Abstract
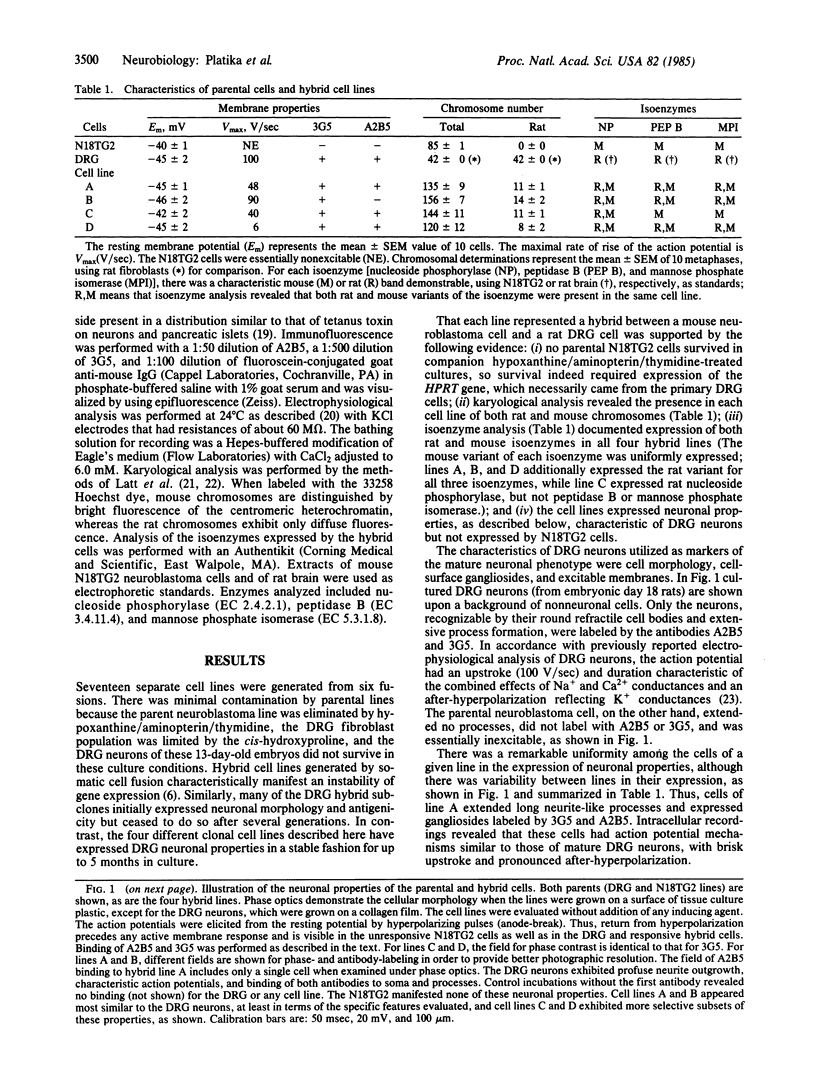
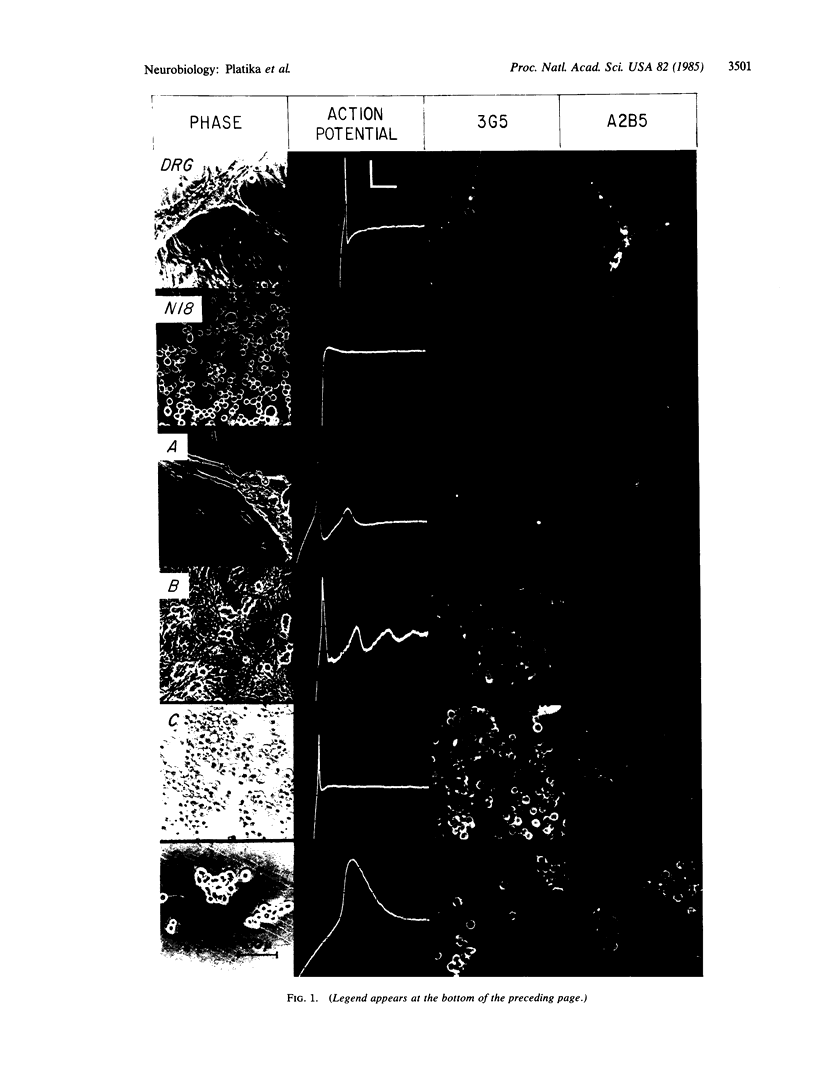
In an attempt to immortalize the gene products of single neurons, somatic cell hybrids were produced by fusion of embryonic rat dorsal root ganglion (DRG) neurons with mouse neuroblastoma cells. Embryonic day 13 rat DRGs were fused with mouse neuroblastoma cells deficient in hypoxanthine phosphoribosyltransferase (HPRT; IMP:pyrophosphate phosphoribosyltransferase, EC 2.4.2.8). The hybrid cells were selected in medium with 100 microM hypoxanthine/1 microM aminopterin/12 microM thymidine to eliminate the neuroblastoma cells and with cis-hydroxyproline to retard fibroblast growth. Of the 17 lines derived, 4 manifested neuronal properties and were cloned. These lines retain both rat and mouse chromosomes and synthesize characteristic rat and mouse isoenzymes. Neuronal gangliosides, action potentials, and extensive neurite-like processes are exhibited by these hybrid cells, properties characteristic of DRG neurons but not of the neuroblastoma parent. Each line manifests a unique combination of action-potential properties and cell-surface markers, suggesting the selective expression of subsets of DRG neuronal genes. All of these neuronal properties are expressed constitutively, without the need for chemical induction or mitotic inhibition, and stably, without diminution after at least 5 months in culture. These lines may prove useful in the identification and isolation of gene products that characterize individual or small subsets of DRG neurons.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augusti-Tocco G., Sato G. Establishment of functional clonal lines of neurons from mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):311–315. doi: 10.1073/pnas.64.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck L., Stein R., Palazzolo M., Anderson D. J., Axel R. Gene expression and the diversity of identified neurons. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):485–492. doi: 10.1101/sqb.1983.048.01.053. [DOI] [PubMed] [Google Scholar]
- Davidson R. L. Genetics of cultured mammalian cells, as studied by somatic cell hybridization. Natl Cancer Inst Monogr. 1978 May;(48):21–30. [PubMed] [Google Scholar]
- Edgell C. J., McDonald C. C., Graham J. B. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth G. S., Walsh F. S., Nirenberg M. Monoclonal antibody to a plasma membrane antigen of neurons. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4913–4917. doi: 10.1073/pnas.76.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman M. C., Spector I. Potassium current suppression by quinidine reveals additional calcium currents in neuroblastoma cells. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5245–5249. doi: 10.1073/pnas.78.8.5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fougère C., Weiss M. C. Phenotypic exclusion in mouse melanoma-rat hepatoma hybrid cells: pigment and albumin production are not reexpressed simultaneously. Cell. 1978 Nov;15(3):843–854. doi: 10.1016/0092-8674(78)90269-6. [DOI] [PubMed] [Google Scholar]
- Goodman C. S., Bastiani M. J., Doe C. Q., du Lac S., Helfand S. L., Kuwada J. Y., Thomas J. B. Cell recognition during neuronal development. Science. 1984 Sep 21;225(4668):1271–1279. doi: 10.1126/science.6474176. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Shain W., Chalazonitis A., Breakfield X., Minna J., Coon H. G., Nirenberg M. Neuronal properties of hybrid neuroblastoma X sympathetic ganglion cells. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4923–4927. doi: 10.1073/pnas.72.12.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer E. J., Macdonald R. L. Calcium- and sodium-dependent action potentials of mouse spinal cord and dorsal root ganglion neurons in cell culture. J Neurophysiol. 1982 Apr;47(4):641–655. doi: 10.1152/jn.1982.47.4.641. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Elde R., Johansson O., Luft R., Nilsson G., Arimura A. Immunohistochemical evidence for separate populations of somatostatin-containing and substance P-containing primary afferent neurons in the rat. Neuroscience. 1976;1(2):131–136. doi: 10.1016/0306-4522(76)90008-7. [DOI] [PubMed] [Google Scholar]
- Kao W. W., Prockop D. J. Proline analogue removes fibroblasts from cultured mixed cell populations. Nature. 1977 Mar 3;266(5597):63–64. doi: 10.1038/266063a0. [DOI] [PubMed] [Google Scholar]
- Killary A. M., Fournier R. E. A genetic analysis of extinction: trans-dominant loci regulate expression of liver-specific traits in hepatoma hybrid cells. Cell. 1984 Sep;38(2):523–534. doi: 10.1016/0092-8674(84)90507-5. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Latt S. A. Microfluorometric detection of deoxyribonucleic acid replication in human metaphase chromosomes. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3395–3399. doi: 10.1073/pnas.70.12.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson S. N., Caddy K. W., Biscoe T. J. Development of rat dorsal root ganglion neurones. Studies of cell birthdays and changes in mean cell diameter. Cell Tissue Res. 1974;153(3):399–413. doi: 10.1007/BF00229167. [DOI] [PubMed] [Google Scholar]
- Madan K., Allen J. W., Gerald P. S., Latt S. A. Fluorescence analysis of late DNA replication in mouse metaphase chromosomes using BUdR and 33258 Hoechst. Exp Cell Res. 1976 May;99(2):438–444. doi: 10.1016/0014-4827(76)90604-2. [DOI] [PubMed] [Google Scholar]
- Minna J. D., Yavelow J., Coon H. G. Expression of phenotypes in hybrid somatic cells derived from the nervous system. Genetics. 1975 Jun;79 (Suppl):373–383. [PubMed] [Google Scholar]
- Mével-Ninio M., Weiss M. C. Immunofluorescence analysis of the time-course of extinction, reexpression, and activation of albumin production in rat hepatoma-mouse fibroblast heterokaryons and hybrids. J Cell Biol. 1981 Aug;90(2):339–350. doi: 10.1083/jcb.90.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers A. C., Rabizadeh A., Akeson R., Eisenbarth G. S. Characterization of monoclonal antibody 3G5 and utilization of this antibody to immobilize pancreatic islet cell gangliosides in a solid phase radioassay. Endocrinology. 1984 Apr;114(4):1338–1343. doi: 10.1210/endo-114-4-1338. [DOI] [PubMed] [Google Scholar]
- Sonderegger P., Fishman M. C., Bokoum M., Bauer H. C., Nelson P. G. Axonal proteins of presynaptic neurons during synaptogenesis. Science. 1983 Sep 23;221(4617):1294–1297. doi: 10.1126/science.6612344. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G., Milner R. J., Bloom F. E. Cellular localization and function of the proteins encoded by brain-specific mRNAs. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):477–484. doi: 10.1101/sqb.1983.048.01.052. [DOI] [PubMed] [Google Scholar]
- Szpirer J., Szpirer C., Wanson J. C. Control of serum protein production in hepatocyte hybridomas: immortalization and expression of normal hepatocyte genes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6616–6620. doi: 10.1073/pnas.77.11.6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. G., Southgate E., Thomson J. N., Brenner S. Factors that determine connectivity in the nervous system of Caenorhabditis elegans. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):633–640. doi: 10.1101/sqb.1983.048.01.067. [DOI] [PubMed] [Google Scholar]




