Cornelissen for his revision of this manuscript. We are grateful to Dr. Francesco de Bello and the two anonymous reviewers for their valuable comments to improve the quality and clarity of the manuscript.
Funding Statement
This project was supported by the NSFC grant (grant no. 31261120580, 39825106) and the Innovative R & D grant of Hangzhou Normal University (grant no. PD12002002004001) to MD, and by the NSFC grants (grant no. 31100333) to GL. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.de Kroon H, van Groenendael JM (1997) The ecology and evolution of clonal plants.Backhuys Publishers, Leiden.
- 2.Jackson J, Buss L, Cook R (1985) Population biology and evolution of clonal organisms. Yale University Press, New Haven.
- 3. Stuefer JF, Erschbamer B, Huber H, Suzuki JI (2001) The ecology and evolutionary biology of clonal plants: an introduction to the proceedings of Clone-2000. Evolutionary Ecology 15: 223–230. [Google Scholar]
- 4. Cornelissen JHC, Lavorel S, Garnier E, Díaz S, Buchmann N, et al. (2003) A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany 51: 335–380. [Google Scholar]
- 5.Klimeš L, Klimešová J, Hendriks R (1997) Clonal plant architecture: A comparative analysis of form and function. In: de Kroon H, van Groenendael JM, editors. The ecology and evolution of clonal plants. Leiden: Backhuys.pp. 1–29.
- 6. Dong M, de Kroon H (1994) Plasticity in morphology and biomass allocation in Cynodon dactylon, a grass species forming stolons and rhizomes. Oikos 70: 99–106. [Google Scholar]
- 7. Fischer M, Van Kleunen M (2001) On the evolution of clonal plant life histories. Evolutionary Ecology 15: 565–582. [Google Scholar]
- 8. van Groenendael JM, Klimeš L, Klimešová J, Hendriks RJJ (1996) Comparative ecology of clonal plants. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences 351: 1331–1339. [Google Scholar]
- 9. Prach K, Pyšek P (1994) Clonal plants - what is their role in succession. Folia Geobotanica & Phytotaxonomica 29: 307–320. [Google Scholar]
- 10. Song MH, Dong M, Jiang GM (2001) Clonal plants along NECT and relation of theirimportance to environmental conditions. Acta Ecologica Sinica 21: 1095–1103. [Google Scholar]
- 11. Oborny B, Bartha S (1995) Clonality in plant communities: an overview. Abstracta Botanica 19: 115–127. [Google Scholar]
- 12. Fogel BN, Crain CM, Bertness MD (2004) Community level engineering effects of Triglochin maritima (seaside arrowgrass) in a salt marsh in northern New England, USA. Journal of Ecology 92: 589–597. [Google Scholar]
- 13.Herben T, Nováková Z, Klimešová J (2014) Clonal growth and plant species abundance. Annals of Botany doi: 10.1093/aob/mct308. [DOI] [PMC free article] [PubMed]
- 14. Zobel M, Moora M, Herben T (2010) Clonal mobility and its implications for spatio-temporal patterns of plant communities: what do we need to know next? Oikos 119: 802–806. [Google Scholar]
- 15. Klimeš L (2003) Life-forms and clonality of vascular plants along an altitudinal gradient in E Ladakh (NW Himalayas). Basic and Applied Ecology 4: 317–328. [Google Scholar]
- 16. Klimešová J, Doležal J, Dvorský M, de Bello F, Klimeš L (2011) Clonal Growth Forms in Eastern Ladakh, Western Himalayas: Classification and Habitat Preferences. Folia Geobotanica 46: 191–217. [Google Scholar]
- 17. Song MH, Dong M (2002) Clonal plants and plant species diversity in wetland ecosystems in China. Journal of Vegetation Science 13: 237–244. [Google Scholar]
- 18. Sosnová M, van Diggelen R, Klimešová J (2010) Distribution of clonal growth forms in wetlands. Aquatic Botany 92: 33–39. [Google Scholar]
- 19. Klimešová J, Doležal J (2011) Are clonal plants more frequent in cold environments than elsewhere? Plant Ecology & Diversity 4: 373–378. [Google Scholar]
- 20. Song MH, Dong M, Jiang GM (2002) Importance of clonal plants and plant species diversity in the Northeast China Transect. Ecological Research 17: 705–716. [Google Scholar]
- 21. Moles AT, Warton DI, Warman L, Swenson NG, Laffan SW, et al. (2009) Global patterns in plant height. Journal of Ecology 97: 923–932. [Google Scholar]
- 22.Dong M, Wang YF, Kong FZ, Jiang GM, Zhang ZB (1996) Survey,observation and analysis of terrestrial biocommunities. Standards Press of China, Beijing.
- 23.Editorial Committee for Flora Reipublicae Popularis Sinicae (1961–2004) Flora reipublicae popularis sinicae. Science Press, Beijing.
- 24. Klimešová J, de Bello F (2009) CLO-PLA: the database of clonal and bud bank traits of Central European flora. Journal of Vegetation Science 20: 511–516. [Google Scholar]
- 25. Del Tredici P (2001) Sprouting in temperate trees: A morphological and ecological review. Botanical Review 67: 121–140. [Google Scholar]
- 26. Hosaka N, Gómez S, Kachi N, Stuefer JF, Whigham DF (2005) The ecological significance of clonal growth in the understory tree, pawpaw (Asimina triloba). Northeastern Naturalist 12: 11–22. [Google Scholar]
- 27. Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25: 1965–1978. [Google Scholar]
- 28. Grace JB, Kelley JE (2006) A structural equation model analysis of postfire plant diversity in California shrublands. Ecological Applications 16: 503–514. [DOI] [PubMed] [Google Scholar]
- 29. Lamb EG (2008) Direct and indirect control of grassland community structure by litter, resources, and biomass. Ecology 89: 216–225. [DOI] [PubMed] [Google Scholar]
- 30. Liu JS, Bai YG, Lamb EG, Simpson D, Liu GF, et al. (2013) Patterns of cross-continental variation in tree seed mass in the Canadian boreal forest. Plos One 8: e61060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCullagh P, Nelder J (1989) General linear models. Chapman and Halls, London.
- 32.R Development Core Team (2012) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria.
- 33. Lamb EG, Shirtliffe SJ, May WE (2011) Structural equation modeling in the plant sciences: An example using yield components in oat. Canadian Journal of Plant Science 91: 603–619. [Google Scholar]
- 34.Muthén LK, Muthén BO (2010) Mplus: Statistical analysis with latent variables: User's guide: Muthén & Muthén.
- 35. Moles AT, Ackerly DD, Tweddle JC, Dickie JB, Smith R, et al. (2007) Global patterns in seed size. Global Ecology and Biogeography 16: 109–116. [Google Scholar]
- 36. de Bello F, Doležal J, Ricotta C, Klimešová J (2011) Plant clonal traits, coexistence and turnover in East Ladakh, Trans-Himalaya. Preslia 83: 315–327. [Google Scholar]
- 37. Wellstein C, Kuss P (2011) Diversity and frequency of clonal traits along natural and land-use gradients in grasslands of the Swiss Alps. Folia Geobotanica 46: 255–270. [Google Scholar]
- 38. Evette A, Bédécarrats A, Bornette G (2009) Environmental constraints influence clonal traits of herbaceous plant communities in an Alpine massif. Folia Geobotanica 44: 95–108. [Google Scholar]
- 39. Cheplick GP (1997) Responses to severe competitive stress in a clonal plant: Differences between genotypes. Oikos 79: 581–591. [Google Scholar]
- 40. Oborny B, Kun A, Czárán T, Bokros S (2000) The effect of clonal integration on plant competition for mosaic habitat space. Ecology 81: 3291–3304. [Google Scholar]
- 41. Woodward FI (1997) Life at the edge: a 14-year study of a Verbena officinalis population's interactions with climate. Journal of Ecology 85: 899–906. [Google Scholar]
- 42. Mikesell JE (1997) Influences on the life cycle in Pharbitis nil Choisy (Convolvulaceae) induced by low temperature. International Journal of Plant Sciences 158: 306–312. [Google Scholar]
- 43. Simon EW, Minchin A, Mcmenamin MM, Smith JM (1976) Low temperature limit for seed germination. New Phytologist 77: 301–311. [Google Scholar]
- 44. Dornbos DL, Mullen RE, Shibles RM (1989) Drought stress effects during seed fill on soybean seed germination and vigor. Crop Science 29: 476–480. [Google Scholar]
- 45. de Kroon H, Fransen B, vanRheenen JWA, vanDijk A, Kreulen R (1996) High levels of inter-ramet water translocation in two rhizomatous Carex species, as quantified by deuterium labelling. Oecologia 106: 73–84. [DOI] [PubMed] [Google Scholar]
- 46. Klimešová J, Doležal J, Prach K, Košnar J (2012) Clonal growth forms in arctic plants and their habitat preferences: a study from Petuniabukta, Spitsbergen. Polish Polar Research 33: 421–442. [Google Scholar]
- 47. Shaver GR, Billings WD (1976) Carbohydrate accumulation in tundra graminoid plants as a function of season and tissue age. Flora 165: 247–267. [Google Scholar]
- 48. Yu FH, Wang N, He WM, Chu Y, Dong M (2008) Adaptation of rhizome connections in drylands: Increasing tolerance of clones to wind erosion. Annals of Botany 102: 571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Suzuki JI, Stuefer J (1999) On the ecological and evolutionary significance of storage in clonal plants. Plant Species Biology 14: 11–17. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Coordinates of sites and their species richness and proportion of clonal plants.
(XLS)