Abstract
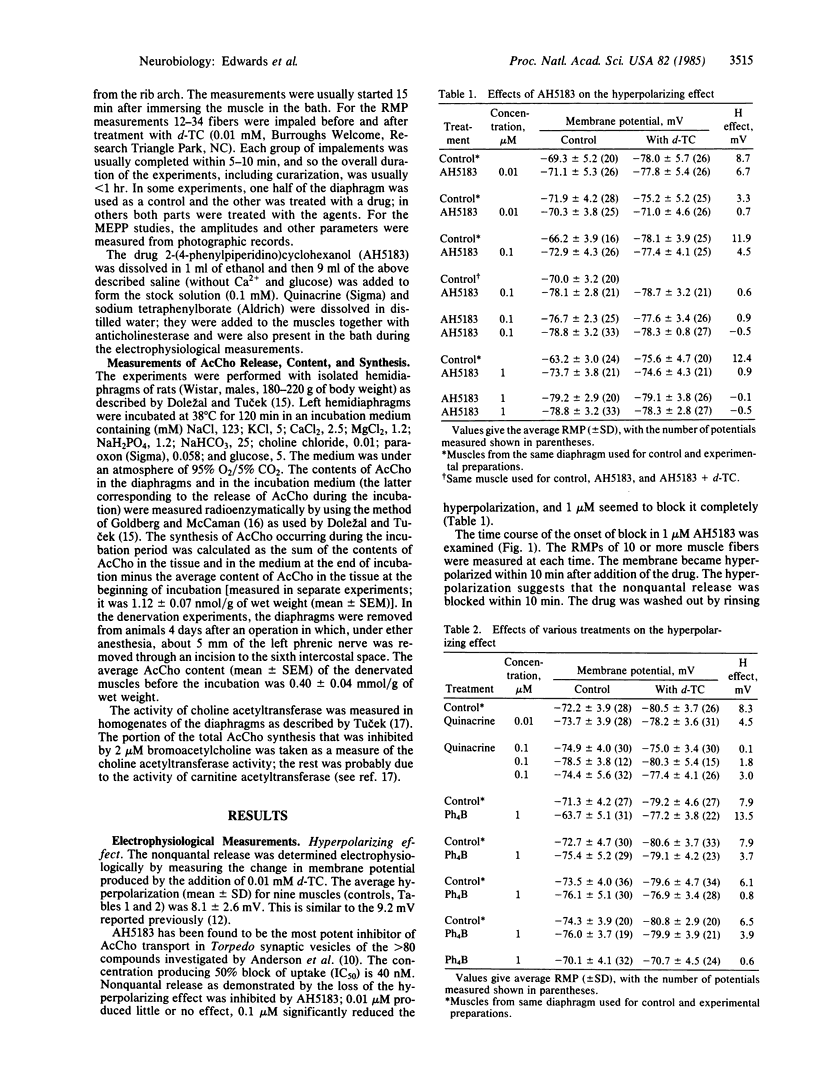
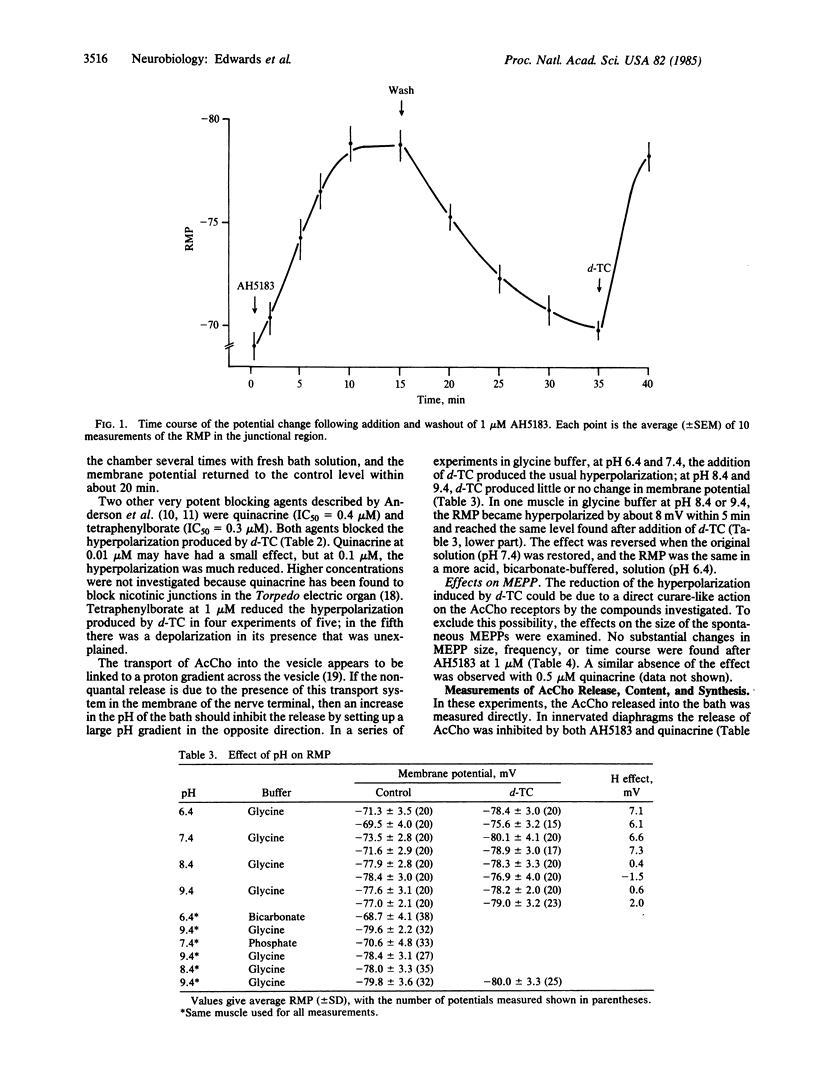
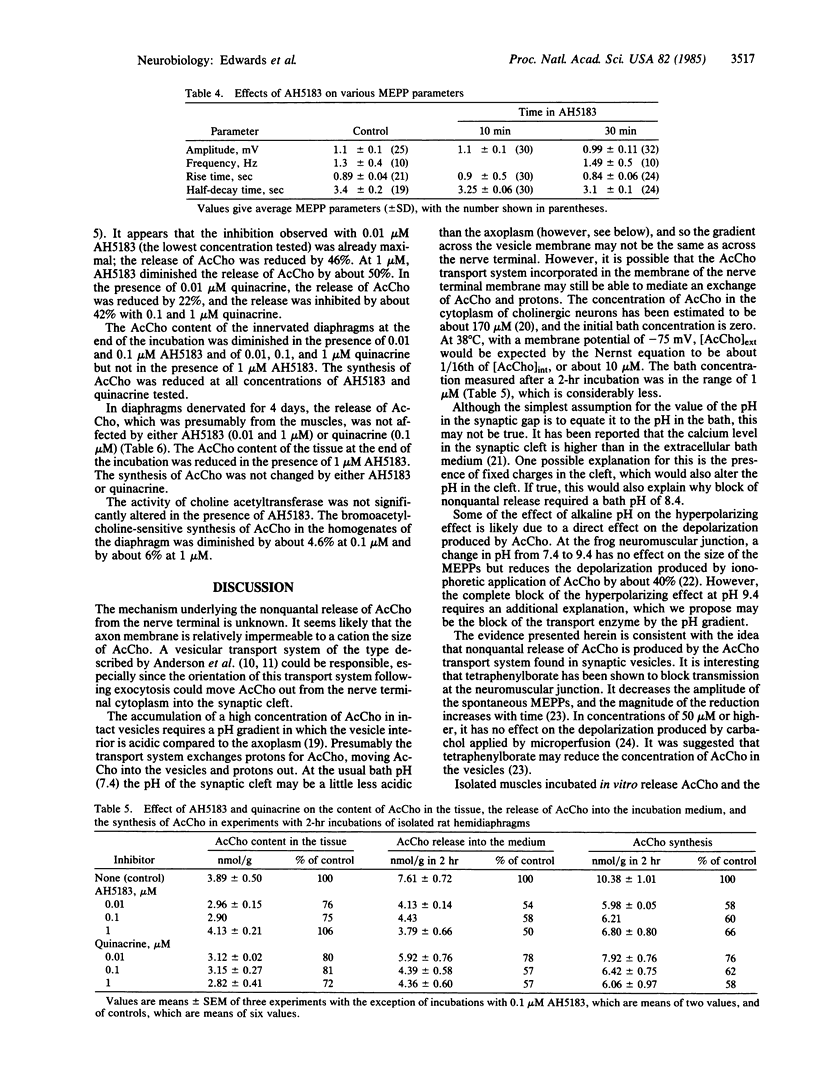
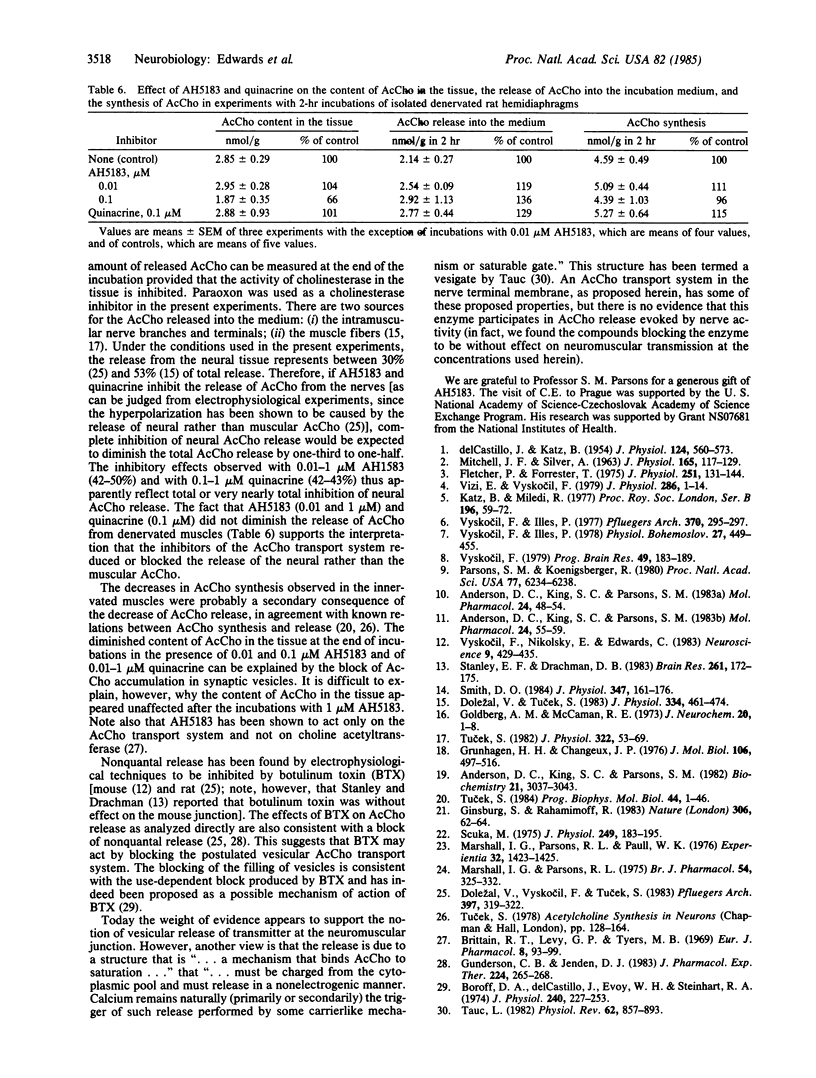
Experiments were performed to investigate the effects on the spontaneous, nonquantal release of acetylcholine (AcCho) from motor nerve terminals of substances known to inhibit the AcCho transport system present in cholinergic synaptic vesicles. In mouse diaphragms, the hyperpolarization normally produced by d-tubocurarine in the endplate area of muscle fibers that had been treated by an anticholinesterase was partly or completely blocked by 2-(4-phenylpiperidino)cyclohexanol (AH5183, 0.1-1 microM), quinacrine (0.1 microM), and tetraphenylborate (1 microM). Since the sensitivity of the subsynaptic area to AcCho was not changed, the block of the hyperpolarizing action of d-tubocurarine indicated in inhibition of the spontaneous, nonquantal release of AcCho. This was confirmed in experiments on rat diaphragm using direct radioenzymatic measurement of the AcCho released into the incubation medium. The release of AcCho from the innervated diaphragm was decreased by about 50% in the presence of AH5183 (0.01-1 microM) and by 42% in the presence of quinacrine (0.1-1 microM). The AcCho released was presumably neural, since the release of AcCho from 4-day denervated diaphragms was not diminished by either AH5183 or quinacrine. The results indicate that the spontaneous release of AcCho from the motor nerve terminals is highly sensitive to low concentrations of specific inhibitors and is probably mediated by a carrier. It is proposed that spontaneous release is due to the incorporation into the membrane of the nerve terminal during exocytosis of the vesicular transport system responsible for moving AcCho into the vesicle.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., King S. C., Parsons S. M. Inhibition of [3H]acetylcholine active transport by tetraphenylborate and other anions. Mol Pharmacol. 1983 Jul;24(1):55–59. [PubMed] [Google Scholar]
- Anderson D. C., King S. C., Parsons S. M. Pharmacological characterization of the acetylcholine transport system in purified Torpedo electric organ synaptic vesicles. Mol Pharmacol. 1983 Jul;24(1):48–54. [PubMed] [Google Scholar]
- Anderson D. C., King S. C., Parsons S. M. Proton gradient linkage to active uptake of [3H]acetylcholine by Torpedo electric organ synaptic vesicles. Biochemistry. 1982 Jun 22;21(13):3037–3043. doi: 10.1021/bi00256a001. [DOI] [PubMed] [Google Scholar]
- Boroff D. A., del Castillo J., Evoy W. H., Steinhardt R. A. Observations on the action of type A botulinum toxin on frog neuromuscular junctions. J Physiol. 1974 Jul;240(2):227–253. doi: 10.1113/jphysiol.1974.sp010608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain R. T., Levy G. P., Tyers M. B. The neuromuscular blocking action of 2-(4-phenylpiperidino) cyclohexanol (AH 5183). Eur J Pharmacol. 1969 Oct;8(1):93–99. doi: 10.1016/0014-2999(69)90133-2. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolezal V., Tucek S. The synthesis and release of acetylcholine in normal and denervated rat diaphragms during incubation in vitro. J Physiol. 1983 Jan;334:461–474. doi: 10.1113/jphysiol.1983.sp014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P., Forrester T. The effect of curare on the release of acetylcholine from mammalian motor nerve terminals and an estimate of quantum content. J Physiol. 1975 Sep;251(1):131–144. doi: 10.1113/jphysiol.1975.sp011084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg S., Rahamimoff R. Is extracellular calcium buffering involved in regulation of transmitter release at the neuromuscular junction? Nature. 1983 Nov 3;306(5938):62–64. doi: 10.1038/306062a0. [DOI] [PubMed] [Google Scholar]
- Goldberg A. M., McCaman R. E. The determination of picomole amounts of acetylcholine in mammalian brain. J Neurochem. 1973 Jan;20(1):1–8. doi: 10.1111/j.1471-4159.1973.tb12097.x. [DOI] [PubMed] [Google Scholar]
- Grünhagen H. H., Changeux J. P. Studies on the electrogenic action of acetylcholine with Torpedo marmorata electric organ. IV. Quinacrine: a fluorescent probe for the conformational transitions of the cholinergic receptor protein in its membrane-bound state. J Mol Biol. 1976 Sep 25;106(3):497–516. doi: 10.1016/0022-2836(76)90249-7. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Jenden D. J. Spontaneous output of acetylcholine from rat diaphragm preparations declines after treatment with botulinum toxin. J Pharmacol Exp Ther. 1983 Feb;224(2):265–268. [PubMed] [Google Scholar]
- Katz B., Miledi R. Transmitter leakage from motor nerve endings. Proc R Soc Lond B Biol Sci. 1977 Feb 11;196(1122):59–72. doi: 10.1098/rspb.1977.0029. [DOI] [PubMed] [Google Scholar]
- Marshall I. G., Parsons R. L., Paull W. K. Depletion of synaptic vesicles at the frog (Rana pipiens) neuromuscular junctions by tetraphenylboron. Experientia. 1976 Nov 15;32(11):1423–1426. doi: 10.1007/BF01937413. [DOI] [PubMed] [Google Scholar]
- Marshall I. G., Parsons R. L. The effects of tetraphenylboron on neuromuscular transmission in the frog. Br J Pharmacol. 1975 Jul;54(3):325–332. doi: 10.1111/j.1476-5381.1975.tb07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. F., Silver A. The spontaneous release of acetylcholine from the denervated hemidiaphragm of the rat. J Physiol. 1963 Jan;165(1):117–129. doi: 10.1113/jphysiol.1963.sp007046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons S. M., Koenigsberger R. Specific stimulated uptake of acetylcholine by Torpedo electric organ synaptic vesicles. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6234–6238. doi: 10.1073/pnas.77.10.6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuka M. The amplitude and the time course of the end-plate current at various pH levels in the frog sartorius muscle. J Physiol. 1975 Jul;249(2):183–195. doi: 10.1113/jphysiol.1975.sp011010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. O. Acetylcholine storage, release and leakage at the neuromuscular junction of mature adult and aged rats. J Physiol. 1984 Feb;347:161–176. doi: 10.1113/jphysiol.1984.sp015059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. F., Drachman D. B. Botulinum toxin blocks quantal but not non-quantal release of ACh at the neuromuscular junction. Brain Res. 1983 Feb 14;261(1):172–175. doi: 10.1016/0006-8993(83)91300-8. [DOI] [PubMed] [Google Scholar]
- Tauc L. Non vesicular release of neurotransmitter. Physiol Rev. 1982 Jul;62(3):857–893. doi: 10.1152/physrev.1982.62.3.857. [DOI] [PubMed] [Google Scholar]
- Tucek S. Problems in the organization and control of acetylcholine synthesis in brain neurons. Prog Biophys Mol Biol. 1984;44(1):1–46. doi: 10.1016/0079-6107(84)90011-7. [DOI] [PubMed] [Google Scholar]
- Tucek S. The synthesis of acetylcholine in skeletal muscles of the rat. J Physiol. 1982 Jan;322:53–69. doi: 10.1113/jphysiol.1982.sp014022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizi E. S., Vyskocil F. Changes in total and quantal release of acetylcholine in the mouse diaphragm during activation and inhibition of membrane ATPase. J Physiol. 1979 Jan;286:1–14. doi: 10.1113/jphysiol.1979.sp012603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyskocil F., Illés P. Electrophysiological examination of transmitter release in non-quantal form in the mouse diaphragm and the activity of membrane ATP-ase. Physiol Bohemoslov. 1978;27(5):449–455. [PubMed] [Google Scholar]
- Vyskocil F., Illés P. Non-quantal release of transmitter at mouse neuromuscular junction and its dependence on the activity of Na+-K+ ATP-ase. Pflugers Arch. 1977 Sep 16;370(3):295–297. doi: 10.1007/BF00585542. [DOI] [PubMed] [Google Scholar]
- Vyskocil F., Nikolsky E., Edwards C. An analysis of the mechanisms underlying the non-quantal release of acetylcholine at the mouse neuromuscular junction. Neuroscience. 1983 Jun;9(2):429–435. doi: 10.1016/0306-4522(83)90305-6. [DOI] [PubMed] [Google Scholar]
- Vyskocil F. The regulatory role of membrane Na+-K+-ATPase in non-quantal release of transmitter at the neuromuscular junction. Prog Brain Res. 1979;49:183–189. doi: 10.1016/S0079-6123(08)64632-4. [DOI] [PubMed] [Google Scholar]



