Abstract
Protein phosphatase 2A (PP2A), a major Serine/Threonine protein phosphatase, consists of three subunits; a highly conserved structural subunit A, a catalytic subunit C, and a highly variable regulatory subunit B which determines the substrate specificity. Although the functional mechanism of PP2A in signaling transduction in Arabidopsis is known, their physiological roles in wheat remain to be characterized. In this study, we identified a novel regulatory subunit B, TaPP2AbB"-α, in wheat (Triticum aestivum L.). Subcellular localization indicated that TaPP2AbB"-α is located in the cell membrane, cytoplasm and nucleus. It interacts with both TaPP2Aa and TaPP2Ac. Expression pattern analyses revealed that TaPP2AbB"-α is strongly expressed in roots, and responds to NaCl, polyethylene glycol (PEG), cold and abscisic acid (ABA) stresses at the transcription level. Transgenic Arabidopsis plants overexpressing TaPP2AbB"-α developed more lateral roots, especially when treated with mannitol or NaCl. These results suggest that TaPP2AbB"-α, in conjunction with the other two PP2A subunits, is involved in multi-stress response, and positively regulates lateral root development under osmotic stress.
Introduction
Environmental stresses, such as water deficit and high salinity, are major challenges for plant growth and development. Plants adapt to severe environments by regulating morphogenesis and physiological reactions. Increased lateral root formation is one strategy for plants to survive in unfavorable conditions [1], [2]. More lateral roots permit plants to absorb nutrients and water more easily in order to overcome stress damage, especially under osmotic stress [3]–[5]. Many genes are involved in plant stress response [6]; among them, protein kinases and protein phosphatases are basic components of stress signal transduction [7]. For example, ABA-activated SNF1-related protein kinases (SnRK) phosphorylate downstream substrates to enhance drought tolerance in Arabidopsis, whereas, SnRKs are inhibited by protein phosphatases in the absence of ABA [8]–[10]. Protein kinases have been well described in wheat, but the roles of protein phosphatases (PPs) have not been investigated as widely [11], [12].
PP2A is one of the most important Serine/Threonine (Ser/Thr) protein phosphatases that regulate many cellular processes, such as transcription, translation, the cell cycle, metabolism, and apoptosis [13], [14]. PP2A positively regulates salt stress response through modulating polar auxin transport (PAT). It dephosphorylates auxin efflux carrier PIN proteins, which can be regulated by Ser/Thr kinase PINOID, and influences the distribution of auxin [15]–[17].
As a heterotrimeric phosphase, PP2A consists of a scaffolding subunit A (PP2Aa), a regulatory subunit B (PP2Ab), and a catalytic subunit C (PP2Ac). In Arabidopsis, PP2Aa has three isoforms, PP2Aa1, PP2Aa2 and PP2Aa3, which are composed of tandem HEAT repeats that form a hook-like structure for binding PP2Ac and PP2Ab [18]. Auxin transportation is affected in the mutant rcn1 (root curl in naphthylphthalamic acid1), which exhibits a root coiling phenotype and belongs to the PP2Aa group [19]. Two single mutants (pp2aa2 and pp2aa3) and one double mutant (pp2aa2 pp2aa3) in Arabidopsis develop normally, but plants exhibit serious developmental aberrations when PP2Aa1 is absent [15]. PP2Ac has five isoforms grouped into two subfamilies based on their conserved sequences. Subfamily I (PP2Ac1, PP2Ac2, and PP2Ac5) is involved in stress response, and the ABA and brassinosteroid (BR) signaling pathways. Subfamily II (PP2Ac3 and PP2Ac4) participates in dephosphorylation of PIN proteins in the auxin signaling pathway [20]. Methylated PP2Ac dephosphorylates receptor BRI1 to attenuate BR signaling [21]. Regulatory subunit B of PP2A (PP2Ab) determines multiple roles of PP2A in different signaling pathways.
Most researches on PP2Abs have been performed on animals where four gene families encode PP2Abs, including B (B55, PR55, PPP2R2), B′ (B56, PR61, PPP2R5), B″ (PR72, PPP2R3), and B′″(PR93/PR110) [22], [23]. Down-regulation of PR55/Bδ resulted in a diminished eye and enlarged trunk in gold fish [24]. Knockdown of PR61/B′δ in mice led to decreased glycogen synthase kinase 3β (GSK3β) activity, increased cyclin-dependent kinase 5 (CDK5) activity in the brain, and reduced ability to perform beam walking [25]. PP2AbB″ containing two calcium binding sites formed by EF-hands is involved in the adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) signaling pathway through which it regulates the activity of PP2A in pig [26]. In Arabidopsis, at least 17 PP2Ab have been reported, including two PP2AbB, nine PP2AbB′, five PP2AbB″ and TAP46 [27]. Only a few PP2Ab are well studied. The expression of TAP46 is induced by low temperature suggesting a role in cold stress signaling in Arabidopsis [28]. TON2, a member of the Arabidopsis PP2AbB″ subfamily, has a role in determining the geometry of microtubule nucleation and organization of the cortex [29]. Some members of B' regulatory subunits of PP2A dephosphorylate BZR1 thus promoting plant growth [30]. Although PP2Abs determine substrate specificity and subcellular location of PP2A [31], detailed studies in Arabidopsis are restricted to TAP46 and TON2. PP2Ab in wheat has not been well characterized. TaB-β (PP2AbB) was isolated and shown to be involved in a stress signaling pathway [32]. Some sequence information on the regulatory B″-α subunit was characterized in Oryza sativa, Setaria italica and Brachypodium distachyon, but the functions of these genes are largely unknown.
The present research focused on the function of a novel PP2A regulatory subunit B″ gene TaPP2AbB"-α in wheat. We isolated TaPP2AbB"-α based on information from the wheat D genome sequence and the candidate sequence from Oryza sativa. We found that TaPP2AbB"-α interacted with PP2Aa and PP2Ac, which were isolated from wheat. Expression of TaPP2AbB"-α was up-regulated by multiple stresses. Arabidopsis lines overexpressing TaPP2AbB"-α developed more lateral roots and grew better than the wild type under the medium osmotic stress, presenting a possibility of using TaPP2AbB"-α in transgenic breeding to improve abiotic stress tolerance in crop plants.
Materials and Methods
Plant materials, growth conditions and stress treatments
Drought-tolerant common wheat (Triticum aestivum L.) cultivar Hanxuan 10 was used as the plant material. Ten-day-old seedlings (two-leaf stage) grown in 1/2 MS liquid culture at 23°C in a 12 h light/12 h darkness photoperiod were subjected to the following four treatments: 16.1% PEG (−0.5 MPa), 250 mM NaCl, 4°C, and 50 µM ABA. The leaf (seedlings sprayed with ABA) and root samples (treated with NaCl, PEG and 4°C) were harvested at 0, 0.5, 1, 1.5, 2, 3, 6, 12, 24, 48 and 72 h after each treatment, and tissues were stored at −70°C for RNA isolation and transcription level detection. Samples harvested at 0 h were used as controls for the corresponding treatments. Total RNAs was extracted using Trizol reagent.
Cloning and sequencing of full-length TaPP2AbB"-α cDNA
The putative full-length cDNA of TaPP2AbB"-α was obtained using the candidate sequence from Oryza sativa (NM_001071385.1) and sequence information for a dehydration-inducible cDNA library of wheat D genome-bearing Aegilops tauschii [33]–[35]. The full-length cDNA of TaPP2AbB"-α was amplified using primers: TaPP2AbB"-α F: 5’ TTGACGGCATGGAGGTG 3’, TaPP2AbB"-α R: 5’ GTAGCACTCACATGATTCAGAAT 3’. The cDNA of Hanxuan 10 as templates, and PCR products were ligated with pEASY-blunt simple vectors (TransGen; Beijing, China). The ligated products were transformed into E-coli DH5α, and then positive recombinants were sequenced with an ABI 3730XL 96-capillary DNA analyzer (Lifetech; USA).
A Neighbor–Joining tree was constructed by MEGA5.05 software [36]. AtPP2AbB"-β (NP_851089.1), GmPP2AbB"-α like (XP_003556377.1), VvPP2AbB"-α (XP_002272394.1), SiPP2AbB"-α like (XP_004982873.1), OsPP2AbB"-α (NP_001064850.1), MtPP2AbB"-γ (XP_003609619.1), BdPP2AbB"-γ like (XP_003568929.1), GmPP2AbB"-γ like (XP_003529585.1), Xenopus laevis PR74 (AAK98641), X. laevis PR130 (NP_001082623.1), Mus musculus PP2AbB" isoform 1 (NP_001154834.1), Homo sapiens PP2AbB" isoform 1 (NP_002709.2), Macaca mulatta PP2AbB"-α (NP_001244537.1) and TaPP2AbB"-α were used to construct the tree.
Database (nucleotide and protein blasts) searches were performed through the NCBI website. Sequence alignments and comparisons were implemented by the MegAlign program in DNAStar and DNAman. Protein predictions were performed using Softberry (http://www.softberry.com).
Subcellular localization of TaPP2AbB"-α protein
The ORF of TaPP2AbB"-α without the termination codon was amplified using forward primer: 5’ CCCAAGCTTATGGAGGTGGAAGCGGC 3’ (Hind III site in bold italics), and reverse primer: 5’ ACGCGTCGACGAATGGAGCTTCAAGTGACTCG 3’ (Sal I site in bold italics), then inserted into the pJIT163-GFP vector using T4 ligase.
The 10-day-old wheat seedling was used for protoplast isolation. The leaf strips of 0.5–1 mm length were cut from the middle part of leaves, and digested in cellulose R10 and macerozyme R10 enzyme solution buffer for 3 h to obtain wheat mesophyll protoplast cell. The fusion construct (TaPP2AbB"-α-GFP) and control (GFP) were transformed into wheat mesophyll protoplast cells by the PEG-mediated method [37]. After incubation in W5 nutrient solution at 23°C for 16 h, the protoplast cells were examined using a laser scanning confocal microscope (Leica TCS-NT; Germany). GFP auto-fluorescence was collected in the range of 500–570 nm wavelength. For chloroplast auto-fluorescence, the wavelength range measured was 630–700 nm. Under which wavelength, the chloroplasts were shown in red color.
Interactions of TaPP2AbB"-α with TaPP2Aa and TaPP2Ac
Yeast two-hybrid and firefly luciferase complementation imaging assays were carried out to identify physical interaction of TaPP2AbB"-α with both TaPP2Aa and TaPP2Ac.
The TaPP2AbB"-α coding region was amplified using primer pair: Forward: 5’ CACCATGGCCGATGGATGGAGGTGGAAGCGGC 3’ (Nco I site in bold italics) Reverse: 5’ ACGCGTCGACTCAGAATGGAGCTTCAAGTGAC 3’ (Sal I site in bold italics) for the pGBKT7 vector construct. Full length cDNAs of TaPP2Aa and TaPP2Ac were separately sub-cloned into the pGADT7 vector. Two pairs of vectors, TaPP2AbB"-α-pGBKT7/TaPP2Aa-pGADT7, and TaPP2AbB"-α-pGBKT7/TaPP2Ac-pGADT7 were transformed into yeast strain AH109. Transformations were selected on SD/–Trp/–Leu screening medium with X-β-gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside). The interaction was tested on SD/–Trp/–Leu/–His plates containing 5 mM 3-AT (3-amino-1,2,4-triazole). Yeast cells on SD/–Trp/–Leu plates were used as controls for co-transformation.
For the firefly luciferase complementation imaging assay, which is an effective method to detect protein-protein interaction [38], full length cDNAs of TaPP2AbB"-α and TaPP2Aa were separately PCR-amplified and ligated into the pCAMBIA-cLuc and pCAMBIA-nLuc vectors. The constructed vectors were transformed into Agrobacterium tumefaciens strain GV3101, and further transformed into Nicotiana benthamiana leaves. After 3 days, the leaves were sprayed with 1 mM luciferin, and kept in darkness for 10 min. A camera fitted with a low-light cooled charge-coupled device was employed to capture the LUC (luciferase) image (Nikon-L936, Andor Tech; UK). The exposure time for LUC images was 15 min. ABA-insensitive 1 (ABI1) and its substrate Open Stomata 1 (OST1), from Arabidopsis were used as positive controls [39].
Expression pattern of TaPP2AbB"-α in wheat
Quantitative real-time PCR (qRT-PCR) was performed to determine the expression pattern of TaPP2AbB"-α. Actin transcript was used as an internal control to quantify the relative transcript level. The qRT-PCR was performed in triplicate with an ABI PRISMH 7900 system (Applied Biosystems, USA) using the SYBR Green PCR master mix kit (Takara, Japan). The specific primers (F: 5’ CTGGCTCTCCCCGTGTTATG 3’, R: 5’ AGAGGAGATCCCAAGGATGATG 3’) were designed according to the full-length of the TaPP2AbB"-α cDNA sequence. The relative level of gene expression was detected using the 2−△△CT method [40]. △△C T = (C T, Target - C T, Actin)Time x - (C T, Target - C T, Actin)Time 0. The C T (cycle threshold) values for both the target and internal control genes were the means of triplicate independent qRT-PCRs. Time x represented the treatment time point (0.5, 1, 1.5, 2, 3, 6, 12, 24, 48 and 72 h), and time 0 h represented the time immediately prior go treatment. Expression of TaPP2AbB"-α in seedling leaves was regarded as standard due to its lowest expression level in that tissue. The corresponding formula was modified to △△C T = (C T, Target - C T, Actin)leaf - (C T, Target - C T, Actin)root, to identify the expression pattern of TaPP2AbB"-α in leaf and root tissues from wheat seedlings.
Generation of transgenic plants in Arabidopsis
TaPP2AbB"-α cDNA containing the entire ORF was inserted into pCHF3 under control of the CaMV 35S promoter and NOS terminator, using primers 5’ GGGGTACCATGGAGGTGGAAGCGGC 3’ (Kpn I site in bold italics) and 5’ CGGGATCCTCAGAATGGAGCTTCAAGTGAC 3’ (BamH I site in bold italics). The p35S-TaPP2AbB"-α-NOS construct and the p35S-NOS vector were separately transformed into Agrobacterium strain GV3101 and then infected into wild type Arabidopsis plants by floral infiltration. Positive T3 generation transgenic Arabidopsis plants overexpressing TaPP2AbB"-α were screened by kanamycin plates and PCR amplification.
Morphological characterization of transgenic Arabidopsis
Arabidopsis seeds were sown on MS medium solidified with 0.8% agar. Seeds were vernalized for 36 h at 4°C before being transferred to a growth chamber. To examine root morphology, the 8-day-old seedlings vertically grown on normal MS solid medium were replanted to MS medium plates containing NaCl (50, 100 and 150 mM), and mannitol (100, 200 and 300 mM). Root system morphologies were examined 3 days after replanting. The root length of Arabidopsis was measured with a ruler. The number of lateral root was captured with EXPSON EXTRESSION 10000XL (EXPSON, Japan) and counted with winRHIZO software.
Results
Isolation and sequence analysis of TaPP2AbB"-α
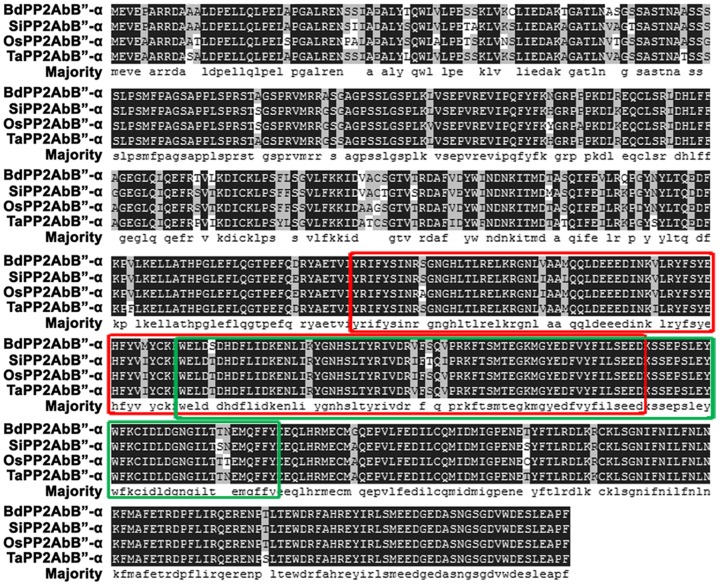
TaPP2AbB"-α is 1,648 bp in length, consisting of an 8 bp 5’ untranslated region, a 1,626 bp ORF, and a 14 bp 3’ untranslated region. The ORF encodes 541 amino acid residues (AAR) with a calculated molecular mass of 62 kDa and predicted pI of 4.92. TaPP2AbB"-α has two domain structures, characterized by the EF-Hand-5 (330–422 AAR) and FRQ1 (272-391 AAR) domains that belong to the EF-hand superfamily. The deduced amino acid sequence shows high homology with counterpart PP2AbB"-α family members from other plant species, viz. Oryza sativa (NP_001064850.1), Setaria italica (XP_004982873.1) and Brachypodium distachyon (XP_003574057.1) (Figure 1). We also performed protein blast in the website of TAIR (http://www.arabidopsis.org/Blast/index.jsp). Several amino acid sequences were identified, sharing about 70% similarity with TaPP2AbB"-α. But, the classification of these amino acid sequences from Arabidopsis was unclear.
Figure 1. Alignment of the predicted amino acid sequences of PP2AbB"-α from different plant species.
Alignment was performed according to DNAman. Common identical amino acid residues are shown in black background. The FRQ1 domain is marked in red rectangles, and the EF-hand-5 domain is marked in green rectangles. Abbreviations on the left side of each sequence: Bd, Brachypodium distachyon; Si, Setaria italica; Os, Oryza sativa; Ta, Triticum aestivum.
Phylogenetic analysis
A phylogenetic tree was constructed with the putative amino acid sequences of TaPP2AbB"-α and members of the PP2AbB" family in other species. PP2AbB"-α from animals and plants were in two distinct clades (Figure 2). TaPP2AbB"-α and its counterparts from plants were placed into Group 1; PP2AbB"-γ in Group 2 were in one clade, and PP2AbB"-α from animals were in another (Group 3).
Figure 2. Phylogenetic tree of TaPP2AbB" from wheat and PP2Ab from other species.
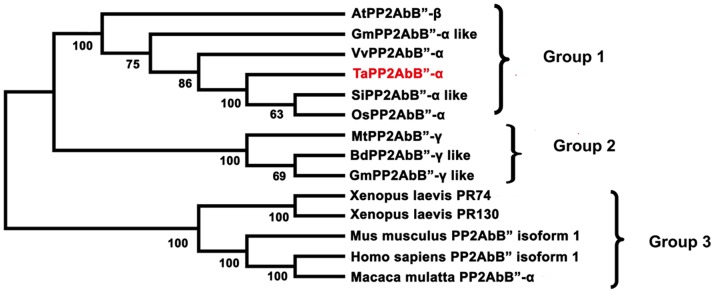
This phylogenetic tree performed by MEGA 5.05 is based on putative amino acid sequences. Bootstrap values are in percentages. There are three distinct isoform groups in the phylogenetic tree. At, Arabidopsis thaliana; Gm, Glycine max; Vv, Vitis vinifera; Ta, Triticum aestivum; Si, Setaria italica; Os, Oryza sativa; Mt, Medicago truncatula; Bd, Brachypodium distachyon.
Subcelluar localization of TaPP2AbB"-α protein
Wheat protoplast cells were used to check subcellular localization of TaPP2AbB"-α. The deduced amino acid sequence contains no putative nuclear localization sequence (NLS) site or transmembrane region. The fusion construct TaPP2AbB"-α::GFP driven by the CaMV 35S promoter was transiently expressed in living wheat protoplast cells. As predicted, TaPP2AbB"-α-GFP was present in the cell membrane, cytoplasm and nucleus (Figure 3).
Figure 3. Subcellular localization of PP2AB”-α in wheat protoplast cells.
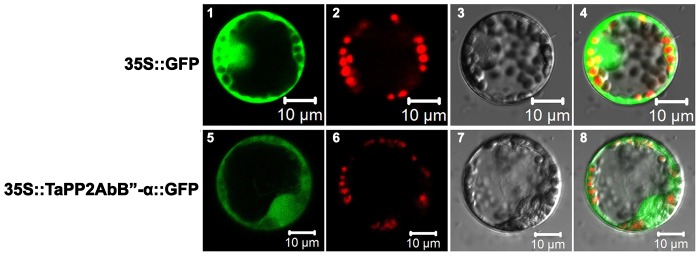
GFP and TaPP2AbB"-α-GFP fusion proteins were transiently expressed under control of the CaMV 35S promoter in wheat protoplast cells and observed with a laser scanning confocal microscope. Images are taken in dark field for green fluorescence (1, 5). The chloroplasts (2, 6) are indicated by red autofluorescence. The cell outline (3, 7) and the combination (4, 8) are photographed in bright field. Scale bar = 10 µm.
Phenotypes of overexpression lines of TaPP2AbB"-α in Arabidopsis
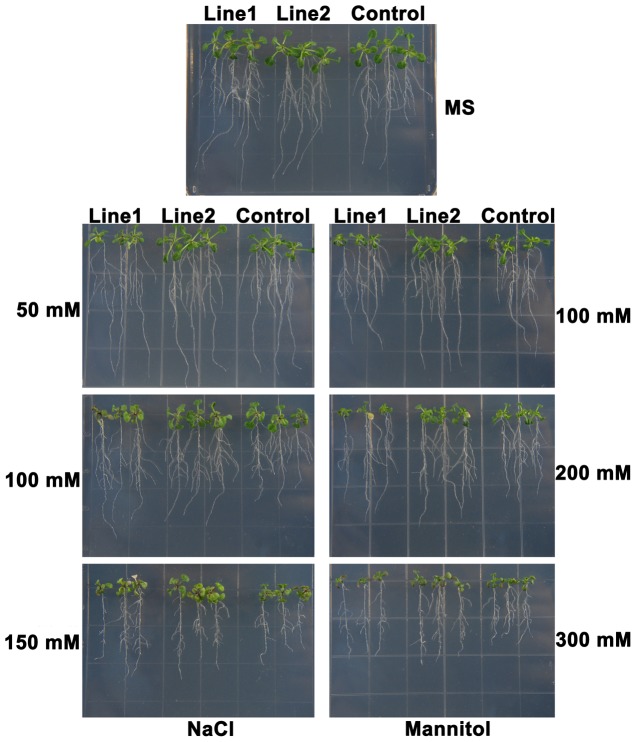
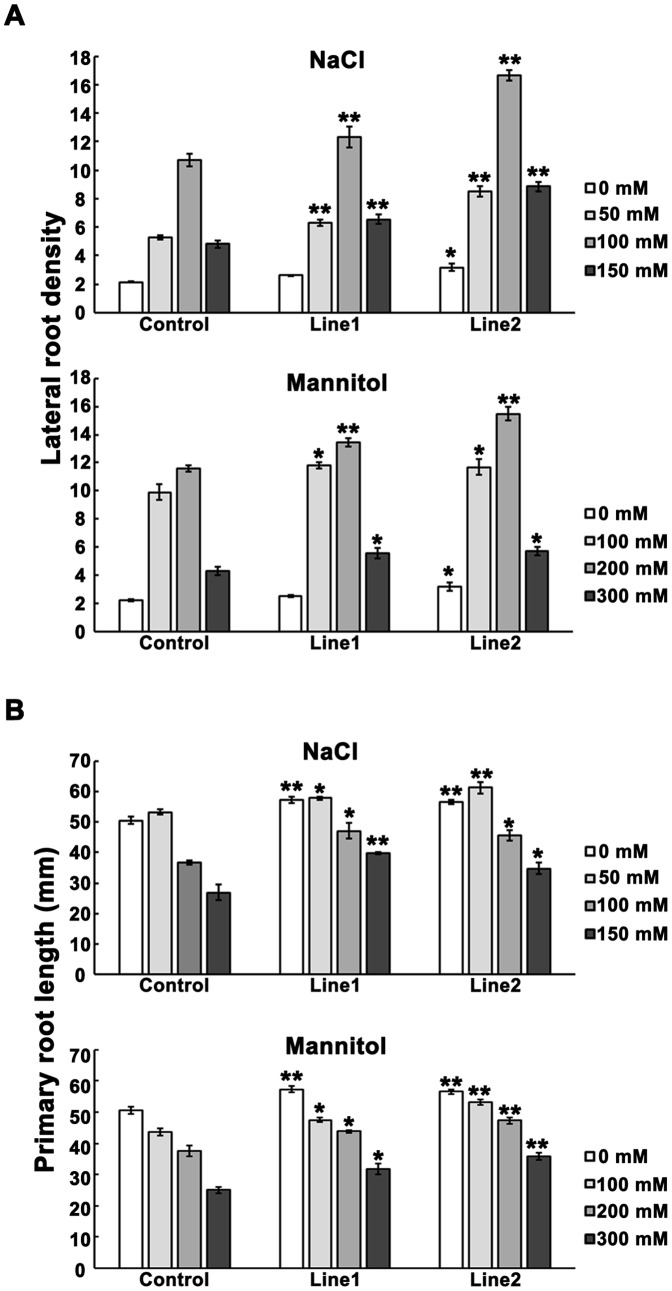
To evaluate the possible function of TaPP2AbB"-α, transgenic plants were assessed under different abiotic stress conditions. Morphological assays indicated differences in root systems between transgenic and control plants (the p35S-NOS vector plants) (Figure 4). Overexpression lines grew better, and developed more lateral roots, especially under the stresses of mannitol and NaCl (Figure 5A). Primary roots of overexpression lines were significantly longer than those of control plants under osmotic stress (Figure 5B). These results suggested that TaPP2AbB"-α may be involved in stress response and regulation of root development, especially lateral root densities under 100 mM NaCl and 200 mM mannitol treatments.
Figure 4. Overexpression of TaPP2AbB"-α promotes root growth in Arabidopsis.
Root morphologies of TaPP2AbB"-α overexpression lines and control (p35S-NOS vector plants) grown on MS medium, and MS medium with added NaCl and mannitol for 3 days after replanting.
Figure 5. Overexpression of TaPP2AbB"-α promotes root growth in Arabidopsis as exhibited by more lateral roots and longer primary roots.
(A) Lateral root density (number of lateral roots/cm primary root) of TaPP2AbB"-α overexpression lines and control. (B) Primary root length of TaPP2AbB"-α overexpression lines and control. *, significantly different at P = 0.05; **, significantly different at P = 0.01. Data represent mean ± SE (n = 9).
Expression pattern of TaPP2AbB"-α in wheat
TaPP2AbB"-α was expressed strongly in roots, and weakly in leaves of wheat seedlings (Figure 6A). Various up-regulated expression patterns occurred under diverse abiotic stresses (Figure 6B). Under NaCl stress, the expression of TaPP2AbB"-α decreased during the first 2 hours, the transcription level increased and peaked at 12 h, and then decreased. Under PEG stress, the transcription level decreased during the first hour, increased from 12 h, slightly decreased at 48 h, and increased again at 72 h. Under cold stress, expression increased rapidly in the first hour, and the level peaked at 3 h, 24 h and 48 h. With ABA treatment, the expression level of TaPP2AbB"-α increased rapidly and reached a maximum at 3 h, then quickly decreased to the 0 h level.
Figure 6. Expression patterns of TaPP2AbB"-α in different wheat seedling tissues after subjection to various stresses.
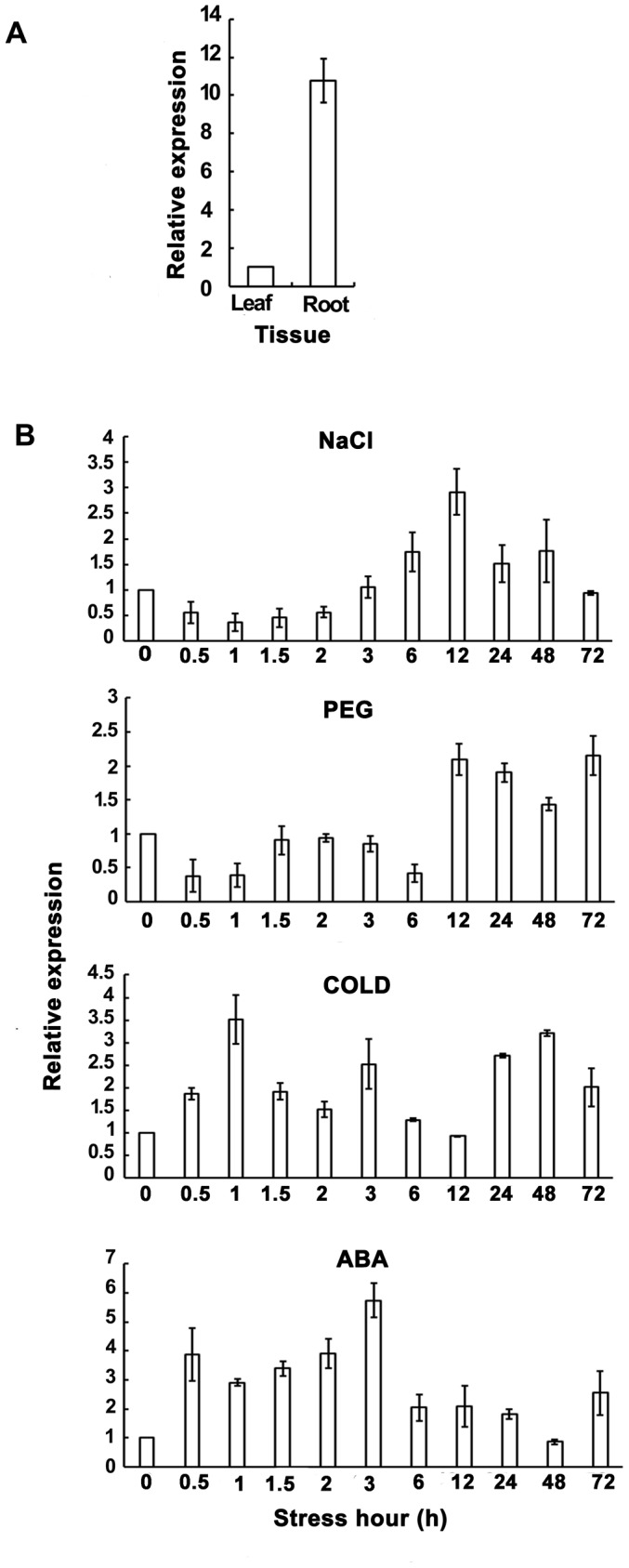
(A) Expression pattern of TaPP2AbB"-α in roots and leaves of wheat seedlings. (B) Expression patterns of TaPP2AbB"-α under various stress conditions. Actin was used as an internal control. Vertical columns indicate relative transcript levels. Data represent mean ± SE of three replicates.
TaPP2AbB"-α interacts with both TaPP2Aa and TaPP2Ac
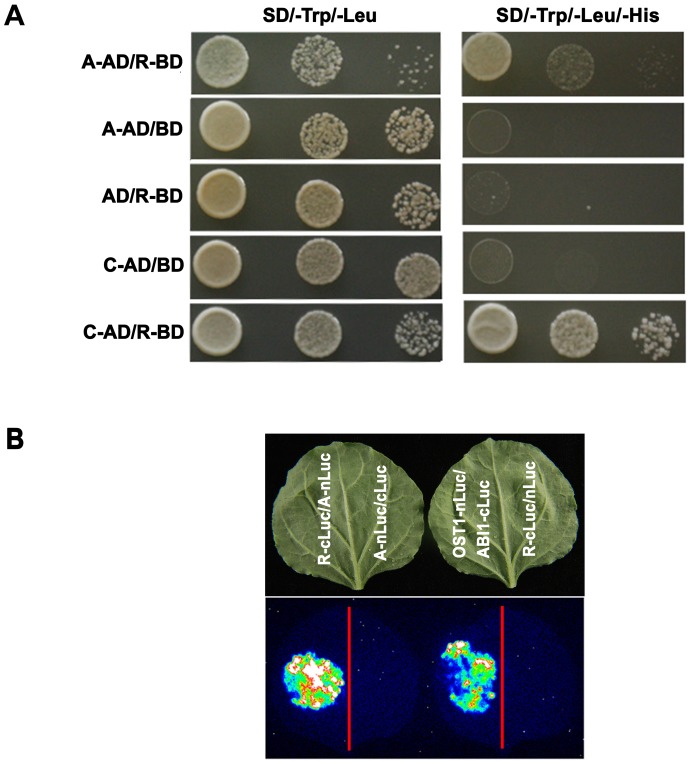
Yeast cells co-transformed with the TaPP2AbB"-α-pGBKT7/TaPP2Aa-pGADT7, TaPP2AbB"-α-pGBKT7/TaPP2Ac-pGADT7, and TaPP2AbB"-α-pGBKT7/pGADT7 vector, and with TaPP2Aa-pGADT7/pGBKT7 and TaPP2Ac-pGADT7/pGBKT7 grew well on SD/–Trp/–Leu plates. Yeast cells co-transformed with the TaPP2AbB"-α-pGBKT7/pGADT7 vector, TaPP2Aa-pGADT7/pGBKT7 and TaPP2Ac-pGADT7/pGBKT7, could not grow on SD/–Trp/–Leu/–His with 5 mM 3-AT (Figure 7A). These results indicated that TaPP2AbB"-α interacted with both TaPP2Aa and TaPP2Ac. In the firefly luciferase complementation imaging assay, the image of LUC was captured during co-transformation of TaPP2AbB"-α-cLuc/TaPP2Aa-nLuc as well as the positive control in Nicotiana benthamiana leaves (Figure 7B). Different fluorescence intensity resulted in the color-mixed spots. These results confirmed that TaPP2AbB"-α interacts with TaPP2Aa.
Figure 7. TaPP2AbB"-α interacts with TaPP2Aa and TaPP2Ac.
(A) Yeast two-hybrid assay shows interactions between TaPP2AbB"-α and subunits A and C of PP2A. Abbreviations on the left side are: AD, pGADT7 vector; BD, pGBKT7 vector; R, TaPP2AbB"-α; A, TaPP2Aa; C, TaPP2Ac. (B) Interaction of TaPP2AbB"-α with TaPP2Aa revealed by firefly luciferase complementation imaging assay in Nicotiana benthamiana leaves. A, TaPP2Aa; R, TaPP2AbB"-α. Different fluorescence intensity resulted in the color-mixed spots.
Discussion
The PP2A regulatory subunit is responsible for substrate specificity and localization of PP2A [41]. Approximately fifteen and seventeen B subunits have been identified in vertebrates and plants, respectively. All B subunit members are derived from four diverse gene families, and little sequence similarity exists between families while maintaining high sequence similarity within families [22]. In this study, the identified wheat PP2Ab, designated as TaPP2AbB"-α, has the same EF-hand domain as PP2AbB" from animals and plants.
We used the cDNA sequence of TaPP2AbB"-α to blast in wheat A genome and D genome. There are 12 regions in TaPP2AbB"-α matching with scaffold59526 of A genome and scaffold72594 of D genome, respectively. The 12 regions of TaPP2AbB"-α almost covered the whole coding region. Most part of the 12 regions have 97% identity with scaffold59526 and scaffold72594. We presumed that TaPP2AbB"-α was exist in A genome and D genome. What’s more, we did not find other scaffolds similar to TaPP2AbB"-α. This phenomenon may imply that the TaPP2AbB"-α has no homologue in wheat.
Our data indicates that transgenic Arabidopsis plants overexpressing TaPP2AbB"-α have a more developed root system than control plants under normal conditions. Previous studies showed that root growth can be affected by environmental conditions and genetic characters [42], such as moderate soil drying which promotes the elongation of lateral roots [43]. Overexpression of TaSnRK2.3–GFP in Arabidopsis also generated an improved root system, expressed by longer primary root and more lateral roots [12]. In this study, TaPP2AbB"-α-overexpressing Arabidopsis lines developed more lateral roots and stronger primary roots than the control under normal conditions, suggesting that TaPP2AbB"-α is involved in regulation of root growth and development.
Under medium stresses, i.e. 100 mM NaCl and 200 mM mannitol, both transgenic lines developed more lateral roots (Figure 5), implying that the plants can spontaneously adapt to certain environmental stress, and that this phenotype may also represent a common adaptive response to diverse stresses among plants. As in rice, water deficit also results in increased lateral root formation [44]. In addition, more lateral roots were produced under salt stress in wild-type Arabidopsis [45]. Previous studies showed that Arabidopsis developed more lateral roots under 100 mM NaCl, but less lateral roots under 125 mM NaCl [17]. A more developed root system helps plants to improve the efficiency of water and nutrient uptake, especially under abiotic stress [46]. With more lateral roots and longer primary roots, more water and nutrient uptake may be available in transgenic Arabidopsis. This could be the reason why overexpression lines of TaPP2AbB"-α grew better than control plants, even under NaCl and mannitol stresses.
The transcript expression level of TaPP2AbB"-α is stronger in roots than in leaves of wheat seedlings, suggesting that TaPP2AbB"-α mainly functions in the root system. This inference also matches the phenotypes of overexpression lines of TaPP2AbB"-α in Arabidopsis. The expression of TaPP2AbB"-α is up-regulated by multiple stresses (Figure 6). Previous studies showed that PP2A, or specific PP2A subunits, function as transducers of osmotic stress and in low temperature signaling [47]. In Saccharomyces cerevisiae, PP2AbB′ is an essential activating factor for stress gene transcription, such as CTT1 (encoding cytosolic catalase T), HSP12 (encoding a small heat shock protein), and PGM2 (encoding phosphoglucomutase 2) [48]. Moreover, expression of genes encoding PP2Ac subfamily I is induced by drought and NaCl stress [49], [50]. TaPP2Ac1, which clades in PP2Ac subfamily II, is induced by NaCl, PEG, low temperature and ABA [51]. Considering these findings, the role of PP2Ac in stress signaling matches with the expression of TaPP2AbB"-α under multiple environmental stresses. Together with the phenotypic data for TaPP2AbB"-α overexpression lines, we surmise that transgenic plants develop more lateral roots when the transcript level of TaPP2AbB"-α is induced by osmotic stress.
PP2A is highly conserved and the core enzyme interacts with a variable regulatory subunit to form a PP2A holoenzyme. Human PP2Aa interacts with PP2Ac to form a stable AC core dimer through its intra-repeat loops and inner helices, and B56 (PP2AbB′) interacts with both PP2Aa and PP2Ac [52]. Here, TaPP2AbB"-α exhibits the same subcellular localization pattern with TaPP2Aa (unpublished data) and TaPP2Ac [51]. As PP2A is a heterotrimeric protein, we detected interactions of TaPP2AbB"-α with PP2Aa and PP2Ac. As shown in Figure 7, the interacting relationships of TaPP2AbB"-α with TaPP2Aa and TaPP2Ac were verified in both yeast and tobacco leaves. Moreover, we found that TaPP2Aa has high homology with RCN 1 in Arabidopsis (Figure S1). The mutant line of rcn1 (pp2aa1) is sensitive to osmotic stress and developed less lateral root than the normal plants [53]. RCN1 modulates polar auxin transport (PAT), and dephosphorylates auxin efflux carrier PIN proteins to influence the distribution of auxin accompanied with Ser/Thr kinase PINOID [15], [16]. TaPP2Ac is in the same clade with PP2Ac subfamily II (PP2Ac3 and PP2Ac4) in Arabidopsis (Figure S2), and a double mutant of PP2Ac, pp2ac3/pp2ac4 exhibits defective root development in Arabidopsis. PP2Ac subfamily II is also involved in the auxin signaling pathway [20]. Interaction and the phenotype of PP2Aa and PP2Ac suggest that positive regulation of TaPP2AbB"-α in root development may be associated with auxin signaling.
In this study, TaPP2AbB"-α enhanced root development in overexpressing Arabidopsis under normal conditions. With onset of environmental stress, TaPP2AbB"-α is induced to promote increased root growth, and thus enhanced absorbsion efficiency in the uptake of water and nutrients for survival. In this process, PP2Aa and PP2Ac may interact with PP2AbB"-α and then function as a holoenzyme to act on certain substrates. More evidence needs to be obtained to uncover the molecular mechanism by which TaPP2AbB"-α enhances osmotic-stress tolerance.
Supporting Information
Alignment of the amino acid sequences of PP2AbB"-α from wheat and Arabidopsis . Alignment was performed according to DNAman. The accession number of AtPP2Aa1 is NP_173920.1, the accession number of TaPP2Aa is AEB40165.1. Common identical amino acid residues are shown in black background. The HEAT motif is marked in red rectangles. Abbreviations on the left side of the sequence are: At, Arabidopsis thaliana; Ta, Triticum aestivum.
(TIF)
Phylogenetic tree of TaPP2Ac from wheat and PP2Ac from Arabidopsis . This phylogenetic tree is performed by MEGA 5.05. TaPP2Ac, accession number: ABO16371.1; AtPP2Ac-1, NP 176192.1; AtPP2Ac-2; NP 172514.1; AtPP2Ac-3, NP 567066.1; AtPP2Ac-4, NP 565974.1; AtPP2Ac-5, NP 172514.1. Bootstrap values are in percentages. Abbreviations on the right side of the tree are: At, Arabidopsis thaliana; Ta, Triticum aestivum.
(TIF)
Acknowledgments
We thank Professor Robert A. McIntosh (Plant Breeding Institute, University of Sydney, NSW, Australia) for critical reading and comments on the manuscript.
Funding Statement
This study was supported by the National Natural Science Foundation of China (31201206, http://www.nsfc.gov.cn/), and Science and Technology Project for Outstanding Ph.D. Supervisor of Beijing (YB20088210101). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Zhao Y, Wang T, Zhang W, Li X (2011) SOS3 mediates lateral root development under low salt stress through regulation of auxin redistribution and maxima in Arabidopsis. New Phytol 189: 1122–1134. [DOI] [PubMed] [Google Scholar]
- 2. Lavenus J, Goh T, Roberts I, Guyomarc’h S, Lucas M, et al. (2013) Lateral root development in Arabidopsis: fifty shades of auxin. Trends Plant Sci 18: 450–458. [DOI] [PubMed] [Google Scholar]
- 3. Malamy JE, Ryan KS (2001) Environmental regulation of lateral root initiation in Arabidopsis. Plant Physiol 127: 899–909. [PMC free article] [PubMed] [Google Scholar]
- 4. Malamy J (2005) Intrinsic and environmental response pathways that regulate root system architecture. Plant, Cell Environ 28: 67–77. [DOI] [PubMed] [Google Scholar]
- 5. Ditengou FA, Teale WD, Kochersperger P, Flittner KA, Kneuper I, et al. (2008) Mechanical induction of lateral root initiation in Arabidopsis thaliana . Proc Natl Acad Sci USA 105: 18818–18823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kosová K, Vítámvás P, Prášil IT, Renaut J (2011) Plant proteome changes under abiotic stress - contribution of proteomics studies to understanding plant stress response. J Proteomics 74: 1301–1322. [DOI] [PubMed] [Google Scholar]
- 7. Schweighofer A, Hirt H, Meskiene I (2004) Plant PP2C phosphatases: emerging functions in stress signaling. Trends Plant Sci 9: 236–243. [DOI] [PubMed] [Google Scholar]
- 8. Fujii H, Chinnusamy V, Rodrigues A, Rubio S, Antoni R, et al. (2009) In vitro reconstitution of an abscisic acid signalling pathway. Nature 462: 660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Park S-Y, Fung P, Nishimura N, Jensen DR, Fujii H, et al. (2009) Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324: 1068–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, et al. (2009) Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324: 1064–1068. [DOI] [PubMed] [Google Scholar]
- 11. Mao X, Zhang H, Tian S, Chang X, Jing R (2010) TaSnRK2. 4, an SNF1-type serine/threonine protein kinase of wheat (Triticum aestivum L.), confers enhanced multistress tolerance in Arabidopsis . J Exp Bot 61: 683–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tian S, Mao X, Zhang H, Chen S, Zhai C, et al. (2013) Cloning and characterization of TaSnRK2. 3, a novel SnRK2 gene in common wheat. J Exp Bot 64: 2063–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Janssens V, Goris J (2001) Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem Journal 353: 417–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Høyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, et al. (2007) Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-β, and Bcl-2. Mol Cell 25: 193–205. [DOI] [PubMed] [Google Scholar]
- 15. Michniewicz M, Zago MK, Abas L, Weijers D, Schweighofer A, et al. (2007) Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 130: 1044–1056. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Mehdi S, Topping J, Friml J, Lindsey K (2013) Interaction of PLS and PIN and hormonal crosstalk in Arabidopsis root development. Front Plant Sci 4. http://hdl.handle.net/1854/LU-4159896 [DOI] [PMC free article] [PubMed]
- 17. Chávez-Avilés MN, Andrade-Pérez CL, de la Cruz HR (2013) PP2A mediates lateral root development under NaCl-induced osmotic stress throughout auxin redistribution in Arabidopsis thaliana . Plant Soil 368: 591–602. [Google Scholar]
- 18. Groves MR, Hanlon N, Turowski P, Hemmings BA, Barford D (1999) The structure of the protein phosphatase 2A PR65/A subunit reveals the conformation of its 15 tandemly repeated HEAT motifs. Cell 96: 99–110. [DOI] [PubMed] [Google Scholar]
- 19. Garbers C, DeLong A, Deruere J, Bernasconi P, Söll D (1996) A mutation in protein phosphatase 2A regulatory subunit A affects auxin transport in Arabidopsis . EMBO J 15: 2115–2124. [PMC free article] [PubMed] [Google Scholar]
- 20. Ballesteros I, Domínguez T, Sauer M, Paredes P, Duprat A, et al. (2012) Specialized functions of the PP2A subfamily II catalytic subunits PP2A-C3 and PP2A-C4 in the distribution of auxin fluxes and development in Arabidopsis. Plant J 73: 862–872. [DOI] [PubMed] [Google Scholar]
- 21. Wu G, Wang X, Li X, Kamiya Y, Otegui MS, et al. (2011) Methylation of a phosphatase specifies dephosphorylation and degradation of activated brassinosteroid receptors. Sci Signal 4: ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slupe AM, Merrill RA, Strack S (2011) Determinants for substrate specificity of protein phosphatase 2A. Enzyme Res 2011. http://dx.doi.org/10.4061/2011/398751 [DOI] [PMC free article] [PubMed]
- 23. Leung HT, Wang N (2013) Neuronal function of protein phosphatase 2A. Am J Neuro 4: 46–55. [Google Scholar]
- 24. Zhao JQ, Xie SS, Liu WB, Xiao YM, Zeng XM, et al. (2010) Molecular cloning of the genes encoding the pR55/Bβ/δ regulatory subunits for pp-2A and analysis of their functions in regulating development of goldfish, Carassius auratus . Gene Regul Syst Biol 4: 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Louis JV, Martens E, Borghgraef P, Lambrecht C, Sents W, et al. (2011) Mice lacking phosphatase PP2A subunit PR61/B’δ (Ppp2r5d) develop spatially restricted tauopathy by deregulation of CDK5 and GSK3β. Proc Natl Acad Sci USA 108: 6957–6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park S, Scheffler T, Rossie S, Gerrard D (2013) AMPK activity is regulated by calcium-mediated protein phosphatase 2A activity. Cell Calcium 53: 217–223. [DOI] [PubMed] [Google Scholar]
- 27. Farkas I, Dombradi V, Miskei M, Szabados L, Koncz C (2007) Arabidopsis PPP family of serine/threonine phosphatases. Trends Plant Sci 12: 169–176. [DOI] [PubMed] [Google Scholar]
- 28. Harris DM, Myrick TL, Rundle SJ (1999) The Arabidopsis homolog of yeast TAP42 and mammalian α4 binds to the catalytic subunit of protein phosphatase 2A and is induced by chilling. Plant Physiol 121: 609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kirik A, Ehrhardt DW, Kirik V (2012) TONNEAU2/FASS regulates the geometry of microtubule nucleation and cortical array organization in interphase Arabidopsis cells. The Plant Cell Online 24: 1158–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tang W, Yuan M, Wang R, Yang Y, Wang C, et al. (2011) PP2A activates brassinosteroid-responsive gene expression and plant growth by dephosphorylating BZR1. Nat Cell Biol 13: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ballesteros I, Domínguez T, Sauer M, Paredes P, Duprat A, et al. (2013) Specialized functions of the PP2A subfamily II catalytic subunits PP2A-C3 and PP2A-C4 in the distribution of auxin fluxes and development in Arabidopsis. Plant J 73: 862–872. [DOI] [PubMed] [Google Scholar]
- 32. Liu S, Wang C, Mao X, Liu H, Li A, et al. (2010) Cloning of protein phosphatase 2A regulatory subunit gene TaB β -1 and its expression patterns under abiotic stresses in wheat. Scientia Agricultura Sinica 43: 2197–2208. [Google Scholar]
- 33. Jia J, Zhao S, Kong X, Li Y, Zhao G, et al. (2013) Aegilops tauschii draft genome sequence reveals a gene repertoire for wheat adaptation. Nature 496: 91–95. [DOI] [PubMed] [Google Scholar]
- 34. Shi JF, Mao XG, Jing RL, Pang XB, Wang YG, et al. (2010) Gene expression profiles of response to water stress at the jointing stage in wheat. Agricultural Sciences in China 9: 325–330. [Google Scholar]
- 35. Pang X, Mao X, Jing R, Shi J, Gao T, et al. (2007) Analysis of gene expression profile responsed to water stress in wheat (Triticum aestivum L.) seedling. Acta Agron Sin 33: 333–336. [Google Scholar]
- 36. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yoo S-D, Cho Y-H, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572. [DOI] [PubMed] [Google Scholar]
- 38. Chen H, Zou Y, Shang Y, Lin H, Wang Y, et al. (2008) Firefly luciferase complementation imaging assay for protein-protein interactions in plants. Plant Physi 146: 368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brandt B, Brodsky DE, Xue S, Negi J, Iba K, et al. (2012) Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proc Natl Acad Sci USA 109: 10593–10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C T method. Nat Protoc 3: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 41. Gentry MS, Hallberg RL (2002) Localization of Saccharomyces cerevisiae protein phosphatase 2A subunits throughout mitotic cell cycle. Mol Biol Cell 13: 3477–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Muñoz-Romero V, Benítez-Vega J, López-Bellido RJ, Fontán JM, López-Bellido L (2010) Effect of tillage system on the root growth of spring wheat. Plant Soil 326: 97–107. [Google Scholar]
- 43. Ito K, Tanakamaru K, Morita S, Abe J, Inanaga S (2006) Lateral root development, including responses to soil drying, of maize (Zea mays) and wheat (Triticum aestivum) seminal roots. Physiol Plant 127: 260–267. [Google Scholar]
- 44. Yang L, Zheng B, Mao C, Qi X, Liu F, et al. (2004) Analysis of transcripts that are differentially expressed in three sectors of the rice root system under water deficit. Mol Gen Genomics 272: 433–442. [DOI] [PubMed] [Google Scholar]
- 45. He XJ, Mu RL, Cao WH, Zhang ZG, Zhang J, et al. (2005) AtNAC2, a transcription factor downstream of ethylene and auxin signaling pathways, is involved in salt stress response and lateral root development. Plant J 44: 903–916. [DOI] [PubMed] [Google Scholar]
- 46. Péret B, De Rybel B, Casimiro I, Benková E, Swarup R, et al. (2009) Arabidopsis lateral root development: an emerging story. Trends Plant Sci 14: 399–408. [DOI] [PubMed] [Google Scholar]
- 47. Antolín-Llovera M, Leivar P, Arró M, Ferrer A, Boronat A, et al. (2011) Modulation of plant HMG-CoA reductase by protein phosphatase 2A: positive and negative control at a key node of metabolism. Plant Signal Behav 6: 1127–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reiter W, Klopf E, De Wever V, Anrather D, Petryshyn A, et al. (2013) Yeast protein phosphatase 2A-Cdc55 regulates the transcriptional response to hyperosmolarity stress by regulating Msn2 and Msn4 chromatin recruitment. Mol Cell Biol 33: 1057–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yu RMK, Zhou Y, Xu Z-F, Chye M-L, Kong RYC (2003) Two genes encoding protein phosphatase 2A catalytic subunits are differentially expressed in rice. Plant Mol Biol 51: 295–311. [DOI] [PubMed] [Google Scholar]
- 50. Yu RMK, Wong MML, Jack RW, Kong RYC (2005) Structure, evolution and expression of a second subfamily of protein phosphatase 2A catalytic subunit genes in the rice plant (Oryza sativa L.). Planta 222: 757–768. [DOI] [PubMed] [Google Scholar]
- 51. Xu C, Jing R, Mao X, Jia X, Chang X (2007) A wheat (Triticum aestivum) protein phosphatase 2A catalytic subunit gene provides enhanced drought tolerance in tobacco. Ann Bot 99: 439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cho US, Xu W (2006) Crystal structure of a protein phosphatase 2A heterotrimeric holoenzyme. Nature 445: 53–57. [DOI] [PubMed] [Google Scholar]
- 53. Blakeslee JJ, Zhou H, Heath JT, Skottke KR, Barrios JAR, et al. (2008) Specificity of RCN1-mediated protein phosphatase 2A regulation in meristem organization and stress response in roots. Plant Physiol 146: 539–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alignment of the amino acid sequences of PP2AbB"-α from wheat and Arabidopsis . Alignment was performed according to DNAman. The accession number of AtPP2Aa1 is NP_173920.1, the accession number of TaPP2Aa is AEB40165.1. Common identical amino acid residues are shown in black background. The HEAT motif is marked in red rectangles. Abbreviations on the left side of the sequence are: At, Arabidopsis thaliana; Ta, Triticum aestivum.
(TIF)
Phylogenetic tree of TaPP2Ac from wheat and PP2Ac from Arabidopsis . This phylogenetic tree is performed by MEGA 5.05. TaPP2Ac, accession number: ABO16371.1; AtPP2Ac-1, NP 176192.1; AtPP2Ac-2; NP 172514.1; AtPP2Ac-3, NP 567066.1; AtPP2Ac-4, NP 565974.1; AtPP2Ac-5, NP 172514.1. Bootstrap values are in percentages. Abbreviations on the right side of the tree are: At, Arabidopsis thaliana; Ta, Triticum aestivum.
(TIF)