Abstract
Objective
Hepatitis C virus (HCV) and human immunodeficiency virus (HIV) co-infection has been proved to be a growing public health concern. The prevalence and genotypic pattern vary with geographic locations. Limited information is available to date with regard to HCV genotype and its clinical implications among those former commercial blood donor communities. The aims of this study were to genetically define the HCV genotype and associated clinical characteristics of HIV/HCV co-infected patients from a region with commercial blood donation history in central China.
Methods
A cross sectional study, including 164 HIV infected subjects, was conducted in Shanxi province central China. Serum samples were collected and HCV antibody testing, AST and ALT testing were performed. Seropositive samples were further subjected to RT-PCR followed by direct sequence coupled with phylogenetic analysis of Core-E1 and NS5B regions performed in comparison with known reference genotypes.
Findings
A total of 139 subjects were HCV antibody positive. Genotype could be determined for 88 isolates. Phylogenetic analysis revealed that the predominant circulating subtype was HCV 1b (65.9%), followed by HCV 2a (34.1%). The HCV viral load in the subjects infected with HIV1b was significantly higher than those infected with HCV 2a (P = 0.006). No significant difference for HCV RNA level was detected between ART status, CD4+ cell count level and HIV RNA level. Serum AST and ALT level were likely to increase with HCV RNA level, although no significance was observed. Those who had conducted commercial donation later than 1991 (OR 3.43, 95% CI: 1.12–10.48) and had a short duration of donation (OR 0.35, 95% CI: 0.13–0.96) were more likely to be infected with HCV 1b.
Conclusion
These results suggest that HCV subtype 1b predominates in this population, and the impact of HIV status and ART on HCV disease progression is not significantly correlated.
Introduction
Hepatitis C virus (HCV) was first identified in 1989 as the principal cause of post transfusion of non-A non-B hepatitis [1]. About 3% of the world population is infected with HCV, with a total of about 170 million carriers. Due to shared risk factors for transmission, co-infection with HIV and HCV is common [2]. Unregulated commercial blood collection among farmers occurred in central China, including Henan, Anhui and Shanxi provinces, during the 1990s resulting in the second major epidemic of HIV in the country. Previous studies have indicated that co-infection of HIV and HCV is very common in former blood donors [3], [4]. The first outbreak of HCV infection among plasma donors in China was reported in the last century [5], and since then the issue of HIV co-infection with HCV have been studied extensively, with most of the studies demonstrating high seroprevalence of HCV in this former commercial blood donors society [6], [7], [8], [9].
Understanding HIV/HCV co-infection and their clinical characteristics is of significant public health importance. Co-infection with HIV and HCV has been shown to increase HCV viral loads and may accelerate the natural course of chronic hepatitis C infection, increase the risk for the development of cirrhosis, hepatocellular carcinoma (HCC), and results in hastening progression to end-stage liver disease (ESLD) [10], [11], [12], [13], [14], [15], although whether co-infection HIV and HCV results in a faster progression to AIDS remains controversial [16], [17]. Unfortunately, such studies have rarely been done in China. Furthermore, combined intervention and treatment for HCV/HIV co-infection has not been taken into concerns and been rarely addressed.
HCV is a genetically diverse RNA virus with a single strand, positive sense genome, and is classified into 6 major genotypes with closely related isolates being grouped into subtypes [18], [19]. To date, genotypes 1, 2 and 3 have a worldwide distribution, while other genotypes have been found in more restricted regions only [20]. Genotyping is an important tool for epidemiologic studies, since HCV genotypes may vary according to epidemic history in different geographic regions with different genotypes manifesting substantial differences in clinical characteristics, such as response to treatment, which is of central importance in disease treatment and prevention. In China, the existing literature suggests that the prevalence of HCV genotype is highly heterogeneous with regard to infection sources and epidemic region. Over the years, despite the widespread evidence of co-infection with HIV and HCV, clinical characteristics of HCV/HIV co-infection in China has rarely specifically addressed, especially in terms of the association between HCV genotypes and clinical characteristics.
Therefore, a cross sectional study was designed and conducted to determine the prevalence of HCV genotypes within a sample of HIV infected patients from a rural Chinese community and to examine correlations between genotypes and the clinical implications, such as HCV RNA viral load distribution and serum enzyme levels, of these HCV/HIV co-infected patients. Our observations could bring new insight into the management of HIV/AIDS patients to reduce their morbidity and mortality.
Material and Methods
Ethics Statement
This study was approved by the Institutional Review Board of Fudan University, China. Written consent was obtained from all the adult participants before any procedures were performed. Interview data did not bear the names of respondents and all data were anonymized before analysis.
Study sites and participants
The present study was conducted in a county in the southern part of Shanxi province in central China, which was once reported to have a high prevalence rate of HIV attributed to former commercial blood donors [7], [21], and harbors a large number of former commercial blood donors attributed to the establishment of an commercial blood donation center in 1995.
A total number of 164 HIV positive subjects who have been registered to the National HIV/AIDS Information System were randomly selected into the present study. Data for participants' socio-demographic characteristics and HIV transmission modes were extracted from the National HIV/AIDS Information System using a standard questionnaire form. Free antiretroviral treatment (ART) has been available for HIV/AIDS patients since 2003 in the study area.
Serological testing
HCV serology
ELISA for Anti-HCV immunoglobulin G (IgG) antibody was tested to determine HCV infection status according to the manufacturer's protocol (Wantai Biomedical, Beijing, China). All the sera samples were assayed blindly in duplicate. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were evaluated with commercial reagent kits (Shanghai Kehua Bioengineering Co. Ltd., China). Normal values were considered as: 10–40 IU/L for AST, and 5–40 IU/L for ALT.
HCV RNA extraction
HCV-RNA extraction was carried out from 200 ml of serum using QIAamp1 Viral RNA Kit (QIAGEN, Valencia, CA), following the manufacturer's instructions. The synthesis of the complementary DNA (cDNA) was done immediately after RNA extraction and store −80°C. To avoid false-positive results, rigorous procedures proposed for nucleic acid amplification diagnostic techniques were followed.
Complimentary DNA (cDNA) synthesis
Reverse transcriptase reaction was performed using the Moloney Murine Leukemia Virus Reverse Transcriptase (MMLV-RT) and random primers. The final volume of the reaction was 60 μl in the following concentrations: 50 mM Tris–HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 10 mM DTT, 0.5 mM of each dNTP, 450 ng random primers, 30URNAse enzyme inhibitor (RNase OUTTM), and 300 U MMLV-RT. Samples were submitted to the following temperature cycles: 70°C for 10 min, 25°C for 15 min, 37°C for 60 min, and 95°C for 15 min in a thermocycler (Eppendorf Mastercycler 1, Eppendorf, Hamburg, Germany).
Amplification & Sequencing of HCV NS5B, C/E1 region
Polymerase chain reaction (PCR)
Polymerase chain reactions (PCR) were done in two stages to increase sensitivity. HCV NS5B and C/E1 were amplified for genotyping and sequence analysis. Specific primers used for amplification and amplification cycles were as described [22]. The final PCR amplicons were separated by agarose gel electrophoresis, the correct bands were excised from agarose gel and extracted using the GFXTM PCR DNA and Gel Band purification kit (Amersham Bioscience UK Limited, Little Chalfont, United Kingdom). The second round PCR primers were used for the direct sequencing reactions using Bigdye® Terminator v3.1 Cycle Sequencing kit (Applied Biosystems, Foster City, CA) following the manufacturer's protocol. The resulting sequence data were checked by hand.
Quantification of HCV RNA
serum HCV viral load was determined by using the one-step quantitative HCV RT-PCR kit (Shenzhen Piji Co. Ltd., China) with the standard-curve Taqman probe method. All tests were performed according to the manufacture protocol. (Piji Bio-Tech Company, Shenzhen., China)
Phylogenetic Analysis
Sequence alignments were created using MUSCLE and manually edited [24]. Sequences were then classified into phylogenetic groups (i.e. genotypes and subtypes) using the REGA HCV subtyping tool (version 2.0, available at: www.bioafrica.net) [23]. In addition, maximum likelihood phylogenies were constructed using PhyML with reference strains obtained from the HCV sequence database (http://hcv.lanl.gov/content/hcv-db/index) provided by the Los Alamos National Laboratory with the following accession numbers: 1a.US.H77.NC_004102, 1a.US.HCV-H.M67463, 1a.US5003.EF407419, 1a.LTD6-2-XF224.AF511950, 1b.BR.03.EF032892, 1b.CNAY587016.AY587016, 1b.JPJT.D11355, 1c.IDHC-G9.D14853, 1c.INAY051292.AY051292, 1g.ES1804.AM910652, 2a.JPAY746460.AY746460, 2a.JPHC-J6.D00944, 2a.JPJFH-1.AB047639, 2b.JPHC-J8.D10988, 2b.JPUT971017.AB030907, 2b.MD2B-1.AF238486, 2c.BEBE1.D50409, 2i.VND54.DQ155561, 2j.VE.05.HM777359, 2j.VE.06.HM777358, 2k.MDVAT96.AB031663, 3a.DEHCVCENS1.X76918, 3a.CB.AF046866, 3a.NZL1.NC_009824, 3b.JPHCV-Tr.D49374, 3i.IN.02.FJ407092, 3k.IDJK049.D63821, 4a.EGED43.Y11604, 4a.USF7157.DQ418788, 4b.CAQC264.FJ462435, 4b.PTP026.FJ025854, 4b.PTP212.FJ025855, 4b.PTP245.FJ025856, 4c.CAQC381.FJ462436, 4d.CAQC382.FJ462437, 4d.ES24.DQ516083, 4d.US03-18.DQ418786, 4d.PS2.EU392172, 4f.FRIFBT84.EF589160, 4f.FRIFBT88.EF589161, 4f.CM_DAV9905.EU392169, 4f.CM_SP1578.EU392170, 4f.PS4.EU392174, 4f.PS6.EU392175, 4g.CAQC193.FJ462432, 4k.CAQC383.FJ462438, 4k.PB65185.EU392171, 4k.PS3.EU392173, 4l.CAQC274.FJ839870, 4m.CAQC249.FJ462433, 4n.CAQC97.FJ462441, 4o.CAQC93.FJ462440, 4p.CAQC139.FJ462431, 4q.CAQC262.FJ462434, 4r.CAQC384.FJ462439, 4t.CAQC155.FJ839869, 5a.GBEUH1480.NC_009826, 5a.ZASA13.AF064490, 6.CNGZ52557.DQ278892, 6a.HK6a35.DQ480513, 6b.Th580.NC_009827, 6c.THTh846.EF424629, 6d.VNVN235.D84263, 6e.CNGX004.DQ314805, 6f.THC-0044.DQ835760, 6g.HKHK6554.DQ314806, 6h.VNVN004.D84265, 6i.THC-0159.DQ835762, 6j.THC-0667.DQ835761, 6k.CNKM45.DQ278891, 6l.VND33.EU246933, 6m.THC-0185.DQ835765, 6n.CNKM42.AY878652, 6o.VND85.EU246934, 6p.CAQC216.EF424626, 6q.CAQC99.EF424625, 6r.CAQC245.EU408328, 6s.CAQC66.EU408329, 6t.VND49.EU246939, 6u.CNDH012.EU408330, 6v.CN.NK46.EU158186, 6w.TWHCV-6-D140.EU643834, 7a.CAQC69.EF108306.
Statistical analysis
Original questionnaire data and laboratory results were entered and managed with EpiData 3.0 and then transferred to SAS v8.2 (SAS institute Inc., Cary, North Carolina, USA) for further analysis. Descriptive statistics and univariate analysis were performed to examine the correlates of HCV seropositivity. Multiple logistic regression analyses were conducted to identify potential factors associated with HCV subtype 1b infection compared to those with HCV subtype 2a infection. Crude odds ratio and odds ratio adjusted for gender and age group were generated to determine whether a variable was associated with HCV infection. All p-values ≤0.05 were considered to be statistically significant.
Results
Sociodemographic Characteristics of Study Participants
A total of 164 HIV positive subjects were finally recruited into the present study. Of them, 139 (84.7%) were HCV/HIV co-infected while 25 (15.3%) were HIV mono-infected (Table 1). Among the study subjects, those who participated in HCV/HIV co-infected group were more likely to be male (P = 0.019) and older (P = 0.013) (Table 1). No significant differences were reported between the co-infected and mono-infected groups in martial status, education, family income, smoking habits or consumption of alcohol. Eighty-six per cent of all participants acquired HIV from commercial blood donation while in the HIV mono-infected group transmission of HIV was more likely to occur from blood transfusion and sexual contact (24% vs. 3.6% for transfusion and 28% vs. 2.2% for sexual transmission). In addition approximately 78.7% of all study participants had received ART. The major demographic characteristics of all participants are summarized in Table 1.
Table 1. Socio demographic characteristics of HIV mono-infected and HIV/HCV co-infected subjects in the study.
| Characteristics | HIV | HIV/HCV | Total |
| N = 25 | N = 139 | N = 164 | |
| No. (%) | No. (%) | No. (%) | |
| Gender (P = 0.019) | |||
| Male | 11 (44.0) | 95 (68.3) | 106 (64.6) |
| Female | 14 (56.0) | 44 (31.7) | 58 (35.4) |
| Age group (P = 0.013) | |||
| ≤20 | 2 (8.0) | 1 (0.7) | 3 (1.8) |
| 21–49 | 20 (80.0) | 98 (70.5) | 118 (72.0) |
| ≥50 | 3 (12.0) | 40 (28.8) | 43 (26.2) |
| Marital status (P = 0.147) | |||
| Single | 4 (16.0) | 10 (7.2) | 14 (8.5) |
| Ever married | 21 (84.0) | 129 (92.8) | 150 (91.5) |
| Education (P = 0.460) | |||
| Illiterate | 2 (8.0) | 13 (9.4) | 15 (9.1) |
| Primary school | 10 (40.0) | 54 (36.8) | 64 (39.0) |
| Middle school | 10 (40.0) | 66 (47.5) | 76 (46.3) |
| Senior middle | 3 (12.0) | 5 (4.3) | 9 (5.5) |
| Family income (P = 0.830) | |||
| <1000 | 17 (68.0) | 83 (59.7) | 100 (61.0) |
| 1001∼2000 | 3 (12.0) | 20 (14.4) | 23 (14.0) |
| 2001∼3000 | 2 (8.0) | 10 (7.2) | 12 (7.3) |
| >3001 | 3 (12.0) | 26 (18.7) | 29 (17.7) |
| Alcoholic Drink (P = 0.329) | |||
| Yes | 3 (12.0) | 9 (6.5) | 12 (7.3) |
| No | 22 (88.0) | 130 (93.5) | 152 (92.7) |
| Smoking (P = 0.152) | |||
| Yes | 8 (32.0) | 66 (47.5) | 74 (45.1) |
| No | 17 (68.0) | 73 (52.5) | 90 (54.9) |
| Routes of HIV transmission (P<0.001) | |||
| Commercial blood donation | 13 (52.0) | 126 (90.6) | 139 (84.8) |
| Transfusion | 4 (16.0) | 6 (4.3) | 10 (6.1) |
| Sexual transmitted | 7 (28.0) | 7 (5.0) | 14 (6.1) |
| Mother to Child | 1 (4.0) | 0 (0.0) | 1 (0.6) |
| Antiretroviral therapy (P = 0.052) | |||
| Yes | 16 (64.0) | 113 (81.3) | 129 (78.7) |
| No | 9 (36.0) | 26 (18.7) | 35 (21.3) |
| CD4+ counts (P = 0.569) | |||
| <200 | 7 (28.0) | 47 (33.8) | 54 (32.9) |
| ≥200 | 18 (72.0) | 92 (66.2) | 110 (67.1) |
| HIV viral load (P = 0.946) | |||
| <1000 | 7 (28.0) | 38 (27.3) | 45 (27.4) |
| ≥1000 | 18 (72.0) | 101 (72.7) | 119 (72.6) |
Hepatitis C Virus Sequence Amplification and Classification
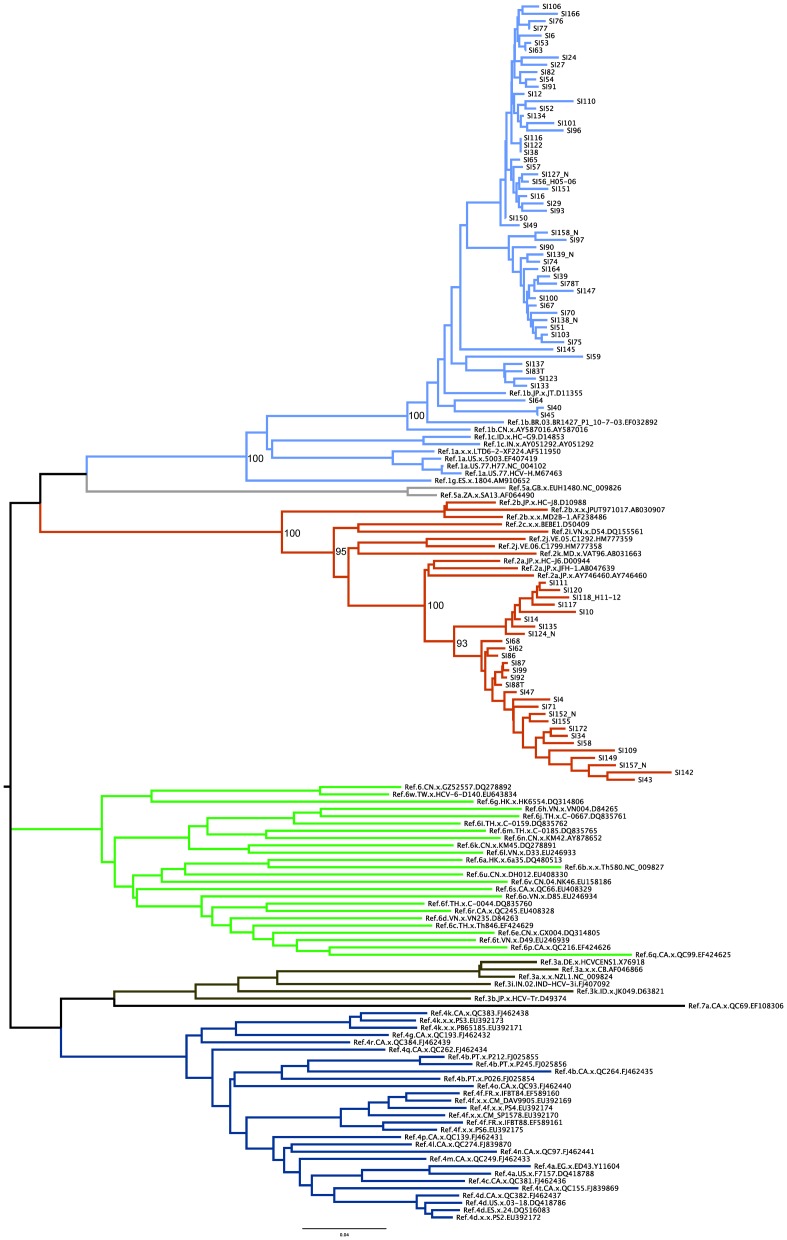
Of the 139 HCV seropositive patients included in this study, 88 were also positive for HCV RNA. NS5B and C/E1 sequences were successfully amplified from 84 and 87 participants, respectively. HCV genotypes were determined using the REGA HCV Subtyping tool (version 2), which uses phylogenetic methods to identify the subtype against a panel of HCV subtype reference sequences. HCV genotyping was determined for the NS5B gene with 1b predominating in 56 individuals while 28 donors were found to be infected with genotype 2a (Figure 1). ML phylogenetic analysis also demonstrates significant clustering with high bootstrap supports (100%) for sequences belonging to the genotypes 1b and 2a clades respectively. REGA subtyping confirmed this observation for the C/E1 region with 57 patients infected with 1b and 30 infected with 2a. In addition, a similar phylogenetic pattern was observed for the C/E1 region with significant bootstrap support values (data not shown). No other genotypes were detected in this study. The genotyping results were consistent when analyzed with either NS5B or C/E1 region (measurement of agreement Kappa = 0.881, P<0.001).
Figure 1. Maximum-Likelihood phylogeny estimated from NS5B sequences (H77 positions 8261–8639).
Bootstrap support values are only shown for the major ancestral nodes of interest. Reference HCV sequences are indicated by genotype followed by their Genbank accession numbers. Similar phylogenetic patterns were observed for C/E1 (not shown).
HCV viral load distribution
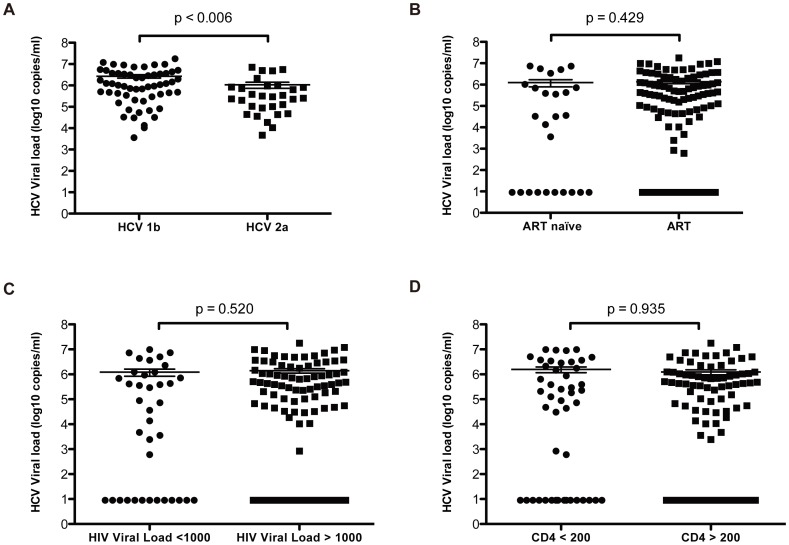
Among those HCV RNA positive subjects, HCV viral loads were determined and compared. The overall median HCV viral load was 5.84log10copies/ml (interquartile range (IQR), 5.12–6.94 log10copies/ml). Furthermore, HCV viral load differences between genotypes, ART status, HIV viral load and CD4+count were explored. No significant differences were observed between the ART group (median 4.59 log10copies/ml; IQR, 0.95–5.89 log10copies/ml) and the ART naïve group (median 5.31 log10copies/ml; IQR, 0.95–6.06 log10copies/ml) (P = 0.429) (Figure 2). Similarly, HCV viral load was not significantly different between the HIV viral loads ≤1000 copies/ml group and HIV viral loads >1000 copies/ml group (P = 0.520) and low CD4 count group and high CD4 high counts group (P = 0.935). However, the HCV viral load of subtype 1b subjects (median 6.03 log10copies/ml; IQR, 5.50–6.56 log10copies/ml) was significantly higher than subtype 2a subjects (median 5.43; IQR, 4.87–5.93 log10copies/ml) (P = 0.006) (Figure 2).
Figure 2. Box and Whisker Plots of HCV viral load distribution by related factors.
(A) HCV viral load stratified by subtype 1b and 2a. (B) HCV viral load between ART naïve and ART treated subjects. (C) Distribution of HCV viral load by HIV viral load <1000 and >1000. (D) HCV viral load between CD4 counts <200 and CD4 counts >200.
Relationship between HCV genotype and serum enzyme level
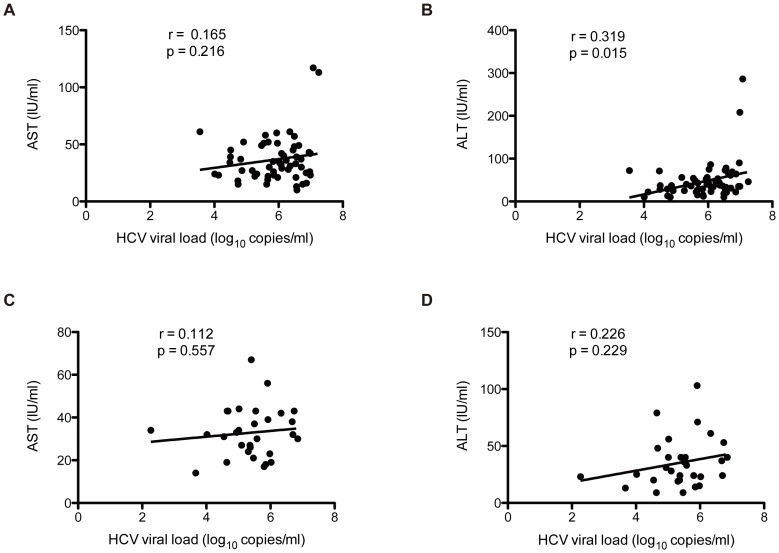
The serum AST and ALT levels were further analyzed for HCV genotypes 1b and 2a. The mean of AST and ALT were 36.72 UI/L and 47.21 UI/L for subtype 1b subjects, whereas the means of AST and ALT were 32.86 UI/L and 35.26 UI/L for subtype 2a subjects. As shown in Figure 3A and 3B, abnormal AST and ALT level (normal range 5–40 UI/L) were detected among 32.8% (19/58) and 41.4% (24/58) HCV subtype 1b infected subjects, with ALT were significantly increased with HCV viral load (PAST = 0.216, PALT = 0.015). Similarly, serum AST and ALT level in the HCV subtype 2a infected subjects tended to be increased with HCV viral load though statistical significance was not observed (PAST = 0.557 and PALT = 0.229), and the abnormal AST and ALT level frequency among subtype 2a subjects were 26.7% (8/30) and 23.3% (7/30), respectively.
Figure 3. Correlation of serum enzyme levels and HCV viral load.
(A) Association of AST and HCV viral load in subjects infected with subtype 1b. (B) Association of ALT and HCV viral load in subjects infected with subtype 1b. (C) Association of AST and HCV viral load in subjects infected with subtype 2a. (D) Association of ALT and HCV viral load in subjects infected with subtype 2a.
Potential risk factors associated with HCV subtype 1b infection
Analysis for potential risk factors was restricted to HCV subtype 1b and 2a cases. As shown in table 2, univariate analysis revealed that first donation time (P = 0.020), duration of commercial blood donation (P = 0.036) was significantly associated with subtype 1b infection. Multivariate Logistic analysis adjusted by age and gender indicated those who donated blood later than the year 1991 were more likely to be HCV subtype 1b infection (AOR = 3.43, 95% CI: 1.12–10.48), whereas if the duration of commercial blood donation was more than 3 years then the participants were less likely to be HCV 1b infection (AOR = 0.35, 95% CI: 0.13–0.96). No significant differences were found between subtype and route of transmission.
Table 2. Multivariate Logistic regression analysis of potential factors associated with HCV subtype 1b infection, compared to those with HCV subtype 2a infection (n = 88).
| Characteristics/Risk factor | Subtype 2a (N = 30) | Subtype 1b (N = 58) | ||
| N (%) | N (%) | COR (95%CI) | AOR* (95%CI) | |
| First blood donation time # | ||||
| <1990 | 10 (34.5) | 7 (12.5) | 1.00 | |
| ≥1991 | 19 (65.5) | 49 (87.5) | 3.68 (1.22–11.08) | 3.43 (1.12–10.48) |
| Duration of commercial donation # | ||||
| <3 | 17 (58.6) | 45 (80.4) | 1.00 | |
| ≥3 | 12 (41.4) | 11 (19.6) | 0.35 (0.13–0.93) | 0.35 (0.13–0.96) |
| Routes of HIV transmission | ||||
| Commercial blood donation | 29 (96.7) | 56 (96.6) | 1.00 | |
| Transfusion | 1 (3.3) | 2 (3.4) | 1.03 (0.09–11.09) | 0.92 (0.07–11.03) |
some subject have not donated.
*AOR adjusted for gender and age group.
Discussion
During the early 1990s, commercial plasma and blood collection activities were once common in rural areas of central China and commercial donation for money seemed to be an easy way for those rural farmers to augment their income at that time [25]. However, due to the unhygienic process of pooling blood and the reinfusion of compatible red blood cells to permit more frequent donations prompted exposing the donors to a range of pathogens. The nature of such practices led to high HCV infections rates in blood and plasma donors with enhanced risk of HIV transmission in addition to other opportunistic infections [25], [26].
Several studies on HCV co-infection in former blood donors from other areas in China have shown similar results demonstrating that the HCV prevalence can be as high as 78.6% to 86.3% among HIV positive subjects [7], [8], [27]. Our results further confirm that dual HIV and HCV infection is relatively common. This is of public health importance, given that HCV co-infection may complicate, antiretroviral therapy (ART) and the use of the different regimens should be closely monitored in this former commercial blood donation region.
To elucidate the epidemiologic picture of circulating viral strains, HCV NS5B and C/E1, two reliable and most commonly used regions, were selected as targets to infer the genotype distribution in the present study [28]. The genotypes from two assays showed high consistency with no recombination detected. Overall, genotyping data showed that two main HCV genotypes, 1b and 2a, are circulating within those who are infected with HIV in central China. These results are in direct agreement with previous reports on HCV and HIV co-infection among commercial blood donor from neighboring provinces, such as Henan and Hubei in China [8], [29], [30]. Actually, subtype 1b and 2a are two of the most prevalent genotypes in China, with 1b prevailing in southern China while 2a is more common in northern China [29]. Interestingly, although a similar genotype pattern, consisting of subtypes 1b and 2a, was observed, the frequency of HCV subtypes differs across geographic regions. This altered distribution on genotype frequency may indeed corroborate recent reports that subtype 2a infections in China have been reduced [31].
The practice of risk behaviors is knowingly an important determinant of HCV transmission. Since the majority of study subjects had a history of commercial blood donation, HCV blood borne transmission should be of importance. In the current study, HCV 1b infection was associated with first donation time, while an inverse correlation has been observed from duration of commercial donation. It is speculated that subtype 1b has entered and become a predominate strain in this population after the year 1991. Conversely, those who had conducted commercial blood donation earlier (<1990) are more likely to be exposed to HCV subtype 2a.Moreover, those who and had a long duration of illegal blood donation often means donated earlier and are tend to be infected with subtype 2a.
To determine whether the HIV infection and ART affect nature course of chronic HCV infection, HCV viral load were compared between HIV RNA level, ART and CD4+ cell level. Currently, some discrepancy exists with previous data regarding HIV/HCV co-infection and the impact of ART on HCV progression [32], [33]. Data from the present study indicates that no significant difference was observed in HCV viral loads when the comparisons above were taken into account. Conversely, HCV viral loads were significantly higher in patients infected with subtype 1b than patients infected with subtype 2a (P = 0.006). There is discrepancy between our results and the studies by Liu et al. which indicated that patients infected with subtype 1b showed a lower HCV viral load compared with subtype 2a [30]. However, in general HCV 1b has been linked to severe chronic liver disease with results from this study supporting this fact that subtype 1b might be more aggressive and may be associated with high serum HCV levels.
Meanwhile, host responses of chronic HCV infection in those HIV positive subjects, in particular ALT and AST, have also been explored. Data from current study indicates that the majority of the HCV infected subjects' serum AST and ALT level are within normal range. Moreover, as previously been reported HCV viral load may not correlate with serum enzyme level in either subtype [34]. Furthermore, it is unlikely that the measurement of such enzymes at a single timepoint will be representive of the ALT/AST profile over time. Therefore, longitudinal data will better aid in supporting these conclusions.
In conclusion, the present study demonstrates that HCV/HIV co-infection is common in the former commercial blood donation community, with HCV 1b and 2a the two predominate subtypes. Although, HCV viral loads were higher in the subjects infected with subtype 1b than those who were infected with 2a, there is no correlation between HIV viral load, ART status, CD4+ cell counts, and HCV viral levels. Moreover, whether those specific subtypes could contribute to elevation of AST and ALT levels remains unclear. Prospective studies on HCV subtypes profile and clinical manifestation might be helpful in elucidating this understanding.
Funding Statement
This study was supported by Program of excellent public health young talent of Shanghai (08GWQ058), National Nature Science Foundation of China (81072345), Shanghai Leading Disciplinary Project (grant no. GWZX0101), National Science Foundation of China (grant no. 81373062) and Doctoral Fund of Ministry of Education of China (Grant No. 20120071120050 to TZ). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, et al. (1989) Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244: 359–362. [DOI] [PubMed] [Google Scholar]
- 2. Alter MJ (2002) Prevention of spread of hepatitis C. Hepatology 36: S93–98. [DOI] [PubMed] [Google Scholar]
- 3. Sherman KE, Rouster SD, Chung RT, Rajicic N (2002) Hepatitis C Virus prevalence among patients infected with Human Immunodeficiency Virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis 34: 831–837. [DOI] [PubMed] [Google Scholar]
- 4. Sulkowski MS, Mast EE, Seeff LB, Thomas DL (2000) Hepatitis C virus infection as an opportunistic disease in persons infected with human immunodeficiency virus. Clin Infect Dis 30 Suppl 1: S77–84. [DOI] [PubMed] [Google Scholar]
- 5. Sun YD, Meng ZD, Wang SY, Chen XR, Sun DG, et al. (1991) Epidemiologic investigation on an outbreak of hepatitis C. Chin Med J (Engl) 104: 975–979. [PubMed] [Google Scholar]
- 6. Zhang M, Sun XD, Mark SD, Chen W, Wong L, et al. (2005) Hepatitis C virus infection, Linxian, China. Emerg Infect Dis 11: 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qian HZ, Vermund SH, Kaslow RA, Coffey CS, Chamot E, et al. (2006) Co-infection with HIV and hepatitis C virus in former plasma/blood donors: challenge for patient care in rural China. AIDS 20: 1429–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu P, Xiang K, Tang H, Zhang W, Wang X, et al. (2008) Molecular epidemiology of human immunodeficiency virus type 1 and hepatitis C virus in former blood donors in central China. AIDS Res Hum Retroviruses 24: 1–6. [DOI] [PubMed] [Google Scholar]
- 9. Zhang L, Chen Z, Cao Y, Yu J, Li G, et al. (2004) Molecular characterization of human immunodeficiency virus type 1 and hepatitis C virus in paid blood donors and injection drug users in china. J Virol 78: 13591–13599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Danta M, Semmo N, Fabris P, Brown D, Pybus OG, et al. (2008) Impact of HIV on host-virus interactions during early hepatitis C virus infection. J Infect Dis 197: 1558–1566. [DOI] [PubMed] [Google Scholar]
- 11. Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, et al. (1999) Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology 30: 1054–1058. [DOI] [PubMed] [Google Scholar]
- 12. Soto B, Sanchez-Quijano A, Rodrigo L, del Olmo JA, Garcia-Bengoechea M, et al. (1997) Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol 26: 1–5. [DOI] [PubMed] [Google Scholar]
- 13. Bonacini M, Govindarajan S, Blatt LM, Schmid P, Conrad A, et al. (1999) Patients co-infected with human immunodeficiency virus and hepatitis C virus demonstrate higher levels of hepatic HCV RNA. J Viral Hepat 6: 203–208. [DOI] [PubMed] [Google Scholar]
- 14. Graham CS, Baden LR, Yu E, Mrus JM, Carnie J, et al. (2001) Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis 33: 562–569. [DOI] [PubMed] [Google Scholar]
- 15. Ragni MV, Belle SH (2001) Impact of human immunodeficiency virus infection on progression to end-stage liver disease in individuals with hemophilia and hepatitis C virus infection. J Infect Dis 183: 1112–1115. [DOI] [PubMed] [Google Scholar]
- 16. Sabin CA, Telfer P, Phillips AN, Bhagani S, Lee CA (1997) The association between hepatitis C virus genotype and human immunodeficiency virus disease progression in a cohort of hemophilic men. J Infect Dis 175: 164–168. [DOI] [PubMed] [Google Scholar]
- 17. Piroth L, Duong M, Quantin C, Abrahamowicz M, Michardiere R, et al. (1998) Does hepatitis C virus co-infection accelerate clinical and immunological evolution of HIV-infected patients? AIDS 12: 381–388. [DOI] [PubMed] [Google Scholar]
- 18. Simmonds P, Bukh J, Combet C, Deleage G, Enomoto N, et al. (2005) Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology 42: 962–973. [DOI] [PubMed] [Google Scholar]
- 19. Simmonds P, Holmes EC, Cha TA, Chan SW, McOmish F, et al. (1993) Classification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS-5 region. J Gen Virol 74 (Pt 11): 2391–2399. [DOI] [PubMed] [Google Scholar]
- 20. Zein NN (2000) Clinical significance of hepatitis C virus genotypes. Clin Microbiol Rev 13: 223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shi XM, Yang ZM, Qian HZ, Qiao XC, Gao JH, et al. (2006) [A cross-sectional survey on human immunodeficiency virus infection in a former commercial blood donating community, Shanxi Province]. Zhonghua Yu Fang Yi Xue Za Zhi 40: 427–432. [PubMed] [Google Scholar]
- 22. Murphy DG, Willems B, Deschenes M, Hilzenrat N, Mousseau R, et al. (2007) Use of sequence analysis of the NS5B region for routine genotyping of hepatitis C virus with reference to C/E1 and 5' untranslated region sequences. J Clin Microbiol 45: 1102–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Oliveira T, Deforche K, Cassol S, Salminen M, Paraskevis D, et al. (2005) An automated genotyping system for analysis of HIV-1 and other microbial sequences. Bioinformatics 21: 3797–3800. [DOI] [PubMed] [Google Scholar]
- 24. Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu Z, Liu Z, Detels R (1995) HIV infection in commerical plasma donors in China. Lancet 346: 61–62. [DOI] [PubMed] [Google Scholar]
- 26. Cohen J (2004) HIV/AIDS in China. An unsafe practice turned blood donors into victims. Science 304: 1438–1439. [DOI] [PubMed] [Google Scholar]
- 27. Liu Z, Xing WG, Zhang YH, Zhang Q, Long XS, et al. (2006) [Study on the epidemiology and HCV genotype distribution of HIV/HCV co-infection among HIV infected blood donors in China]. Zhonghua Gan Zang Bing Za Zh 14: 464–465. [PubMed] [Google Scholar]
- 28. Simmonds P (1999) Viral heterogeneity of the hepatitis C virus. J Hepatol 31 Suppl 1: 54–60. [DOI] [PubMed] [Google Scholar]
- 29. Shang H, Zhong P, Liu J, Han X, Dai D, et al. (2010) High prevalence and genetic diversity of HCV among HIV-1 infected people from various high-risk groups in China. PLoS One 5: e10631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Chen X, Xie Q, Zhang W, Wei L, et al.. (2011) Drug Resistance and HCV Coinfection in Former Blood Donors Infected with HIV Type 1 in China. AIDS Res Hum Retroviruses. [DOI] [PubMed]
- 31. Fu Y, Wang Y, Xia W, Pybus OG, Qin W, et al. (2011) New trends of HCV infection in China revealed by genetic analysis of viral sequences determined from first-time volunteer blood donors. J Viral Hepat 18: 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thein HH, Yi Q, Dore GJ, Krahn MD (2008) Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS 22: 1979–1991. [DOI] [PubMed] [Google Scholar]
- 33. Verma S, Wang CH, Govindarajan S, Kanel G, Squires K, et al. (2006) Do type and duration of antiretroviral therapy attenuate liver fibrosis in HIV-hepatitis C virus-coinfected patients? Clin Infect Dis 42: 262–270. [DOI] [PubMed] [Google Scholar]
- 34. Romeo R, Colombo M, Rumi M, Soffredini R, Del Ninno E, et al. (1996) Lack of association between type of hepatitis C virus, serum load and severity of liver disease. J Viral Hepat 3: 183–190. [DOI] [PubMed] [Google Scholar]