Abstract
Enterohemorrhagic Escherichia coli (EHEC), an emerging food- and water-borne hazard, is highly pathogenic to humans. In the environment, EHEC must survive phosphate (Pi) limitation. The response to such Pi starvation is an induction of the Pho regulon including the Pst system that senses Pi variation. The interplay between the virulence of EHEC, Pho-Pst system and environmental Pi remains unknown. To understand the effects of Pi deprivation on the molecular mechanisms involved in EHEC survival and virulence under Pho regulon control, we undertook transcriptome profiling of the EDL933 wild-type strain grown under high Pi and low Pi conditions and its isogenic ΔphoB mutant grown in low Pi conditions. The differentially expressed genes included 1067 Pi-dependent genes and 603 PhoB-dependent genes. Of these 131 genes were both Pi and PhoB-dependent. Differentially expressed genes that were selected included those involved in Pi homeostasis, cellular metabolism, acid stress, oxidative stress and RpoS-dependent stress responses. Differentially expressed virulence systems included the locus of enterocyte effacement (LEE) encoding the type-3 secretion system (T3SS) and its effectors, as well as BP-933W prophage encoded Shiga toxin 2 genes. Moreover, PhoB directly regulated LEE and stx2 gene expression through binding to specific Pho boxes. However, in Pi-rich medium, constitutive activation of the Pho regulon decreased LEE gene expression and reduced adherence to HeLa cells. Together, these findings reveal that EHEC has evolved a sophisticated response to Pi limitation involving multiple biochemical strategies that contribute to its ability to respond to variations in environmental Pi and to coordinating the virulence response.
Introduction
Two-component systems (TCSs) are signal transduction pathways commonly used by prokaryotes to sense and adapt to stimuli in the environment; as many as 50 different systems exist in bacteria, and at least 36 known TCSs are used by E. coli K-12 [1], [2]. A typical TCS includes a histidine kinase (HK) and a partner response regulator (RR). In response to an input signal, the HK is auto-phosphorylated. Histidine-to-aspartate phosphotransfer to the RR results in transcriptional regulation and a cellular output response.
Inorganic phosphate (Pi) participates in many fundamental cellular processes [3]. In E. coli, the Pst system and the PhoB-R TCS coordinately mediate Pi sensing. The Pst system has two related but distinct functions: high-affinity uptake of Pi and the sensing of Pi levels regulating expression of the Pho regulon [4]. The pst operon (pstSCAB–phoU) encodes a transport complex belonging to the ABC transporter superfamily. The periplasmic protein PstS binds Pi, whereas PstA and PstC form a membrane channel. The ATPase PstB provides the energy for translocation and interacts with PstC [5]. PhoU has no evident role in Pi transport, but is required for control of the Pho regulon. In E. coli, there are at least 40 gene members of the Pho regulon that are primarily involved in phosphate assimilation and metabolism [6]–[9]. Transcription of all these genes is regulated in response to extracellular Pi concentrations via the TCS PhoB-R. During Pi limitation, the histidine-kinase PhoR phosphorylates PhoB. This in turn activates Pho regulon expression by binding to specific Pho box sequences located within the promoters of genes belonging to Pho regulon genes [6]. In addition, null mutations in pst genes disrupt the regulation of PhoB activation and lead to constitutive expression of the Pho regulon independently of environmental Pi availability [4]. Conversely, when Pi is replete, repression of the Pho regulon is mediated by an interaction between the Pst complex and PhoR, preventing PhoR-mediated phosphorylation of PhoB [10]. In these Pi-rich conditions, expressions of the Pst system becomes repressed and the Pit system induced. Reports indicate that in many bacteria, the Pi-limiting environment or the disruption of the Pst system, induce the Pho regulon and sometimes affect bacterial virulence [11]–[17].
Enterohaemorrhagic Escherichia coli (EHEC) serotype O157:H7 is an important pathogen that can cause a variety of clinical symptoms ranging from mild to severe bloody diarrhoea. The main virulence factors of EHEC are Shiga toxins (Stx), responsible for the hemorrhagic syndrome of the infection such as hemolytic uremic syndrome (HUS), and a T3SS through which EHEC translocates effector proteins into host cells, causing intestinal attaching and effacing (A/E) lesions [18], [19]. The genes required for A/E lesions are encoded within a chromosomal pathogenicity island named the locus of enterocyte effacement (LEE) [20]. The LEE is composed of five major operons. The first gene of the LEE1 operon, ler, encodes a transcriptional regulator that positively regulates the expression of the LEE2, LEE3, LEE4 and LEE5 operons [21]–[24]. The LEE encodes the T3SS, an adhesin (the intimin Eae) and its receptor (Tir) required for intimate adherence to epithelial cells, and effector proteins translocated through the T3SS into the host cell. The complex regulation of LEE expression involves many positive and negative regulators [25]. Most of these factors influence LEE expression by directly or indirectly controlling ler expression; however, a variety of extra-transcriptional mechanisms have also been described that modulate the production of T3SS [26]. In EHEC, the genes encoding Stx1 and Stx2 are located in the genomes of lambdoid bacteriophages that can be induced from lysogenic strains. In the temperate state of the lambda (λ) phage, the CI repressor silences the transcription of most phage genes. The removal of repression that can occur when DNA damage activates the bacterial SOS response, leads to prophage induction [27], [28].
EHEC is widely distributed in domestic ruminants, an important route for transmission to humans [29]. Cattle are the main reservoir for EHEC, especially serotype O157:H7 that is transmitted to humans primarily through consumption of contaminated foods. Faecal contamination of water and other foods may also lead to infection. EHEC can survive and persist in different ecological habitats such as soil, manure and aquatic environment (reviewed in [30]).
We have previously shown that during interaction between EHEC and the predacious protozoa Acanthamoeba castellanii, a free-living amoeba mostly found in aquatic environments, the expression of the Pho regulon contributed to adaptation to compete with amoebae in an environment that was limited in nutrients [31]. Indeed in co-culture, the EDL933Δpst strain reduced the growth of A. castellanii even more than the wild-type strain. This is also supported by the observation of an increase in expression of Pho genes in strain EDL933 when facing A. castellanii [32]. The Pho regulon is a global regulatory circuit involved in bacterial Pi management that has the ability to alter other cellular responses and virulence traits that affect bacterial survival when in association with amoebae in the environment [6], [33]. In V. cholerae, the Pho regulon is required for its survival in both fresh water environments and the host small intestine [34].
EHEC, a facultative pathogen, thrives in nutritionally disparate conditions, bovine and human intestines, and in ecological habitats. The essential nutrient Pi is limited in some conditions such as the aquatic ecosystems and terrestrial environments. The concentration of Pi is usually low in natural environments [35], [36]. The availability of Pi in the intestine is variable. It has been estimated from metabolic studies that in healthy adults consuming an average western diet, a Pi concentration of 15 to 30 mM is normally present under homeostatic conditions in the human intestinal lumen [37]–[39]. This level of in vivo Pi concentration should repress the Pho regulon. However, high level expression of some Pho regulon members (UgpB, PhoE, PhoA, Pst and PhoU) has been observed in EHEC O157:H7 isolated from piglet gut [40]. This indicates that EHEC cells can encounter Pi starvation signals in open environments as well as in vivo. Because we and others have observed that either Pi limitation or inactivation of the Pst system can modulate the virulence of intestinal and extra-intestinal bacterial pathogens [11], [12], [14], [16], [41]–[45], we hypothesized that EHEC virulence factors could be modulated in Pi-limited conditions.
Materials and Methods
Bacterial Strains and Culture Media
All strains used in this study are listed in Table 1. We used the EHEC O157:H7 strain EDL933 and its mutants ΔphoB and ΔpstCAB [31]. To measure the expression level of the stx2 promoter, a genetic amplifier reporter system was used as described [46]. This amplification system replaced the chromosomal coding sequence of stx2AB operon with the coding sequence of the T7 RNA polymerase gene (T7pol), resulting in strain EDL933Δstx2::T7pol. This strain was next transformed with the multi-copy reporter plasmid pHL40 [46] that expresses gfp under the control of the T7pol-specific promoter. The Gfp background was determined in the EDL933Δstx strain without T7pol fusion, also carrying the pHL40 plasmid. The fluorescence emission values (λ 520 nm) were normalized with OD600 nm values. As a positive control for stx2 transcription, 500 μg/mL of mitomycin C (MitC; the DNA-damaging agent) [47], was added to wild-type strain grown in high Pi during 3 h to induce the BP933W phage [48].
Table 1. E. coli strains and plasmids used in this study.
| Strain/Plasmid | Description and relevant characteristics a | Source reference |
| EHEC strains | ||
| EDL933WT | E. coli O157:H7; wild-type | [80] |
| EDL933Δpst | EDL933; pstCAB::Km; Pho-regulon constitutive | [31] |
| EDL933ΔphoB | EDL933; phoB::Km; Pho-regulon negative | [31] |
| EDL933ΔescN | EDL933; escN::Km; non-functional T3SS mutant | [56] |
| EDL933Δstx | EDL933; Δstx1; stx2::Km | [55] |
| EDL933Δstx2::T7pol | EDL933; stx1::Km; stx2::T7pol-Cmr | This study |
| E. coli laboratory strains | ||
| ?7213 | SM10λpir ΔasdA4, Kmr | [81] |
| Plasmid | ||
| pAN92 | pACYC184::pst operon Cmr | [82] |
| pHL40 | Reporter plasmid that expresses gfp under the control of a T7 RNA polymerase-specific promoter and Gmr | [46] |
| pHSG575 | Low-copy-number cloning vector | [83] |
| pHSG575-phoBR | pHSG575, phoBR + CmR KmR | This study |
Kmr, kanamycin-resistant; Cmr, chloramphenicol-resistant; Gmr, gentamicin-resistant; Apr; ampicillin-resistant
Pi Starvation, Culture Experiments and Alkaline Phosphatase Assay
EDL933 strains were grown at 37 °C in 3-(Nmorpholino) propanesulfonic acid (MOPS Teknova) minimal medium [49] supplemented with 0.2% (w/v) glucose, thiamine (0.1 μg/mL), and indicated concentrations of Pi (K2HPO4). To test bacterial responses to Pi concentrations, cells from overnight LB cultures were inoculated into 25 mL of MOPS medium containing 1.32 mM KH2PO4 with a starting OD600 nm at 0.15 and grown to an OD600 nm of 0.3. Cells harvested from these fresh cultures were washed once and resuspended in 25 mL MOPS media containing either 1.32 mM (Pi-rich = Pi+) or 1 μM (Pi-limited = Pi−) KH2PO4. Growth was monitored during 16 hours by measuring OD600nm and plating followed by CFU counts. For time-course monitoring of the Pho regulon expression, alkaline phosphatase (AP) activity was measured as described previously [12], [14]. Briefly, aliquots were removed at indicated times, and 4 μg/ml of p-nitrophenyl phosphate (Sigma) was added to cells permeabilized by 50 μl of 0.1% SDS and 50 μl of chloroform. Color development was monitored at 420 nm and AP activity was expressed in Miller units (M.U.), and was calculated as follows: 1000 × [OD420 nm - (1.75 × OD550 nm)] / T (min) × V (ml) × OD600 nm].
In MOPS Pi− medium, the growth curve was similar between the wild-type and ΔphoB strains. However, the growth of the wild-type strain was delayed in Pi− compared to in Pi+. Therefore, we chose the comparison points based on similar CFU numbers (Figure S1A) and this was monitored by the measurement of PhoA activity (Figure. S1B).
RNA Extraction, Microarray Experiments and qRT-PCR
The EDL933WT strain and its isogenic ΔphoB mutant were grown in Pi− and/or in Pi+ conditions until the OD600 nm reached 0.55 to 0.6. Samples equal to 5 × 108 CFU were taken from this mid-log phase and processed for transcriptome analysis. Ten μg of RNAs were extracted and cDNAs were generated using reverse transcriptase. One μg of the fragmented and biotinylated cDNAs was hybridized onto Affymetrix GeneChip E. coli. The data were processed using FlexArray software using Robust Multichip Average (RMA) normalization procedure. The levels of transcription obtained from 3 biological replicates of each experimental condition were compared using the EB (Wright & Simon) algorithm. We then conducted comparisons between EDL933 grown in Pi− and in Pi+ conditions and between EDL933 and EDL933ΔphoB strains both grown in Pi−. The differential expression conditions corresponded to a two-fold change (FC) cut-off and adjusted p-value < 0.05. Microarray results were validated by triplicate qRT-PCR. Each reaction was normalized to the tus housekeeping gene and the variation rate was calculated using the 2−ΔΔCt method. All differential transcripts in both comparisons were functionally classified in COG according to TIGR's Comprehensive Microbial Resource [50].
Production of Constitutively Active PhoB (PhoBCA)
The construction of the gene coding for the constitutively active PhoB (PhoBCA) protein was based on previous work [51] in which the PhoBCA was obtained by the combination of two substitutions: Asp10Ala and Asp53Glu (DADE). PhoBCA was purified and produced by Genscript as follows. Briefly, the phoBCA gene was cloned in a modified pGEX vector that contains a thrombin protease recognition site between the Glutathione S-Transferase (GST) tag and a multiple cloning site. Following the elution of PhoBCA-GST using Glutathione Sepharose resin, the fused protein was cleaved by thrombin protease to remove the GST affinity Tag. The cleavage reaction was incubated again with Glutathione Sepharose 4B beads and the flow-through containing cleaved PhoBCA was collected. The protein concentration was determined by Bradford protein assay with BSA as a standard, and the purity was about 85% as estimated by densitometric analysis of the Coomassie Blue-stained SDS-PAGE gel.
Electrophoretic Mobility Shift Assays (EMSA)
The EMSA assay was adapted from previous reports [34], [52]. The EMSA reaction mix consisted of PhoBCA at the desired concentrations (2.5, 5.0, 7.5 or 10.0 μM), 50 nM final probe concentration, 0.1 mg/mL calf thymus DNA and 0.1 mg/mL BSA in EMSA buffer (50 mM NaCl, 20 mM Tris, pH 7.4, 0.02% v/v sodium azide). Reactions were incubated for 30 minutes at 25°C, and then loaded onto an 8% native polyacrylamide gel running at 120 V in 1x TBE buffer. Fluorescent bands were visualized using the Fusion Suite imaging scanner. For each gene tested, the probes were amplified from the EDL933 genome using primers listed in Table S1. The forward primers used to generate all the probes contained 5′ 6-FAM fluorescein tags, to enable gel detection using a chemiluminescence scanner. The probes tested by EMSA assay represent candidate Pho-related virulence gene promoter regions that contain Pho box-sequences identified by in silico research of the EDL933 genome. To this end, we created a Pho Box matrix based on 12 Pho box-sequences from the known Pho-dependent genes in EDL933. The matrix frequencies were uploaded into the Gibbs algorithm tool at http://ccmbweb.ccv.brown.edu/gibbs/gibbs.html that browses the promoter query (Table S4).
Polyclonal EspB Antibody Production
We used a polyclonal anti-EspB serum obtained by rabbit immunization performed by Genscript (Piscataway, New Jersey, USA). Briefly, 2 rabbits were injected twice at 14-day intervals with a synthetic EspB antigenic peptide “CRRTQDDITTRLRDI” translated from the EDL933 genome. This peptide sequence was chosen according to its predicted antigenic potency and hydrophilic content, as determined by the Jameson and Wolf algorithm. After the second immunization, the serum titer was 1∶512,000 as tested against the peptide by ELISA assay. The anti-EspB serum was then adsorbed overnight at 4°C, at a 1∶1 volume ratio against HB101 E.coli cell extract and incubated at 37°C for 2 hours. Adsorbed material was eliminated by a 45-minute 45,000 rpm ultracentrifugation and 0.05% sodium azide was added to the antibody as a preservative. Unless otherwise indicated, all steps were done at 4 °C to prevent degradation.
Western-blot Analysis
Western-blot analyses were done as previously described [53], [54]. Twenty ml of filtered culture supernatants that were normalized to CFU and OD600 and the total secreted proteins, were precipitated overnight with 10% of trichloroacetic acid (TCA) at 4 °C and then used to measure the secretion of EspB. Protein samples were resuspended in SDS sample buffer, boiled for 5 minutes, run on SDS-PAGE gels and transferred to nitrocellulose membranes. Blots were probed with rabbit polyclonal antiserum against EspB (1∶2000) and then with a goat anti-rabbit HRP-linked secondary antibody (Bio-Rad Laboratories, Hercules, CA). Proteins were detected with ECL-Plus horseradish peroxidase using the Western-blotting detection kit Promega.
Determination of Stx2 Concentrations by ELISA
The production and release of Stx2B was quantified using the ELISA assay as described elsewhere [55]. The EDL933 strain was grown in MOPS/Pi+ or Pi−. Its ΔphoB derivative mutant and the complemented strain (harboring pHSG575-phoBR) were grown in MOPS/Pi−. Twenty mL cultures, normalized to OD600 0.6, were harvested and 8 mL of the supernatants were filtered and concentrated twice through a centrifugal filter (3KDa Amicon Ultra-4) for 25 minutes at 3,700 ×g. The remaining volumes were designed extra-cellular proteins and used to determine the concentration of secreted Stx2. The bacterial pellets were resuspended in 20 mL PBS, lysed by French press/sonication and then centrifuged for 20 minutes at 12,000 rpm. The lysate supernatants were designated whole cell proteins and used to access the amount of Stx2 production.
HeLa cell cultures and infections
The HeLa epithelial cell line was maintained in DMEM-HEPES with 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin at 37 °C under 5% CO2. HeLa cells were seeded into 6-well plates (108 cells/well) and grown for 24 hours. Subsequently cells were washed, and 3 ml of fresh complete medium without antibiotics was added to the wells. Cells were infected with 109 bacteria per well for 5 hours. After 3 washes with PBS, cells were fixed using methanol for 15 minutes and stained with 10% Giemsa solution (Gibco) for 20 minutes. The number of adherent bacteria per 25 cells was determined by direct count under a light microscope (Magnification 640 ×). The TTSS ΔescN strain [56] was used as negative control.
Microarray Accession Numbers
Microarray data have been deposited in the Gene Expression Omnibus database www.ncbi.hlm.nih.gov/projects/geo under accession number GSE50529 [NCBI tracking system #16869052].
Results
Effects of Pi Starvation and phoB Inactivation on the Transcriptomic Profiles of EDL933
EHEC strain EDL933 was grown in MOPS supplemented with low or high Pi at concentrations, respectively 1.0 μM and 1.32 mM. As shown in Figure S1A, a decrease in growth rate was observed when the Pi concentration was limited to 1 μM. We also investigated the role of PhoB in EDL933 adaptation to Pi limitation using the ΔphoB mutant. The growth of the ΔphoB strain was similar to that of the wild-type strain in Pi-limited medium (Figure S1A). The alkaline phosphatase activity of PhoA is commonly used to evaluate the activation state of the Pho regulon [16]. As expected, the production level of PhoA in the wild-type strain was negatively correlated with Pi concentration. In addition, PhoA level was decreased over 100-fold in the ΔphoB mutant compared with the wild-type strain assayed in Pi-limited medium (Figure S1B). Thus, in the wild-type strain, the Pho regulon is highly activated during growth in Pi-limited medium and this activation is abrogated in the ΔphoB mutant.
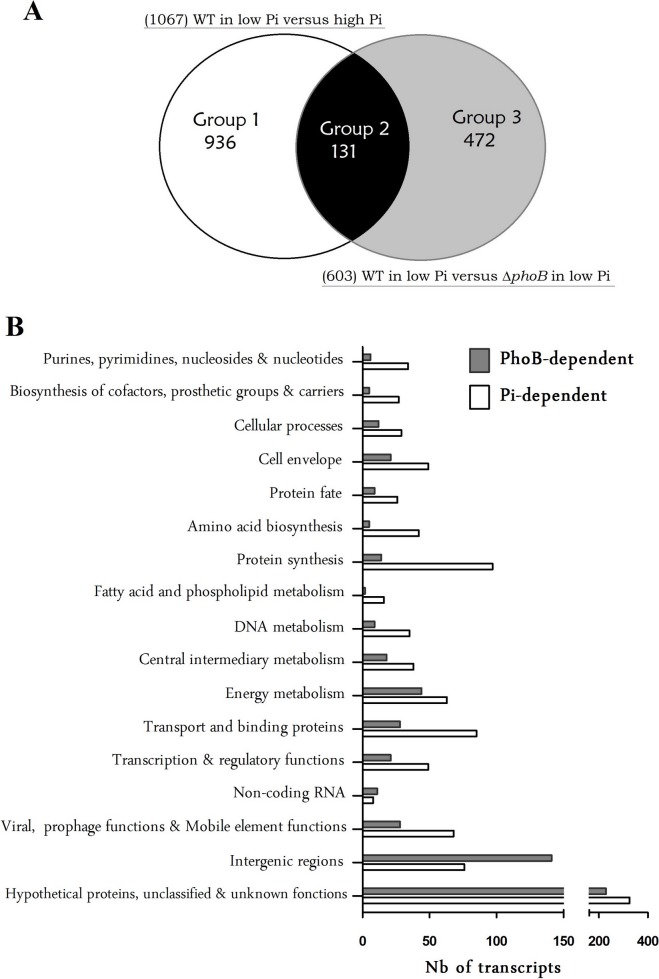
Transcriptomic analysis was performed to identify the regulatory circuit of PhoB in EDL933. Complementary DNAs obtained from total RNA were hybridized onto Affymetrix GeneChip E. coli 2.0 covering the entire EDL933 genome. Compared to the expression profile in high Pi medium, the expression of 1067 genes in the wild-type grown in low Pi medium were changed more than two-fold with an adjusted P value of <0.05 (Figure 1A) (Tables S1 and S2). We also compared the transcriptome of WT and ΔphoB strains cultivated in low Pi medium and found that 603 genes were differentially expressed.
Figure 1. Global analysis of differentially expressed genes in response to Pi starvation or phoB inactivation in EDL933 strain.
A. Classification of genes whose expression levels were altered in Pi-dependent and in PhoB-dependent manners. Left and right circles indicate the differentially expressed genes of wild-type and ΔphoB-mutant strains with expression levels that were altered over 2-fold under Pi limitation. Venn diagram: Group 1 includes 936 PhoB-independent genes that are differentially expressed under Pi limitation in the wild-type strain, but that did not change in the ΔphoB mutant. Group 3 includes 472 PhoB-dependent genes differentially expressed in the ΔphoB mutant but did not change under Pi-limitation in the wild-type strain. Group 2 included 131 PhoB-dependent Pi response genes that are differentially expressed under Pi limitation in the wild-type strain and between the wild-type and the ΔphoB strains. B. Functional classification of genes with altered expression in strain EDL933 grown in Pi-limited conditions compared to cells grown in Pi-rich conditions (Pi-dependent (white bars)) and EDL933 incubated in Pi-limited conditions compared to ΔphoB mutant cells grown in the Pi-limited conditions (PhoB-dependent (gray bars)).
Combining these two transcriptomic analyses, we classified genes into 3 distinct categories as shown in Figure 1A. Group 1 includes 936 genes with expression levels that changed in the wild-type strain between low and high Pi concentration, but did not change between wild-type and ΔphoB strains, therefore corresponding to PhoB-independent Pi response genes. In contrast, Group 3 includes 472 genes with expression levels that changed between wild-type and ΔphoB strains, but with no expression change in the wild-type strain grown in low or high Pi media, indicating that expression of these genes is PhoB-dependent, but not modulated by Pi concentration. Lastly, Group 2 includes 131 genes having expression modulated by both Pi concentration and by the PhoB regulatory protein (Table 2). The high proportion of genes that are differentially expressed between the Pi+ and Pi- regimes could be slightly affected by the difference in growth rate (Figure S1). To minimize that, equivalent CFU amounts were chosen from Pi+ and Pi− conditions that resulted in similar expression patterns of the Pho regulon members presented in Table 2.
Table 2. Genes among upregulated and downregulated PhoB-dependent Pi response genes.
| Functional class and gene name | Known or predicted function | WT/Pi− vs WT/Pi+ | WT/Pi− vs ΔphoB/Pi− | |||
| FC | P value | FC | P value | |||
| DNA metabolism | ||||||
| mutY | Adenine DNA glycosylase | −5.45 | 2.33E-04 | −2.19 | 1.01E-02 | |
| recG | ATP-dependent DNA helicase RecG | −3.02 | 8.04E-03 | −3.46 | 5.12E-03 | |
| Purines, pyrimidines, nucleosides. and nucleotides | ||||||
| gsk | Inosine-guanosine kinase | −2.31 | 4.99E-03 | −2.15 | 9.62E-03 | |
| purT | P-ribosylglycinamide formyltransferase 2 | −3.00 | 6.16E-03 | −2.17 | 8.97E-03 | |
| Energy metabolism | ||||||
| ppc | P-enolpyruvate carboxylase | −3.80 | 5.54E-04 | −2.05 | 1.02E-02 | |
| Central intermediary metabolism | ||||||
| phoA | Alkaline phosphatase (phoA-psiF) | 131.70 | 2.48E-07 | 58.70 | 3.99E-07 | |
| gadB | Glutamate decarboxylase (gadBC) | 10.46 | 9.58E-05 | 2.41 | 1.10E-02 | |
| amn | AMP nucleosidase | 14.54 | 3.29E-06 | 5.24 | 9.48E-04 | |
| Cellular processes | ||||||
| ahpC | Alkyl hydroperoxide reductase (ahpCF) | 6.28 | 1.00E-03 | 11.17 | 1.07E-03 | |
| Amino acid biosynthesis | ||||||
| trpL | Trp operon leader peptide | −9.94 | 1.16E-05 | −2.48 | 4.22E-03 | |
| Cell envelope | ||||||
| pgaB | Biofilm adhesin polysaccharide PGA export lipoprotein | 6.74 | 2.47E-04 | 2.48 | 3.50E-02 | |
| slp | OMP induced after carbon starvation | 2.85 | 4.70E-02 | 2.75 | 7.20E-03 | |
| fhuA | Ferrichrome outer membrane transporter | −4.37 | 6.48E-03 | −2.51 | 1.80E-02 | |
| Protein fate | ||||||
| hdeA | Stress response protein acid-resistance protein | 4.49 | 7.13E-03 | 2.10 | 4.03E-02 | |
| iraP | Anti-RssB factor. RpoS stabilzer during Pi starvation; anti-adapter protein | 3.96 | 3.63E-03 | 2.42 | 7.74E-03 | |
| Protein synthesis | ||||||
| yibD | Predicted glycosyl transferase | 80.87 | 1.56E-06 | 14.99 | 5.68E-05 | |
| Z4332 | Cytotoxin Efa-1 involved in posttranscriptional regulation of T3S proteins | 3.10 | 2.31E-02 | 2.29 | 9.88E-03 | |
| Transcription & regulatory functions | ||||||
| phoB | DNA-binding response regulator (phoBR) | 4.28 | 3.25E-03 | 13.81 | 4.71E-04 | |
| gadE | DNA-binding transcriptional activator | 7.32 | 1.72E-03 | 2.09 | 4.31E-02 | |
| Transport & binding | ||||||
| pstS | High-affinity P-specific transporter (pstSCAB-phoU) | 47.48 | 8.46E-06 | 15.67 | 5.31E-06 | |
| phnC | Phosphonate transporter ATP-binding protein (phnCDEFGHIJKLMNOP) | 81.45 | 1.45E-06 | 11.61 | 7.08E-05 | |
| ugpB | Glycerol-3-phosphate transporter subunit (ugpBAECQ) | 7.92 | 5.46E-04 | 5.67 | 1.74E-03 | |
| phoE | Outer membrane phosphoporin protein E | 40.39 | 1.09E-04 | 11.72 | 1.14E-03 | |
| malE | Maltose transporter periplasmic protein | −2.46 | 2.31E-02 | -2.09 | 2.20E-02 | |
| fadL | Long-chain fatty acid outer membrane transporter | −2.66 | 3.56E-03 | -2.38 | 5.88E-03 | |
| Virulence-related determinants | ||||||
| espH | Z5115 Effector translocated to the host cell membrane by the T3SS. LEE3 operon | 2.42 | 4.89E-03 | 2.56 | 9.88E-03 | |
| escD | LEE-encoded T3SS component | 2.17 | 1.19E-02 | 2.38 | 1.43E-02 | |
| espF | EspF / hypothetical protein. LEE4 operon | 2.02 | 2.79E-02 | 2.28 | 3.15E-02 | |
| Unknown function. unclassified and hypothetical protein | ||||||
| bssR | Repressor of biofilm formation by indole transport regulation | 18.58 | 1.35E-03 | 4.86 | 6.47E-03 | |
| ygaW | Predicted inner membrane protein. stress-responsive | 5.98 | 2.65E-04 | 3.43 | 6.47E-03 | |
| yfiD | Autonomous glycyl radical cofactor GrcA | 5.58 | 1.29E-02 | 3.16 | 1.42E-02 | |
| psiE | P-starvation-inducible protein PsiE | 20.81 | 1.59E-05 | 3.10 | 1.74E-03 | |
| cybC | Cytochrome b562 | 3.56 | 5.29E-04 | 2.01 | 1.41E-02 | |
| uspG | Universal stress protein UP12 | 6.89 | 1.81E-04 | 2.11 | 1.45E-02 | |
| yebE | Inner membrane protein. DUF533 family | 5.19 | 5.75E-05 | 2.34 | 6.47E-03 | |
| yjeI | Conserved protein | 3.72 | 3.01E-04 | 2.65 | 3.35E-03 | |
| matB | Cryptic Mat fimbrillin gene | 2.60 | 9.23E-03 | 2.37 | 1.23E-02 | |
| ffs | Unknown non-coding RNA | 11.99 | 4.59E-05 | 2.19 | 1.20E-02 | |
| Z2828 | Predicted protein YoaI | 5.68 | 1.61E-03 | 4.86 | 1.85E-03 | |
| Z3620 | Hypothetical protein | 7.24 | 4.79E-04 | 3.80 | 2.45E-03 | |
| yheT | Predicted hydrolase | −3.54 | 2.79E-03 | -2.19 | 1.12E-02 | |
| yijP | Conserved inner membrane protein | −5.08 | 1.03E-03 | -2.28 | 4.32E-02 | |
| yejL | Hypothetical conserved protein. UPF0352 family | −4.00 | 3.50E-04 | -2.44 | 6.75E-03 | |
| yhcG | Hypothetical protein | −2.82 | 4.20E-03 | -2.59 | 6.44E-03 | |
| ybdZ | Hypothetical protein | −3.74 | 2.13E-02 | -2.76 | 6.75E-03 | |
| IG | Intergenic gerion IG 696357_696735-r | −2.21 | 1.61E-02 | -2.88 | 8.87E-03 | |
The Cluster of Orthologous Gene (COG) classification was used to designate genes whose expression was altered during Pi starvation and was under the control PhoB (Figure 1B). Upregulated genes include those encoding proteins of unknown function, transport and binding proteins, energy and central intermediary metabolism, and transcription (Table S2). Genes that were downregulated include the unknown function genes, and those involved with protein fate; protein synthesis; DNA metabolism; purine, pyrimidine, nucleoside and nucleotide pathways; and cell envelope proteins (Table S3). A large number of genes involved in stress responses and hypothetical proteins were also differentially expressed as were some virulence genes. In low Pi, there was a coupling between Pho regulon gene expression and the central pathways of carbon and energy metabolism, many of which are also formal pathways in Pi metabolism.
The expression patterns of the genome-wide transcriptional response that was observed shared similarities with transcriptomic analyses of E. coli K-12 during Pi starvation, and with the avian pathogen E.coli (APEC) and Citrobacter rodentium Δpst mutants [7], [11], [12].
The global classification of differentially expressed genes in Pi limitation is shared with the effects of nutrient depletion. Some of these genes coordinate the expression of hundreds of genes across a variety of cellular functions associated with cellular homeostasis and metabolism (Figure 1B). An expression of PhoB regulon genes was effectively induced in the wild-type strain grown in low Pi. In low Pi, the Pho regulon was downregulated in the ΔphoB mutant compared to the wild-type strain. Fold change for Pho regulon genes varied from 190.5- to 2.1-fold (Table 2). Besides those, in low Pi the bssR gene showed the highest induction (18.6-fold), whereas the greatest repression was observed for the trpL gene (9.9-fold) (Tables S2 and S3). Validation of microarray results was achieved using qRT-PCR. Comparison of gene expression by microarray hybridizations and qRT-PCR demonstrated a high level of concordance between the datasets, which is represented by a correlation coefficient of 0.87 in response to low Pi and of 0.82 in response to ΔphoB mutation (Figure S2).
Globally, the transcriptional profile of EDL933 grown in Pi-limited medium indicates that, in addition to up-regulating genes associated with scavenging pathways for Pi acquisition and conservation, many genes dealing directly with global stress were differentially expressed. Many lines of evidence suggest that the Pho regulon and the stress response are interrelated [33]. The Pho regulon and the RpoS regulon are interrelated regulatory networks of the bacterial adaptive response [33]. RpoS is a sigma factor implicated in the cellular response to many stresses. It is also implicated in the stationary phase and the induction of genes in nutrient-limiting environments [57]. The rpoS gene is expressed under a variety of growth conditions, but regulation and RpoS production is largely dependent on post-transcriptional stability [57], [58]. In our study, the rpoS gene was not differentially expressed in strains grown in Pi-limited medium. However, in this condition the RpoS-regulatory gene iraP was induced (4-fold). IraP encodes an anti-adaptor protein that enhances RpoS stability and accumulation by inhibiting its targeting to the ClpXP degradosome [59], [60]. Moreover, Mandel et al., has shown that RpoS translation and stability were significantly increased during Pi starvation [61]. This suggests that in our results the RpoS stability was probably increased in EDL933 grown in Pi-limited medium that could lead to regulatory expression of numerous RpoS-dependent genes. In addition, some genes involved in the oxidative stress and acid stress responses were found to be differentially expressed. Of these, some were modulated in the ΔphoB mutant such as iraP, katE, ahpC, gadE and gadBC. These stress responses were also observed in the Δpst mutant of APEC and C. rodentium strains [11], [12].
Pi-limited Conditions Increased LEE Gene Expression in PhoB-dependent and Independent Manners
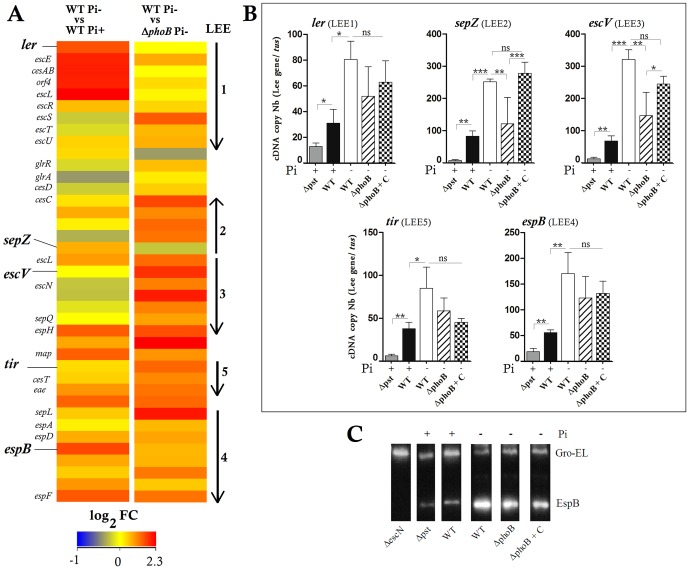
EHEC-specific virulence-associated genes were modulated in Pi-limited medium and in the ΔphoB mutant. Genes from LEE operons were 2.0- to 4.6-fold upregulated in the wild-type strain grown in Pi-limited medium compared to the wild-type strain grown in Pi-rich medium, and compared to the ΔphoB mutant (Fig. 2A). Moreover, several genes encoding LEE regulators such as gadE (+7.3), pchA (+7.6) and ihf (+2.6) were also upregulated in the wild-type strain in response to Pi starvation. However, in the wild-type strain compared to the ΔphoB mutant, genes encoding the non-LEE effectors NleB, NleE, NleH and NleG-1 and the LEE-negative regulator HN-S were upregulated (Tables S2).
Figure 2. Low Pi conditions and deletion of phoB gene increased LEE gene expression and T3SS secretion of EspB in EDL933 strain.
A. A heat map of the expression levels of LEE genes between wild-type strain grown in low or high Pi concentrations, and between wild-type and ΔphoB strain grown in low Pi condition. Expression values were determined from the variance analysis by the EB (Wright & Simon) algorithm and are represented colorimetrically, with red representing up regulation (ratio of +2.3) and blue representing downregulation (ratio of -1) on a log2 scale. The data are represented as the means from three biological replicates. B. Expression level of LEE genes ler, sepZ, escV, tir and espB was analyzed by RT-qPCR in the wild-type, the ΔphoB mutant and its complement (ΔphoB+C) and the Δpst mutant grown in low or high Pi media as indicated. C. Western-blot using anti-EspB antibody against the supernatant of the wild-type, the ΔphoB mutant and its complement and the Δpst mutant grown in low or high Pi media as indicated. The ΔescN mutant grown in DMEM was used as a negative control. One μg/mL of Gro-El was added as a protein precipitation control. Asterisks represent the significant ANOVA p value (*<0.05, **<0.01, ***<0.001). ns: Not significant.
We quantified the transcripts of ler, sepZ, escV, espB and tir encoded by LEE1, 2, 3, 4 and 5 respectively in the wild-type strain grown in Pi-limited and Pi-rich media and in ΔphoB strain grown in Pi-limited conditions. LEE-gene expression in the wild-type strain was 2.0-fold to 4.6-fold higher in response to Pi limitation (Fig. 2B). The tested genes that were downregulated in the absence of PhoB, sepZ and escV were the most significantly affected while the complementation of the ΔphoB mutant restored the sepZ and escV transcripts to wild-type levels (Fig. 2B). This suggests that PhoB is at least partially required for LEE activation in low Pi.
The EDL933 strain cultivated in Pi-limited medium delivers higher level of extracellular proteins compared to when grown in Pi-rich medium (data not shown). Similarly, secretion of the effector protein EspB, encoded by LEE4, was more abundant in the wild-type strain grown in low Pi medium (EspB/GroEL ratio = 3.6 ±0.3) than in high Pi medium (1.2 ±0.3) and to a lesser extent in the ΔphoB mutant in Pi-limited medium (2.6 ±0.2) (Figure 2C). The ΔphoB-complemented strain showed a slight increase of EspB (2.8±0.4) compared to the mutant. These results showed that higher level of the EspB effector as detected in Pi-limited conditions, confirming that Pi limitation can increase LEE gene expression. Moreover Pi-dependent activation was partially PhoB-dependent.
In Pi-limited medium, PhoB Activated the Transcription, Production and Release of Stx2 and Controlled Gene Transcription of BP933W Regulators
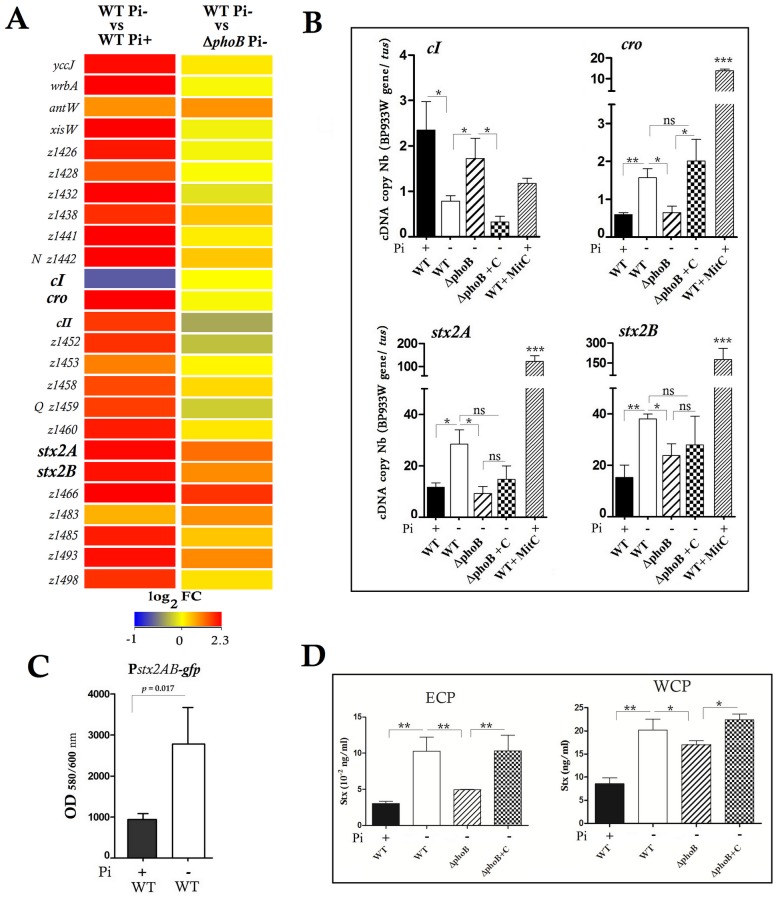
Many BP-933W phage genes were upregulated in response to Pi starvation, including the anti-terminators N and Q, and the stx2 and phage-late genes while expression of the CI repressor gene was downregulated (Figure 3A, Tables S2 and S3). In these Pi-limited conditions, genes encoding RecA and LexA, the SOS response marker and regulators, were not differentially expressed, while a gene encoding an indirect λ-phage regulator PcnB was repressed (Table S3).
Figure 3. Effect of Pi and PhoB on stx2 gene expression and toxin production.
Low Pi and PhoB increased transcription of stx2 and its toxin production and of BP933W genes except for repressor gene cI that was repressed. A. Heat map of the expression levels of BP933W genes between wild-type EDL933 strain grown in low or high Pi conditions and between wild-type and ΔphoB strain grown in low Pi conditions. B. The expression levels of the BP933W genes cI, cro and stx2AB were analyzed by RT-qPCR in the wild-type strain and the ΔphoB mutant grown in low or high Pi media as indicated. The wild-type strain induced by mitomycin C (WT+MitC) was used as a positive control C. Fluorescence of the wild-type EDL933 strain carrying a chromosomal fusion reporting stx2 transcription level grown in Pi+ or Pi- conditions. D. The production of Stx2 measured by ELISA in extra-cellular protein (ECP) and whole cell protein (WCP) fractions of the EDL933 wild-type strain grown in Pi+ or Pi- conditions and in the ΔphoB mutant and its complemented derivative. Asterisks represent the significant ANOVA P value (*<0.05, **<0.01, ***<0.001).
In EDL933, Stx2 is encoded by stx2AB genes in the λ-like lysogenic phage BP933W. The stx2AB operon is located downstream of the late promoter region and upstream of the phage lysis cassette [62]–[64].
Measurement by qRT-PCR confirmed the significant decrease of cI expression and increase of cro and stx2AB genes in Pi-limited medium and in a PhoB-dependent manner (Figure 3B). As a positive control, MitC-induction of the BP933W phage resulted in high expression of cro and stx2 genes while cI repressor gene expression remained at a basal transcription level. The ΔphoB-complemented strain exhibited similar levels of cI and cro transcription as the wild-type, although expression of the stx2 genes was only partially restored. The transcriptional activation of stx2 expression in Pi-limited medium was also observed using a GFP reporter system (Figure 3C).
To evaluate Stx2 synthesis, amounts of Stx2 were quantified in cells and supernatants using an ELISA assay. In response to Pi-limited conditions, the production and release of Stx2B were increased by 2.9-fold (Figure 3D). By contrast, Stx levels were decreased 2-fold in the ΔphoB mutant, whereas complementation of the ΔphoB mutant restored Stx2 level to that of the wild-type parent strain. These results demonstrate that the production of Stx2 toxin by the EDL933 strain is modulated by Pi levels and the PhoB regulatory protein.
PhoB Binds to LEE1, LEE2 and stx2 Promoter Regions in vitro
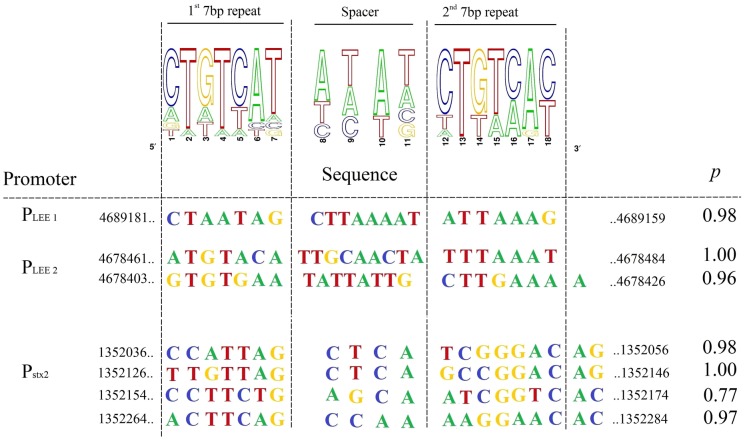
The PhoB binding consensus sequence is defined as a 22-bp region of double-stranded DNA that consists of two direct repeats of 11 bp [65]. We performed in silico analysis to identify putative PhoB-binding sites associated with the LEE promoters (Figure 4). Pho box consensus sequences with high probability were identified in the LEE1 and LEE2 promoter regions. In LEE1, a Pho box was located between the ler distal P1 and proximal P2 promoters [66] and two Pho boxes were identified in the LEE2 promoter region [67].
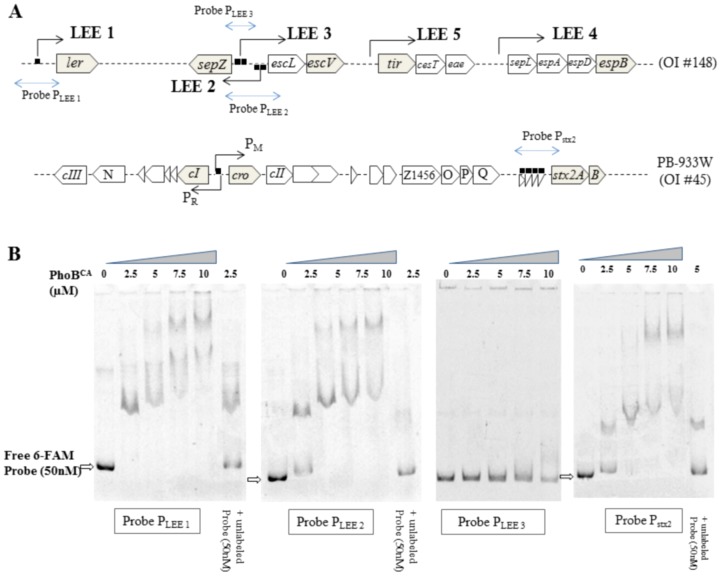
Figure 4. Bio-informatic analyses of PhoB-binding sites.
Sequence logos determined from 12 putative PhoB-binding sites in EDL933 are indicated (upper panel). Potential consensus sequences identified in the promoter regions of LEE 1, LEE 2 operons and upstream stx2AB genes are shown with their statistical scores and genomic positions (lower panel). Pho box prediction probabilities were determined using the matrix frequencies of Table S4 that were uploaded into the Gibbs software algorithm. http://ccmbweb.ccv.brown.edu/gibbs/gibbs.html.
To investigate whether PhoB directly binds to the LEE promoters containing putative PhoB boxes, EMSA experiments were performed using PhoBCA, a constitutively active PhoB mutant that activates Pho regulon promoters in the absence of phosphorylation. EMSA results showed PhoB dose-dependent shifts of the probes designed from the LEE1 and LEE2 promoter regions but not the LEE3 promoter region (Figure 5). As expected, the addition of unlabelled probes (1∶1) reduced the amount of shifted 6-FAM labelled probes. This data indicates that PhoBCA binds specifically to the LEE1 and LEE2 promoter regions in vitro.
Figure 5. PhoB binds in vitro to LEE1, LEE2 and stx2 promoter regions.
A. Schematic representation of LEE and lambdoid prophage BP933W DNA regions. Arrows indicate the orientation of transcription. The blue lines/arrows indicate the probes used for EMSA assays. The square symbol indicates the predicted Pho box. B. Increasing amounts of GST-purified recombinant PhoBCA were used in the EMSA assay to shift the 6-FAM labeled DNA probes amplified from LEE1 (−253 to +64 bp), LEE2 (−228 to +81 bp), LEE3 (−109 to −259 bp) and stx2AB (−402 to −3 bp) promoter regions.
Four Pho box consensus sequences were found in the promoter of the stx2AB operon (Figures 4 and 5A). Using PhoBCA, EMSA experiments demonstrated that PhoB was able to bind the stx2AB promoter region (Figure 5B). Indeed, the probe designed from the stx2 promoter region shifted with a dose-dependent addition of PhoBCA and the addition of unlabelled control probe competed well with the 6-FAM-labelled probe. Although a potential Pho box was identified in the intergenic region between cI and cro that overlaps the PR promoter, no shift was observed by EMSA (data not shown). Thus, the increased expression of stx2 could be a direct effect of PhoB in Pi-limited medium. This would suggest that increased transcription of BP933W genes could result from direct and indirect effects of PhoB, and could also be due to the PhoB-independent effect of Pi-limited conditions.
Constitutive Activation of the Pho Regulon Decreased LEE Gene Expression and Reduced Adherence of strain EDL933 to HeLa Cells
To investigate the influence of the Pho regulon and PhoB on adherence, we evaluated the attachment of Δpst and ΔphoB mutants to HeLa cells in DMEM cell culture medium, which is a Pi-rich medium. The Δpst mutant was used because the inactivation of the Pst system results in constitutive expression of the Pho regulon. Thereby, the Δpst mutant should mimic the behavior of the wild-type strain grown in Pi-limited conditions. Accordingly, the EDL933 Δpst mutant grown in Pi-rich medium demonstrated strong Pho regulon activation as indicated by PhoA activity (350 M.U.) (data not shown). However, expression of LEE genes in the Δpst mutant was decreased, which was in contrast to the wild-type strain in response to Pi starvation, a condition that activates the Pho regulon (Figure 2B). Furthermore, secretion of the EspB effector protein was also reduced in the Δpst mutant confirming the repression of LEE genes in this mutant (Figures 2B and 2C).
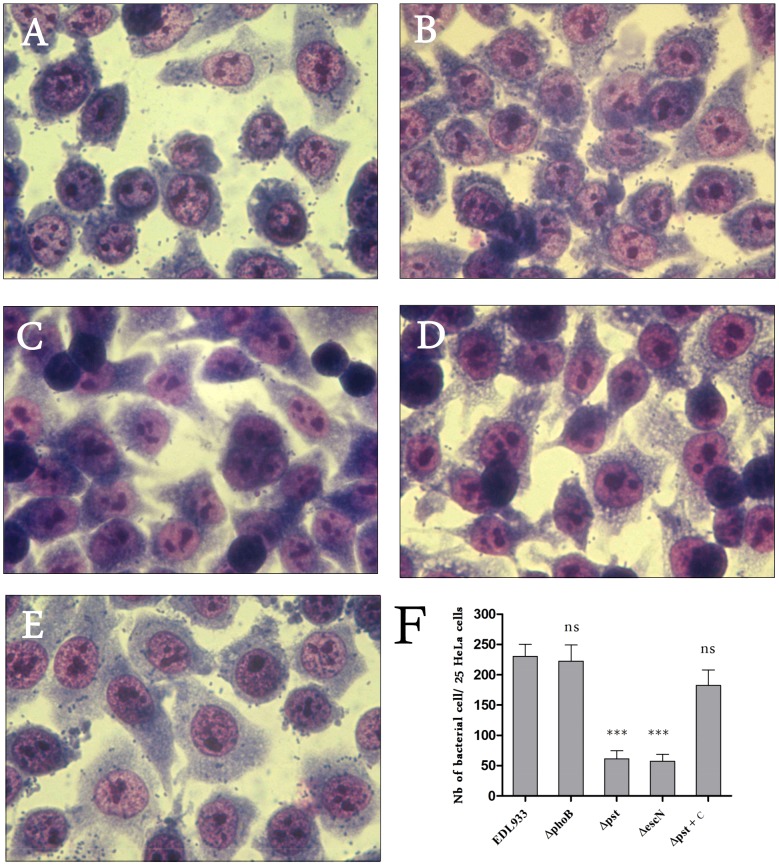
While the adhesion phenotype of the ΔphoB mutant to HeLa cells was similar to that of the wild-type strain (≈ 230 bacteria/25 HeLa cells) (Figure 6), the adhesion of the Δpst mutant was reduced to a level similar to a ΔescN mutant, which lacks a functional T3SS (≈60 bacteria/25 HeLa cells) (Figure 6). Complementation of the Δpst mutant restored the adhesion phenotype of the wild-type parent strain. With regards to LEE gene expression, our results indicate that a Δpst mutant, in which the Pho regulon was highly and unduly activated (phoB/tus transcript ratio ≈ 127), did not reflect the behaviour of the wild-type strain responding to low Pi in which the Pho regulon was moderately activated (phoB/tus transcript ratio ≈ 10.5). Nonetheless, LEE gene expression and EHEC adherence properties are importantly affected in a Δpst mutant, indicating that alteration of the Pho regulon modulates the transcription levels of genes belonging to this pathogenicity island.
Figure 6. Regulation of adhesion of EDL933 to human epithelial cells by the Pho regulon.
HeLa cells were infected with EDL933 and indicated mutants. After 6-type and ΔphoB strains (A and B). The adhesion decreased in the Δpst mutant (P <0.001) to levels similar to that of the ΔescN mutant which lacks functional TTSS (C and D). The attachment phenotype was restored in the trans complemented Δpst mutant (E). The number of adherent bacteria per HeLa cell was determined from 25 cells (F). Magnification 64 ×.
Discussion
In response to Pi limitation, EHEC uses the Pho regulon, a specific regulatory network for phosphate acquisition. The two-component signal transduction system of PhoB-R plays a crucial role in inducing Pho regulon genes to adapt to Pi starvation. Herein, we show that during its adaptation to Pi limiting-conditions, EHEC EDL933 modulates gene expression associated with the acquisition and metabolism of Pi and with mechanisms of general stress responses. In addition, concomitant changes in expression of virulence genes occur. This is also observed in other pathogenic E. coli including C. rodentium that causes EHEC-like intestinal A/E lesions [11]. Changes in global gene expression underlying the overall physiological state of the cells in Pi limitation are also shared with other conditions of nutrient depletion. Some gene subsets responded particularly to Pi limitation stress. The Pho regulon has been shown to contribute to the virulence of atypical enteropathogenic E. coli (EPEC) and C. rodentium through the response regulator PhoB [11], [44].
During growth under Pi limitation, the absence of PhoB affected the expression of genes involved in Pi metabolism and genes involved in stress response and virulence. This indicates that PhoB plays an important role in the survival of EHEC under stressful Pi limiting conditions. Additionally, we show that PhoB is involved in EHEC virulence by regulating the expression of important virulence genes of the LEE and Stx2. Thus, we identified a novel role for PhoB as a transcriptional regulator of importance for both, adaptation to Pi availability and regulation of key virulence systems of EHEC.
Using transcriptional analysis and ELISA assays we showed that Pi-limiting conditions activate the expression and production of virulence genes encoding the LEE T3SS and some effectors and the Stx2 Shiga toxin. By regulating LEE and Stx2 expression, EHEC is able to control expression of major virulence genes under Pi limiting-conditions. The virulence modulation is partly due to PhoB, or PhoB-regulated gene(s), because a ΔphoB mutation affected the expression of some virulence genes in Pi-limiting conditions.
Microarray and qRT-PCR analysis demonstrated for the first time that low Pi-induced signals activated LEE gene expression. Both, low Pi and PhoB increased protein secretion by T3SS. PhoB was required to significantly increase the transcription of LEE2 and LEE3 while the transcription of LEE1 required a low Pi signal rather than PhoB. However, PhoB interacts directly with the promoters of LEE1 and LEE2 operons containing Pho boxes.
The regulation of LEE expression is complex and involves many positive and negative regulators [25]. Most of these factors influence LEE expression through direct or indirect control of ler expression. However, a variety of extra-transcriptional mechanisms have also been described that regulate LEE expression [26]. Our results suggest that PhoB could act on LEE gene expression through its binding to the ler/LEE1 promoter, which in return would activate the whole LEE. In addition, PhoB is involved in expression of LEE2 by direct interaction with its promoter region. However, it also possible that LEE regulators other than PhoB may also be involved in the fine tuning of expression of LEE genes. Indeed, some positive regulators of LEE such as PchA, IHF and the LEE-negative regulator GadE were found to be upregulated in Pi-limited conditions. In addition, the silencer HN-S was repressed in the ΔphoB mutant.
In contrast, the EHEC Δpst mutant showed a reduced expression of LEE and reduced adherence. This is also observed in C. rodentium that also harbors the LEE locus, where the expression of some T3SS genes and the ability to adhere and colonize were reduced in the Δpst mutant [44]. In the Δpst mutant, the Pho regulon is constitutively and more strongly activated than in Pi-limiting condition. Indeed, strong activation of the phoB gene was found in the Δpst background compared to in the wild-type strain in low Pi. This may reflect the super-shift in EMSA LEE1 and such high levels of phospho-PhoB may actually repress the expression of LEE genes. We have previously shown that the level of activation of the Pho regulon influenced the degree of attenuation of APEC [16]. Moreover, it has been shown recently that different Pi conditions have conflicting requirements of PhoB expression levels for optimal cell fitness [68], [69]. Thus, our results suggest that the level of Pho regulon activation influences expression of LEE. In low Pi conditions, LEE is activated, while in the Δpst mutant, LEE is repressed.
In the Sakai strain, Yoshida et al. [70], have demonstrated that the gene cluster esc0540–0544 is positively regulated by PhoB. This gene cluster is homologous to the siiCA-DA operon of Escherichia fergusonii, which encodes a putative RTX toxin and its cognate type I secretion system (T1SS) [70]. These findings taken together with our results suggest that PhoB acts as a virulence gene regulator in EHEC. Moreover, maintenance of proper regulation is critical, since both underexpression and overexpression can affect its virulence functions.
Signals from the host may arise within the intestine, such as changes in metabolite concentration, which allow bacteria to monitor infection and alter their behavior. Pieper et al. [40], have recently shown the activation of the Pho regulon of EHEC in the intestinal environment of gnotobiotic piglets. The signal that leads to induction of the Pho regulon is likely to be Pi limitation. However, we cannot be certain as PhoB can be regulated by a number of signals in addition to Pi concentration [3], [71]–[73].
Many genes of the lysogenic λ phage BP933W were upregulated in response to Pi-limiting conditions including stx2 while the CI repressor gene was inhibited. The repressor system of BP933W maintains the prophage in a quiescent state, [74]. Phage induction is a complex process dependent on bacterial and phage factors. Prophage induction, which leads to lytic growth, results from removal of repression. Ultimately the repressor controls Stx production and/or release. Stx2 production was increased in Pi-limited medium. Factors that regulate the switch between lysogeny and lytic growth, e.g., repressor, operator sites, and associated phage promoters, play important roles in regulating the production and/or release of Stx [64]. In Pi-limited conditions, PhoB indirectly inhibits the transcription of the repressor gene cI, which in return releases the constitutive transcription of cro that should induce a lytic phage cycle, thereby increasing stx2 transcription. Prophage induction ultimately results in Q-modified transcription initiating at PR′ that transcends the tR′ terminator, leading to expression of downstream genes that include stx2 and those encoding lysis functions. Another scenario could be direct PhoB binding onto sequences upstream of the Stx2 promoter that activates stx2 transcription.
Induction of the SOS response that commonly occurs through damage to the bacterial cell's DNA can lead to significantly higher levels of prophage induction. The SOS response refers to the production of a large number of enzymes following damage to DNA [75]. These enzymes include the activated form of RecA protein that facilitates auto-cleavage of many phage repressors and the bacterial LexA protein, which itself controls expression of the SOS response. In Pi-limited conditions, it appears that phage induction is RecA- and LexA-independent. RecA-independent induction also occurs in phages infecting E. coli [76]–[78]. In phage λ, induction does not always imply repressor cleavage by RecA. In some cases, the mechanism involves RcsA, a regulator of colanic acid synthesis, and DsrA [76], [78], a small regulatory RNA that, among other functions, prevents the degradation of RcsA. Imamovic and Muniesa [79] showed that the Mg++ chelator EDTA affects the membrane structure of bacteria and triggers λ phage by RecA-independent mechanisms.
All together, these findings reveal that EHEC has evolved a sophisticated response to Pi availability involving multiple biochemical strategies that are likely critical to its ability to respond to changes in environmental Pi levels and also coordination of its virulence gene response by inducing LEE and stx2 genes and controlling BP933W expression.
Supporting Information
A. Growth (CFU/mL) of EDL933 the WT strain and its isogenic mutants ΔphoB in MOPS medium supplemented with 1.32 mM (Pi+) or 1.0 μM (Pi-) KH2PO4 for 16 h at 37°C. B. PhoA activity monitoring during 6 h cultivation of EDL933 in Pi+ or ΔphoB in Pi- leads to abolition of the Pho regulon while it is activated in WT grown in Pi-. Arrows indicate the 2 comparisons points chosen for the rest of the experiments in this study.
(DOCX)
Microarray results were validated by triplicate qRT-PCR on 13 representative genes. Each reaction was normalized to tus gene and the variation rate was calculated using 2-ΔΔCt method.
(DOCX)
Primers used in this study.
(DOCX)
Functional classification of upregulated genes of microarray data comparing the wild-type strain grown low Pi to in high Pi (Pi-dependent) and comparing the wild-type strain to Δ phoB mutant both grown low Pi (PhoB-dependent).
(DOCX)
Functional classification of downregulated genes of microarray data comparing the wild-type strain grown low Pi to in high Pi (Pi-dependent) and comparing the wild-type strain to Δ phoB mutant both grown low Pi (PhoB-dependent).
(DOCX)
Pho Box matrix based on 12 Pho-Boxes sequences from the known Pho genes in EDL933 identified by Yuan et al . 2006.
(DOCX)
Acknowledgments
We thank Philippe Garneau for excellent technical assistance in microarray experiments. We also thank Rebecca Guy for advice and for reading this manuscript and Judith Kashul for editing this manuscript.
Funding Statement
This work was supported by Discovery grants (250129-07) to C.M.D. and (25120-13) to J.H. from the Natural Sciences and Engineering Research and the strategic grant (RS-170946) Centre de Recherche en Infectiologie Porcine et Aviaire from Fonds de Recherche du Québec Nature et technologies. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Parkinson JS (1993) Signal transduction schemes of bacteria. Cell 73: 857–871. [DOI] [PubMed] [Google Scholar]
- 2. Oshima T, Aiba H, Masuda Y, Kanaya S, Sugiura M, et al. (2002) Transcriptome analysis of all two-component regulatory system mutants of Escherichia coli K-12. Mol Microbiol 46: 281–291. [DOI] [PubMed] [Google Scholar]
- 3.Wanner BL (1996) Phosphorus assimilation and control of the phosphate regulon. In: F. C. Neidhardt RCI, J. L Ingraham, E. C. C Lin, K. B Low, B Magasanik, W. S Reznikoff, M Riley, M Schaechter, and H. E Umbarger. Escherichia coli and Salmonella: Cellular and Molecular Biology. 2 ed. ASM Press, Washington, D.C. pp. 1357–1381.
- 4. Rao NN, Torriani A (1990) Molecular aspects of phosphate transport in Escherichia coli . Mol Microbiol 4: 1083–1090. [DOI] [PubMed] [Google Scholar]
- 5. Chan FY, Torriani A (1996) PstB protein of the phosphate-specific transport system of Escherichia coli is an ATPase. J Bacteriol 178: 3974–3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hsieh YJ, Wanner BL (2010) Global regulation by the seven-component Pi signaling system. Curr Opin Microbiol 13: 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang C, Huang TW, Wen SY, Chang CY, Tsai SF, et al. (2012) Genome-wide PhoB binding and gene expression profiles reveal the hierarchical gene regulatory network of phosphate starvation in Escherichia coli . PloS one 7: e47314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baek JH, Lee SY (2006) Novel gene members in the Pho regulon of Escherichia coli . FEMS Microbiol Lett 264: 104–109. [DOI] [PubMed] [Google Scholar]
- 9. Yoshida Y, Sugiyama S, Oyamada T, Yokoyama K, Makino K (2012) Novel members of the phosphate regulon in Escherichia coli O157:H7 identified using a whole-genome shotgun approach. Gene 502: 27–35. [DOI] [PubMed] [Google Scholar]
- 10. Wanner BL (1994) Molecular genetics of carbon-phosphorus bond cleavage in bacteria. Biodegradation 5: 175–184. [DOI] [PubMed] [Google Scholar]
- 11. Cheng C, Wakefield MJ, Yang J, Tauschek M, Robins-Browne RM (2012) Genome-wide analysis of the Pho regulon in a pstCA mutant of Citrobacter rodentium . PloS one 7: e50682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crepin S, Lamarche MG, Garneau P, Seguin J, Proulx J, et al. (2008) Genome-wide transcriptional response of an avian pathogenic Escherichia coli (APEC) pst mutant. BMC genomics 9: 568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Danhorn T, Hentzer M, Givskov M, Parsek MR, Fuqua C (2004) Phosphorus limitation enhances biofilm formation of the plant pathogen Agrobacterium tumefaciens through the PhoR-PhoB regulatory system. J Bacteriol 186: 4492–4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lamarche MG, Dozois CM, Daigle F, Caza M, Curtiss R 3rd, et al. (2005) Inactivation of the pst system reduces the virulence of an avian pathogenic Escherichia coli O78 strain. Infect Immun 73: 4138–4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zaborina O, Holbrook C, Chen Y, Long J, Zaborin A, et al. (2008) Structure-function aspects of PstS in multi-drug-resistant Pseudomonas aeruginosa . PLoS Pathog 4: e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bertrand N, Houle S, LeBihan G, Poirier E, Dozois CM, et al. (2010) Increased Pho regulon activation correlates with decreased virulence of an avian pathogenic Escherichia coli O78 strain. Infect Immun 78: 5324–5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Crepin S, Chekabab SM, Le Bihan G, Bertrand N, Dozois CM, et al. (2011) The Pho regulon and the pathogenesis of Escherichia coli . Vet Microbiol 153: 82–88. [DOI] [PubMed] [Google Scholar]
- 18. Griffin PM, Tauxe RV (1991) The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev 13: 60–98. [DOI] [PubMed] [Google Scholar]
- 19. Gyles CL (2007) Shiga toxin-producing Escherichia coli: an overview. J Anim Sci 85: E45–62. [DOI] [PubMed] [Google Scholar]
- 20. McDaniel TK, Jarvis KG, Donnenberg MS, Kaper JB (1995) A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad of Sci U S A 92: 1664–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Elliott SJ, Sperandio V, Giron JA, Shin S, Mellies JL, et al. (2000) The locus of enterocyte effacement (LEE)-encoded regulator controls expression of both LEE- and non-LEE-encoded virulence factors in enteropathogenic and enterohemorrhagic Escherichia coli . Infect Immun 68: 6115–6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haack KR, Robinson CL, Miller KJ, Fowlkes JW, Mellies JL (2003) Interaction of Ler at the LEE5 (tir) operon of enteropathogenic Escherichia coli . Infect Immun 71: 384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sperandio V, Mellies JL, Delahay RM, Frankel G, Crawford JA, et al. (2000) Activation of enteropathogenic Escherichia coli (EPEC) LEE2 and LEE3 operons by Ler. Mol Microbiol 38: 781–793. [DOI] [PubMed] [Google Scholar]
- 24. Deng W, Puente JL, Gruenheid S, Li Y, Vallance BA, et al. (2004) Dissecting virulence: systematic and functional analyses of a pathogenicity island. Proc Natl Acad of Sci U S A 101: 3597–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tree JJ, Wolfson EB, Wang D, Roe AJ, Gally DL (2009) Controlling injection: regulation of type III secretion in enterohaemorrhagic Escherichia coli . Trends Microbiol 17: 361–370. [DOI] [PubMed] [Google Scholar]
- 26. Bhatt S, Romeo T, Kalman D (2011) Honing the message: post-transcriptional and post-translational control in attaching and effacing pathogens. Trends Microbiol 19: 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ptashne M (1992) A Genetic Switch: Phage λand Higher Organisms; Publications CPBS, editor. Cambridge, MA.
- 28.Little JW (1995) The SOS regulatory system. In: In Lin E. C. C LAS, editor. Regulation of gene expression in Escherichia coli. R. G. Landis, Georgetown, TX. pp. 453–479.
- 29. Hancock D, Besser T, Lejeune J, Davis M, Rice D (2001) The control of VTEC in the animal reservoir. Int J Food Microbiol 66: 71–78. [DOI] [PubMed] [Google Scholar]
- 30. Chekabab SM, Paquin-Veillette J, Dozois CM, Harel J (2013) The ecological habitat and transmission of Escherichia coli O157:H7. FEMS Microbiol Lett 341: 1–12. [DOI] [PubMed] [Google Scholar]
- 31. Chekabab SM, Daigle F, Charette SJ, Dozois CM, Harel J (2012) Survival of enterohemorrhagic Escherichia coli in the presence of Acanthamoeba castellanii and its dependence on Pho regulon. MicrobiologyOpen 1: 427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carruthers MD, Bellaire BH, Minion FC (2010) Exploring the response of Escherichia coli O157:H7 EDL933 within Acanthamoeba castellanii by genome-wide transcriptional profiling. FEMS Microbiol Lett 312: 15–23. [DOI] [PubMed] [Google Scholar]
- 33. Lamarche MG, Wanner BL, Crepin S, Harel J (2008) The phosphate regulon and bacterial virulence: a regulatory network connecting phosphate homeostasis and pathogenesis. FEMS Microbiol Rev 32: 461–473. [DOI] [PubMed] [Google Scholar]
- 34. Pratt JT, Ismail AM, Camilli A (2010) PhoB regulates both environmental and virulence gene expression in Vibrio cholerae . Mol Microbiol 77: 1595–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magid J, Tiessen H, Condron L (1996) Dynamics of organic phosphorus in soils under natural and agricultural ecosystems. In: In Piccolo A, editor. Humic substances in terrestrial ecosystems. Elsevier, Amsterdam. pp. 429–466.
- 36. Zubkov MV, Mary I, Woodward EM, Warwick PE, Fuchs BM, et al. (2007) Microbial control of phosphate in the nutrient-depleted North Atlantic subtropical gyre. Environ Microbiol 9: 2079–2089. [DOI] [PubMed] [Google Scholar]
- 37. Kayne LH, D'Argenio DZ, Meyer JH, Hu MS, Jamgotchian N, et al. (1993) Analysis of segmental phosphate absorption in intact rats. A compartmental analysis approach. J Clin Invest 91: 915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Walton J, Gray TK (1979) Absorption of inorganic phosphate in the human small intestine. Clinical science 56: 407–412. [DOI] [PubMed] [Google Scholar]
- 39.Lemann J (1993) Intestinal absorption of calcium, magnesium, and phosphorus. In: In Murray J F, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. The American Society for Bone and Mineral Research: Raven Press, New York, N.Y. pp. 46–50.
- 40. Pieper R, Zhang Q, Clark DJ, Parmar PP, Alami H, et al. (2013) Proteomic View of Interactions of Shiga Toxin-Producing Escherichia coli with the Intestinal Environment in Gnotobiotic Piglets. PloS one 8: e66462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Daigle F, Fairbrother JM, Harel J (1995) Identification of a mutation in the pst-phoU operon that reduces pathogenicity of an Escherichia coli strain causing septicemia in pigs. Infect Immun 63: 4924–4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lamarche MG, Harel J (2010) Membrane homeostasis requires intact pst in extraintestinal pathogenic Escherichia coli . Curr Microbiol 60: 356–359. [DOI] [PubMed] [Google Scholar]
- 43. Crepin S, Houle S, Charbonneau ME, Mourez M, Harel J, et al. (2012) Decreased expression of type 1 fimbriae by a pst mutant of uropathogenic Escherichia coli reduces urinary tract infection. Infect Immun 80: 2802–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cheng C, Tennant SM, Azzopardi KI, Bennett-Wood V, Hartland EL, et al. (2009) Contribution of the pst-phoU operon to cell adherence by atypical enteropathogenic Escherichia coli and virulence of Citrobacter rodentium . Infect Immun 77: 1936–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lamarche MG, Kim SH, Crepin S, Mourez M, Bertrand N, et al. (2008) Modulation of hexa-acyl pyrophosphate lipid A population under Escherichia coli phosphate (Pho) regulon activation. J Bacteriol 190: 5256–5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Graveline R (2011) Étude des mécanismes moléculaires influençant la variation de phase des adhésines P, F1651 et CS31A présentes chez des souches d'Escherichia coli pathogènes. Faculty of veterinary medecine: Montreal. 264 p. [Google Scholar]
- 47. Muhldorfer I, Hacker J, Keusch GT, Acheson DW, Tschape H, et al. (1996) Regulation of the Shiga-like toxin II operon in Escherichia coli . Infect Immun 64: 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. de Sablet T, Bertin Y, Vareille M, Girardeau JP, Garrivier A, et al. (2008) Differential expression of stx2 variants in Shiga toxin-producing Escherichia coli belonging to seropathotypes A and C. Microbiology. 154: 176–186. [DOI] [PubMed] [Google Scholar]
- 49. Neidhardt FC, Bloch PL, Smith DF (1974) Culture medium for enterobacteria. J Bacteriol 119: 736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Peterson JD, Umayam LA, Dickinson T, Hickey EK, White O (2001) The Comprehensive Microbial Resource. Nucleic Acids Res 29: 123–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Arribas-Bosacoma R, Kim SK, Ferrer-Orta C, Blanco AG, Pereira PJ, et al. (2007) The X-ray crystal structures of two constitutively active mutants of the Escherichia coli PhoB receiver domain give insights into activation. J Mol Biol 366: 626–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Graveline R, Mourez M, Hancock MA, Martin C, Boisclair S, et al. (2011) Lrp-DNA complex stability determines the level of ON cells in type P fimbriae phase variation. Mol Microbiol 81: 1286–1299. [DOI] [PubMed] [Google Scholar]
- 53. Deng W, Li Y, Hardwidge PR, Frey EA, Pfuetzner RA, et al. (2005) Regulation of type III secretion hierarchy of translocators and effectors in attaching and effacing bacterial pathogens. Infect Immun 73: 2135–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dasanayake D, Richaud M, Cyr N, Caballero-Franco C, Pittroff S, et al. (2011) The N-terminal amphipathic region of the Escherichia coli type III secretion system protein EspD is required for membrane insertion and function. Mol Microbiol 81: 734–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gobert AP, Vareille M, Glasser AL, Hindre T, de Sablet T, et al. (2007) Shiga toxin produced by enterohemorrhagic Escherichia coli inhibits PI3K/NF-kappaB signaling pathway in globotriaosylceramide-3-negative human intestinal epithelial cells. J Immunol 178: 8168–8174. [DOI] [PubMed] [Google Scholar]
- 56. Deng W, Puente JL, Gruenheid S, Li Y, Vallance BA, et al. (2004) Dissecting virulence: systematic and functional analyses of a pathogenicity island. Proc Natl Acad Sci U S A 101: 3597–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hengge-Aronis R (2002) Signal transduction and regulatory mechanisms involved in control of the sigma(S) (RpoS) subunit of RNA polymerase. Microbiol Mol Biol Rev 66: 373-395, table of contents. [DOI] [PMC free article] [PubMed]
- 58. Loewen PC, Hu B, Strutinsky J, Sparling R (1998) Regulation in the rpoS regulon of Escherichia coli . Can J Microbiol 44: 707–717. [DOI] [PubMed] [Google Scholar]
- 59. Bougdour A, Wickner S, Gottesman S (2006) Modulating RssB activity: IraP, a novel regulator of sigma(S) stability in Escherichia coli . Genes & development 20: 884–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Altuvia S, Weinstein-Fischer D, Zhang A, Postow L, Storz G (1997) A small, stable RNA induced by oxidative stress: role as a pleiotropic regulator and antimutator. Cell 90: 43–53. [DOI] [PubMed] [Google Scholar]
- 61. Mandel MJ, Silhavy TJ (2005) Starvation for different nutrients in Escherichia coli results in differential modulation of RpoS levels and stability. J Bacteriol 187: 434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Herold S, Karch H, Schmidt H (2004) Shiga toxin-encoding bacteriophages—genomes in motion. Int J Med Microbiol 294: 115–121. [DOI] [PubMed] [Google Scholar]
- 63. Unkmeir A, Schmidt H (2000) Structural analysis of phage-borne stx genes and their flanking sequences in shiga toxin-producing Escherichia coli and Shigella dysenteriae type 1 strains. Infect Immun 68: 4856–4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tyler JS, Mills MJ, Friedman DI (2004) The operator and early promoter region of the Shiga toxin type 2-encoding bacteriophage 933W and control of toxin expression. J Bacteriol 186: 7670–7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Blanco AG, Sola M, Gomis-Ruth FX, Coll M (2002) Tandem DNA recognition by PhoB, a two-component signal transduction transcriptional activator. Structure 10: 701–713. [DOI] [PubMed] [Google Scholar]
- 66. Kendall MM, Rasko DA, Sperandio V (2010) The LysR-type regulator QseA regulates both characterized and putative virulence genes in enterohaemorrhagic Escherichia coli O157:H7. Mol Microbiol 76: 1306–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mellies JL, Barron AM, Carmona AM (2007) Enteropathogenic and enterohemorrhagic Escherichia coli virulence gene regulation. Infect Immun 75: 4199–4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gao R, Stock AM (2013) Evolutionary tuning of protein expression levels of a positively autoregulated two-component system. PLoS Genet 9: e1003927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gao R, Stock AM (2013) Probing kinase and phosphatase activities of two-component systems in vivo with concentration-dependent phosphorylation profiling. Proc Natl Acad Sci U S A 110: 672–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yoshida Y, Sugiyama S, Oyamada T, Yokoyama K, Makino K (2010) Identification and characterization of novel phosphate regulon genes, ecs0540-ecs0544, in Escherichia coli O157:H7. Mol Genet Genomics 284: 197–205. [DOI] [PubMed] [Google Scholar]
- 71. Fisher SL, Jiang W, Wanner BL, Walsh CT (1995) Cross-talk between the histidine protein kinase VanS and the response regulator PhoB. Characterization and identification of a VanS domain that inhibits activation of PhoB. J Biol Chem 270: 23143–23149. [DOI] [PubMed] [Google Scholar]
- 72. Suziedeliene E, Suziedelis K, Garbenciute V, Normark S (1999) The acid-inducible asr gene in Escherichia coli: transcriptional control by the phoBR operon. J Bacteriol 181: 2084–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wanner BL, Wilmes-Riesenberg MR (1992) Involvement of phosphotransacetylase, acetate kinase, and acetyl phosphate synthesis in control of the phosphate regulon in Escherichia coli . J Bacteriol 174: 2124–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Waldor MK, Friedman DI (2005) Phage regulatory circuits and virulence gene expression. Curr Opin Microbiol 8: 459–465. [DOI] [PubMed] [Google Scholar]
- 75. Wagner PL, Neely MN, Zhang X, Acheson DW, Waldor MK, et al. (2001) Role for a phage promoter in Shiga toxin 2 expression from a pathogenic Escherichia coli strain. J Bacteriol 183: 2081–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rozanov DV, D'Ari R, Sineoky SP (1998) RecA-independent pathways of lambdoid prophage induction in Escherichia coli . J Bacteriol 180: 6306–6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Shkilnyj P, Koudelka GB (2007) Effect of salt shock on stability of lambdaimm434 lysogens. J Bacteriol 189: 3115–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ghosh D, Roy K, Williamson KE, Srinivasiah S, Wommack KE, et al. (2009) Acyl-homoserine lactones can induce virus production in lysogenic bacteria: an alternative paradigm for prophage induction. Appl Environ Microbiol 75: 7142–7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Imamovic L, Muniesa M (2012) Characterizing RecA-independent induction of Shiga toxin2-encoding phages by EDTA treatment. PloS one 7: e32393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Strockbine NA, Marques LR, Newland JW, Smith HW, Holmes RK, et al. (1986) Two toxin-converting phages from Escherichia coli O157:H7 strain 933 encode antigenically distinct toxins with similar biologic activities. Infection and immunity 53: 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kang HY, Srinivasan J, Curtiss R 3rd (2002) Immune responses to recombinant pneumococcal PspA antigen delivered by live attenuated Salmonella enterica serovar typhimurium vaccine. Infect Immun 70: 1739–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jans DA, Fimmel AL, Langman L, James LB, Downie JA, et al. (1983) Mutations in the uncE gene affecting assembly of the c-subunit of the adenosine triphosphatase of Escherichia coli . Biochem J 211: 717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hashimoto-Gotoh T, Franklin FC, Nordheim A, Timmis KN (1981) Specific-purpose plasmid cloning vectors. I. Low copy number, temperature-sensitive, mobilization-defective pSC101-derived containment vectors. Gene 16: 227–235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Growth (CFU/mL) of EDL933 the WT strain and its isogenic mutants ΔphoB in MOPS medium supplemented with 1.32 mM (Pi+) or 1.0 μM (Pi-) KH2PO4 for 16 h at 37°C. B. PhoA activity monitoring during 6 h cultivation of EDL933 in Pi+ or ΔphoB in Pi- leads to abolition of the Pho regulon while it is activated in WT grown in Pi-. Arrows indicate the 2 comparisons points chosen for the rest of the experiments in this study.
(DOCX)
Microarray results were validated by triplicate qRT-PCR on 13 representative genes. Each reaction was normalized to tus gene and the variation rate was calculated using 2-ΔΔCt method.
(DOCX)
Primers used in this study.
(DOCX)
Functional classification of upregulated genes of microarray data comparing the wild-type strain grown low Pi to in high Pi (Pi-dependent) and comparing the wild-type strain to Δ phoB mutant both grown low Pi (PhoB-dependent).
(DOCX)
Functional classification of downregulated genes of microarray data comparing the wild-type strain grown low Pi to in high Pi (Pi-dependent) and comparing the wild-type strain to Δ phoB mutant both grown low Pi (PhoB-dependent).
(DOCX)
Pho Box matrix based on 12 Pho-Boxes sequences from the known Pho genes in EDL933 identified by Yuan et al . 2006.
(DOCX)