Abstract
Stroke is a leading cause of human death and disability in the US and around the world. Shortly after the cerebral ischemia, cell swelling is the earliest morphological change in injured neuronal, glial and endothelial cells. Cytotoxic swelling directly results from increased Na+ (with H2O) and Ca2+ influx into cells via ionic mechanisms evoked by membrane depolarization and a number of harmful factors such as glutamate accumulation and the production of oxygen reactive species (ROS). During the sub-acute and chronic phases after ischemia, injured cells may show a phenotype of cell shrinkage due to complex processes involving membrane receptors/channels and programmed cell death signals. This review will introduce some progress in the understanding of the regulation of pathological cell volume changes and the involved receptors and channels, including NMDA and AMPA receptors, acid-sensing ion channels (ASIC), hemichannels, transient receptor potential (TRP) channels and KCNQ channels. Moreover, accumulating evidence supports a key role of energy deficiency and dysfunction of Na+/K+-ATPase in ischemia-induced cell volume changes and cell death. Specifically, the Na+ pump failure is a prerequisite for disruption of ionic homeostasis including a pro-apoptotic disruption of the K+ homeostasis. Finally, we will introduce the concept of hybrid cell death as a result of the Na+ pump failure in cultured cells and the ischemic brain. The goal of this review is to outline recent understanding of the ionic mechanism of ischemic cytoxicity and suggest innovative ideas for future translational research.
Introduction
According to the American Heart Association (AHA) 2013 updated statistics [1], about 795,000 people experience a new or recurrent stroke each year in the United States. This makes stroke one of the leading causes of human death and long-term disability in the US. Similar stroke occurrence and threats to human life and health are seen in other countries. The economic and social burden of stroke is severe in the US and around the world.
Brain ischemia initially causes oxygen and glucose deprivation and relatively acute (from hours to the first 1–3 days) cell death in the region where cerebral blood flow is arrested or severely reduced (≥80% decrease from the baseline level), eventually leading to an infarction core during this time [2]. The remaining tissue within the peri-infarct region (also known as the penumbra) is partially and chronically (days to weeks) injured. Because of the slow time course of cell death and partial tissue damage in this region, the penumbra has been regarded as savable brain tissue and a preferred therapeutic target in neuroprotective treatments. Although this neuroprotective approach has been effective in protecting the gray matter in animal stroke models, none of neuroprotective treatments has been successfully translated into clinical therapy for stroke patients [3]. The underlying reasons for clinical failures may include, but are not limited to, the narrow therapeutic window of the neuroprotective reagents, anatomic and pathophysiological differences between animal and human strokes (such as the lack of focus on white matter damage in rodent models), the narrow focus on only an individual target (a single signaling pathway/receptor/channel or a single gene), and inappropriate selection/control of stroke subtypes in clinical trials [4–7]. Additionally, it is possible that we still have incomplete understanding of ischemic brain damage. For example, the majority of investigations on cell death mechanisms were carried out in cultured cells, while cells in the ischemic brain are injured or die in a more complicated environment and their fate is affected by interplays of multiple regulatory pathways. It is now clear that in addition to neurons, glial cells, endothelia cells and axonal fibers are susceptible to hypoxia and ischemia, and are of equal importance in ischemic brain damage, treatments and functional recovery [8–10]. For example, investigations from Sun's group and others have implicated the Na+-K+-Cl− cotransporter isoform 1 (NKCC1), an electroneutral cotransporter expressed in astrocytes and the blood brain barrier (BBB), in cerebral edema in rodent models of stroke [4–6, 11]. Pharmacological inhibition or genetic deficiency of NKCC1 decreases ischemia-induced cell swelling, BBB breakdown, cerebral edema, and neurotoxicity. A combination of pharmacological strategies that targets receptors/channels as well as membrane transporters might thus prove beneficial for the treatment of cerebral ischemia. More information on the role of NKCC1 in brain edema and damage mechanisms can be found in previous reviews [6, 11].
The progression of cell death is irreversible in the ischemic core without a timely blood reperfusion. A slower cell death occurs in the peri-infarct region due to the presence of collateral blood perfusion. The active cell death mechanisms crossing the ischemic core and penumbra are characterized by a spectrum of time-dependent pathological events that have been described as ion imbalance, excitotoxicity, acidosis, production of reactive oxygen species (ROS), apoptotic cascade activation, and inflammatory activities. A summary of this ischemic cascade along with stroke risk factors and plasticity for recovery can be learned from previous reviews and other reviews in this special issue [12–14]. Here, we concentrate on ionic mechanisms underlying the cellular toxicity during the acute, sub-acute and chronic stages (hours to days and weeks) after a cerebral ischemic insult. A better understanding of these basic cellular and molecular changes is expected to help in translational stroke research.
Cytotoxic cell swelling
Gray and white matters in the brain are active in electrophysiological activities and chemical signal transmission, thus requiring a high consumption of glucose and oxygen to maintain ionic gradients across the cell membrane. A few minutes after occlusion of cerebral blood flow, ischemic brain tissues become deprived of oxygen and glucose, resulting in mitochondrial dysfunction and reduction of ATP synthesis. In this acute phase after ischemia, a rapid cell swelling (cytotoxic edema) occurs due to excessive Na+ and Ca2+ influx via cation channels/receptors and water flood into intracellular space. Facing the challenge of excessive Na+ and Ca2+ influx as well as K+ efflux, Na+/K+-ATPase, which is the major active transporter for Na+ and K+ homeostasis and the main cellular machinery of ATP consumption, is over-activated to keep the ionic gradients in affected cells. Sooner or later in the pathological process, the activity of Na+/K+-ATPase decreased due to ATP depletion and accumulation of injurious factors. In electrophysiological examinations, we were able to directly record the Na+ pump associated membrane current in cortical neurons and demonstrated that the lack of ATP in combination with the production of ROS severely impaired the activity of Na+/K+-ATPase [15].
Intracellular Na+ accumulation may reverse the operational direction of the Na+/Ca2+ exchanger (NCX) to cause a Na+-dependent Ca2+ uptake [16]. NCX are trans-membrane transporters that exchange 3 Na+ for 1 Ca2+ in forward mode (Ca2+ extrusion) or reverse mode (Ca2+ uptake) depending on the ion gradients and membrane potentials. The ischemia-induced reversed operation of the Na+/Ca2+ exchanger is mostly reported in glial cells and other non-neuronal cells, which seems consistent with the observations that early swelling is more prominent in astrocytes and oligodendrocytes than in neurons. In the ischemic cortex of a rat model, astrocytic swelling and fragmentation can be seen 30 min after MCAO while neuronal cells are less injured at this early stage [17]. In the sub-cortical region, swollen oligodendrocytes and astrocytes can be identified in the ischemic core by electron microscopy 30 min after MCAO. Three hours after ischemia, axonal swelling appears, and oligodendrocytes undergo pyknosis; 12–24 hours later, pyknotic oligodendrocytes show necrotic deterioration [17]. Demyelination of axonal fibers occurs 30 min after ischemia and is still seen in white matter injury 12–24 hours later [17]. Evidence from a sciatic nerve ischemic model also shows that swelling is the earliest morphological change in white matter injury [18]. Numerous studies have shown that increased Na+ and Ca2+ influx into cells is responsible for cytotoxic cell swelling and the ensuing acute injury.
It is believed that the reverse mode of the Na+/Ca2+ exchanger in glial cells contribute to Ca2+ uptake under ischemic conditions. In neuronal cells, however, the situation may be different. Using whole-cell recordings, we directly measured the Na+/Ca2+ exchanger current under normal and ischemic conditions. In cortical neurons we showed that exchangers in these cells might concurrently operate in either the forward or the reverse direction, perhaps in different membrane locations. More importantly, cytotoxic glutamate exposure enhanced the exchanger forward current while the reverse activity was inhibited [19]. Consistent to this observation, knockdown of the exchanger NCKX2 resulted in greater [Ca2+]i increases in cortical neurons. Oxygen-glucose deprivation (OGD)-induced cell death in vitro and ischemic infarct formation in a rat stroke model both dramatically increased in the absence of NCKX2 [20]. In NCX3 knockout mice subjected to transient middle cerebral artery occlusion (MCAO), an enlarged area of brain damage was seen compared to wild type mice [21]. Thus it appears that block of the NCX damages neurons while increasing the NCX activity is neuroprotective [22, 23]. These investigations provide specific evidence that after ischemia the neuronal exchangers remains or even increases its forward operation to remove Ca2+ from the intracellular space and therefore it has a neuroprotective role in the ischemic gray matter.
Apoptotic cell shrinkage
Cell volume change is a dynamic process. When cells swell under physiological conditions, a counteracting cellular process of regulatory volume decrease (RVD) is activated to offset the cell volume increase [24]. In sharp contrast to cell volume increase commonly seen in necrotic cell death, apoptosis is characterized by cell volume decrease [24–27]. The cell shrinking process or apoptotic volume decrease (AVD) is a ubiquitous aspect of apoptosis regardless of the cell types involved. Evidence from recent years supports the idea that AVD is an integrated episode of a cell death program that is regulated by specific ion channels and molecules in apoptosis [24, 26, 27]. For example, our early investigations provided the original evidence that apoptotic insults evoked marked upregulation of the delayed rectifier K+ current and excessive K+ efflux [28]. This ionic mechanism is not only responsible for AVD but also constitutes an active early event in the death program ahead of the activation of caspases and nucleases [24, 26, 29, 30]. To some extent, a selective up-regulation of certain K+ channels and excessive K+ efflux in AVD can be regarded as a red flag for cells ready to commit suicide. Thus, an appreciation of ion movement, particularly changes in K+ efflux and intracellular K+ in the dying cell, may hold the key to understanding the ionic mechanism of apoptosis.
Although RVD and AVD are distinct cellular events, they share some common mechanisms; for example both involve K+ efflux and are mediated by activation of certain K+ and/or Cl− flux via Cl− channels [27]. A well-known phenomenon supporting ischemic stimulation of a massive K+ leak from the intracellular space is the elevated extracellular K+ concentration (from ~5 mM raises to over 60 mM) in the ischemic brain [31]. This pathological event acts as the ionic mechanism of “spreading depression” that is widely recognized in the post-ischemic brain and is detrimental to brain tissues [32, 33]. Accompanied with increased extracellular K+, compelling evidence now supports that a marked intracellular K+ loss is a common and critical step leading to cell body shrinkage and apoptotic cell death [28, 34]. In cultured cells, stimulation of K+ efflux and intracellular K+ depletion induces caspase activation, cytochrome c release and DNA degradation. Therefore, under certain circumstances, especially during sub-acute and chronic phases of an ischemic insult, intracellular K+ loss is an ultimate consequence that can lead to activation of apoptotic cascades [29, 35]. This K+-mediated mechanism underlying apoptosis has been demonstrated in neurons and non-neuronal cells after ischemia, hypoxia and many other pathological insults [27, 29, 30, 36, 37] (please see the review from Aizenman’s group in this special issue).
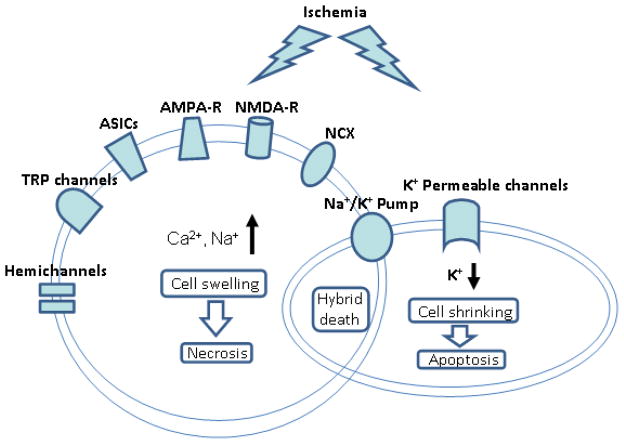
The following paragraphs will introduce membrane molecules (receptors and channels) that can regulate and/or participate in cell volume changes after a hypoxic/ischemic insult (Fig. 1). Many receptors and cation channels have been identified in excitotoxicity for cell swelling. Some may have dual roles in both cell swelling and cell shrinkage, depending on the insult and pathological conditions. For example, although glutamate receptors, including N-methyl-D-aspartate (NMDA) receptors and some α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA)/kainite receptors, are well known for their Ca2+ permeability and responsibility for excitotoxic Ca2+ influx, these non-selective cation receptor channels are also capable of carrying out massive K+ efflux. We have shown that activation of NMDA receptors and even AMAP/kainate receptors in a low Ca2+ condition can lead to significant intracellular K+ reduction (~50% depletion), cell shrinkage and apoptosis [34, 38].
Figure 1. Ionic mechanism of ischemia-induced cell death.
The graphic diagram illustrates a simplified model of ischemia-induced neuronal cell death. Excessive activation of Ca2+ and Na+ permeable channels and receptors leads to intracellular Ca2+ and Na+ accumulation. The resulted cell swelling and destructive consequences are characteristics of necrosis. On the other hand, ischemia can cause over-activation of K+ permeable channels and receptors that mediate pro-apoptotic K+ efflux. The resulted intracellular K+ reduction consequently induces caspase activation and apoptotic cell death. Concurrent activation of these cell death events likely occurs after an ischemic insult and may results in hybrid cell death features in the same cells. The dysfunction of Na+/K+ pump plays a critical role in the development of hybrid cell death. Recent data suggest that excessive autophage also contributes to ischemic cellular damage and is an integrated component of hybrid cell death.
It is worth pointing out that although an excessive K+ efflux occurs as a pro-apoptotic event, the disruption of K+ and Na+ homeostasis can only occur when the Na+/K+-ATPase counterpart mechanism is impaired. We will discuss this issue and a unique hybrid cell death mechanism associated with Na+/K+-ATPase impairment later in this review. In the next few sections, we inspect the most prominent and also some recently identified receptors/channels that mediate excessive Na+ and Ca2+ influx under hypoxic/ischemic conditions (Fig. 1).
Ionotropic glutamate receptors
Glutamate is the primary excitatory neurotransmitter in the brain, playing key roles in synaptic transmission, synaptic plasticity and cell excitotoxicity via activation of glutamate receptors including NMDA, AMPA and kainate receptors. In the ischemic core, sustained cell membrane depolarization causes increased glutamate release and removal of the voltage-dependent Mg2+ block of NMDA receptors. The process of glutamate re-uptake is simultaneously suppressed by ischemia. Accumulation of glutamate in the synaptic cleft and extracellular spaces over-activates ionotropic glutamate receptors including NMDA, AMPA and kainate receptors. It is believed, with some exceptions, that activation of these receptors especially the extrasynaptic NMDA receptors is a major route for excessive Ca2+ influx to trigger cell death [39–42]. The increased intracellular Ca2+ ([Ca2+]i) activates Ca2+-dependent protein kinases and neutral proteases (calpain) that degrade essential proteins in maintenance of cellular and subcellular integrity. This cell death mechanism, known as excitotoxicity, is responsible for acute neuronal death and has been used to guide stroke interventions.
Glutamate-mediated excitotoxicity is also involved in ischemic axonal injury. In axonal damage, activation of AMPA/kainate receptors mediates the excitotoxic effect [43–45]. The AMPA receptor blocker NBQX preserves axonal structure and functional activity in OGD-treated brain slices and is likely due to a secondary effect resulting from protection of oligodendrocytes by NBQX [46]. Blockade of AMPA/Kainate receptors also suppresses OGD-induced Ca2+ entry [47–49]. Aside from in vitro evidence, in situ and in vivo experiments also show that blockade of AMPA/kainate receptors reduces Ca2+-dependent oligodendrocyte death in hypoxic-ischemic acute brain slices and hypoxic-ischemic injury in the developing white matter [46, 50]. Most recent studies show that rat dorsal column axons express glutamate receptor subunit 4 (GluR4) in AMPA receptors and GluR5 and GluR6 in kainate receptors. Application of AMPA/kainate receptor agonists induces progressive elevation of intra-axonal Ca2+ and impairs functional compound action potentials (CAP) in dorsal axons [51, 52].
It has long been thought that the NMDA receptor was not involved in excitotoxic oligodendrocyte death because these cells lack functional expression of NMDA receptors [53–55]. This concept is now challenged by several reports showing the existence of NMDA receptor subunits and their functional expression in mature and immature oligodendrocytes of the cerebellum and corpus callosum. It was shown that activation of NMDA receptors contributes to an ischemia-induced intracellular Ca2+ increase in oligodendrocyte damage [56–58]. Hence, it is likely that NMDA receptors also participate in hypoxic-ischemic injury of oligodendrocytes. The importance of this contribution in ischemic stroke requires further examination in animal experiments and the human brain.
In a retrospective review, glutamate-mediated excitotoxicity was initially investigated in simplified in vitro models and then acute artery ligation animal models. In stroke patients, complex ischemic cascades are activated after years of development of pathphysiological processes prior to the sudden arrest of local blood supply. Although glutamate excitotoxicity is a dominant player in the acute phase of ischemic injury, it may not be responsible for all of the dynamic changes and pathological progression of stroke. Beyond NMDA and AMPA receptors, many other selective and nonselective cation channels have been indicated in the ionic mechanisms responsible for ischemic brain damage [59]. .
Acid-sensing ion channels (ASICs)
Acid-sensing ion channels (ASICs) are members of the degenerin/epithelial Na+ channel (DEG/ENaC) family of cation channels [60, 61]. In the central and peripheral nervous systems, ASICs are gated by extracellular H+ and conduct the acid-evoked inward Na+ currents with a selective sensitivity to amiloride blockade [62–64]. This is particularly important considering that acidosis, e.g. reduction in pH, develops in the ischemic brain region. In ischemic region, deprivation of energy and oxygen leads to an enhanced anaerobic glucose metabolism and accumulation of lactic acid, along with enhanced H+ release from ATP hydrolysis. The pH value in the ischemic core may drop to 6.5 or lower [65]. This acidosis occurs in many pathological conditions such as brain trauma, inflammation, epileptic seizure, multiple sclerosis, and ischemic stroke [66–68]. Electrophysiological characterization reveals a pH50 of 6.18 for acidosis to activate ASIC1a in rodent brain neurons, while the pH50 in human cortical neurons is 6.60 [69].
Molecular cloning has identified six ASIC subunits that arise from four genes: ASIC1a and ASIC1b; ASIC2a and ASIC2b; ASIC3 and ASIC4[66]. Each ASIC protein contains two trans-membrane domains (TM1 and TM2), intracellular C and N terminals, and a large cysteine-rich extracellular loop for sensing protons and other unrecognized ligands [70]. These channels have different pH values of half-maximal activation (pH50); pH5.8–6.8 for ASIC1a, ASIC1b and ASIC3 and pH 4.5–4.9 for ASIC2a. ASIC2b or ASIC4 on their own do not form pH-sensitive homomeric channels [66]. Once activated by extracellular acid, ASICs mediate Na+ influx leading to increased cell excitability and water content. Homomeric ASIC1a channels are also Ca2+ permeable and have been implicated in acidosis-mediated neuronal injury under ischemic conditions [71–73].
Ischemic condition mimicked by OGD increases ASIC-mediated inward currents and decreases desensitization of ASIC activity in cultured neurons [71]. Evidence from Ca2+ imaging experiment shows that acidosis can induce Ca2+ entry via ASIC1a and cytosolic Ca2+ elevation in a glutamate receptor-independent manner. In cultured cortical neurons, ASIC1a activation and its mediated Ca2+ entry are critically involved in acidosis induced, glutamate-independent neuronal damage. Application of ASIC blockers and low Ca2+ significantly prevent this type of injury [74]. In an animal model of ischemic stroke, blockage of ASIC with amiloride or knockout of the ASIC1a gene shows a protective effect by reducing the infarct volume, even where glutamate-mediated excitotoxicity is already suppressed [71]. The therapeutic time frame for ASICs in the stroke brain is much longer than for glutamate receptor antagonists. It can be up to 5 hours and may persist for at least 7 days [71, 75]. Thus, ASICs are identified as a new therapeutic target for ischemic stroke. Some studies have taken the first step to test leading drugs targeting ASICs for stroke treatment [76–79].
Pannexin and connexin hemichannels
There are two major families of proteins, pannexins and connexins contributing to the formation of hemichannels. A Gap junction forms when two opposing connexons or pannexin hemichannels join together between two adjacent cells. Hemichannels in nonjunctional membranes conduct ions and signaling molecules that are smaller than 1 kDa, such as K+, Na+, Ca2+, ATP, glutamate, glucose, NAD+, adenosine and PGE2 [80–82]. Three pannexin members Px1, Px2 and Px3 have been identified in animals and humans. Px1 and Px2 are abundantly expressed in the brain region of hippocampus, neocortex, cerebellum, thalamus and hypothalamus, whereas Px3 is only found in skin and osteoblasts [83]. At the cellular level, Px1 is localized in neurons, astrocytes, micorglia and oligodendrocytes, and Px2 appears only in a subset of GAPDH-positive pyramidal neurons [84–86].
In the central nervous system (CNS), gating of hemichannels is regulated by pH, cations, trans-membrane potential, cytokines/growth factors, neurotransmitters and many other factors [87]. Thus, it is obvious that hemichannels may be involved in trans-membrane signal transduction/transmission and cell-cell communication under physiological and pathological conditions. In glial and neuronal cells, hemichannels have been implicated in cell swelling and disruption of intracellular ionic homeostasis after exposure to hypoxia/ischemia. For example, chemical ischemia increases cell permeability of cortical astrocytes due to opening of Cx43 hemichannels. Na+, Ca2+ and H2O pass through Cx43 hemichannels and causes astrocytic swelling and cell death [88]. Down-regulation of Cx43 function reduced cell swelling and astrocytic death in a rat model of traumatic brain injury [89]. An in vitro experiment performed on acutely isolated hippocampal neurons showed that OGD induced a large inward current at the resting membrane potential [90]. Pharmacological testing and biophysical characterization of this large conductance current indicated that it is mediated by Px1 hemichannels, but not voltage-dependent Na+ channels, glutamate receptor channels, ASICs and TRP channels. It was proposed that Px1 hemichannels are activated under ischemic conditions and mediate up-regulated Ca2+ entry, glucose and ATP efflux, thus contributing to ionic imbalance and cytoxicity. The gap junction/hemichannel blocker carbenoxolone (Cbx) was shown to prevent ischemic insult in stroke models [91, 92]. A double-knockout of Px1 and Px2 in mice leads to smaller infarct size in the ischemic brain [93]. Pannexin or connexin hemichannels are emerging as a new molecular target that has therapeutic potential for protecting brain from ischemic attack.
TRP channels
TRP channels are nonselective cation channels containing six families: TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPP (polycystin), TRPML (mucolipin), and TRPA (ankyrin) [94, 95]. The first type of TRP channel was identified in the Drosophila eye. Now TRP channels are found widely distributed in many organs and tissues of mammals, including the nervous system [96, 97]. The TRP channels function as tetramers and conduct different cations (Na+, K+, and Ca2+ ) when responding to local changes in their environment such as temperature, mechanical pressure, ion changes, pH changes and many other physical and chemical changes. Opening of TRP channels depolarizes the cell membrane and thereby activates voltage-dependent Na+ and Ca2+ channels, leading to intracellular Na+ and Ca2+ accumulation [97]. Therefore, TRP channels are destined to regulate membrane potential and manipulate intracellular Na+, K+ and Ca2+ contents in both excitable and non-excitable cells. Several TRP channel members have been investigated in ischemic/anoxic conditions and implicated in the cytotoxicity of secondary injury.
In order to detect novel ischemic cell death mechanisms beyond glutamate-mediated excitotoxicity, Arts and his colleagues reported that OGD of 1.5 hours or longer evoked an intracellular Ca2+ increase in cultured cortical neurons. This Ca2+ increase cannot be eliminated by the mixture of blockers containing nimodipine, MK801 and CNQX and thus is not mediated by L-type Ca2+ channels, NMDA or AMPA/kainate glutamate receptors [98]. Protection of this anti-excitotoxic combination is confined to 1-hour OGD exposure and allowed intracellular Ca2+ to return close to basal level. Their experimental data suggested that prolonged Ca2+ entry and cytotoxicity mainly resulted from activation of TRPM7 channels. Inhibition of TRPM7 channels by its blockers or siRNA-mediated knockdown significantly prevents [Ca2+]i accumulation during prolonged OGD and protected anoxic neuronal death [98]. It was also showed that TRPM7 channels are activated by ROS from nitric oxide (NO) signaling. Evidence from an in vivo study demonstrated that down-regulation of TRPM7 in rat hippocampus protected neurons against brain ischemia and also preserved behavioral deficits [99]. TRPM7 channels seem to be a potential target for treating cerebral ischemia although the effect of TRPM7 blockers on ischemic infarct formation remains to be verified [99].
TRPM4 channels are also recommended as a mediator for ischemic injury. TRPM4 channels are activated by intracellular Ca2+, closed by ATP, permeable to Na+ ,K+, Cs+ and Li+, but impermeable to Ca2+ [99]. Study of TRPV4 in rat hippocampal astrocytes shows that cerebral hypoxia/ischemia increases TRPV4 expression and its Ca2+ permeability along with the development of astrocytosis [100]. TRPV4 blockade suppresses intracellular Ca2+ elevation and attenuates astroglial reactivity, suggesting a mediating role of TRPV4 in ischemic insult. The role of the TRPC channel family in Ca2+ homeostasis regulation related to cell survival and growth has received more and more attention [101–103]. An in vivo study on TRPC channel knockout mice demonstrated that the activity of TRPC1/4 and TRPC5 channels are involved in pilocarpine-induced seizure and secondary excitotoxicity in the brain [104]. Opening of TRPC1/4 channels contributes to the depolarizing plateau potential in some neurons and TRPC5 is sensitive to and inhibited by intracellular ATP [99]. These studies suggest TRPC1/4 and TRPC5 may play a role in epilepsy and stroke. TRPC6 channels are critical for neuronal survival by mediating the trophic action of brain-derived neurotrophic factor (BNDF) and Ca2+ influx as growth signals [105]. The expression level of TRPC6 protein is much lower than TRPC4 and TRPC5 in rodent brain [99]. A recent study reported that neuronal TRPC6 proteins are degraded in ischemic brain via a NMDA receptor–dependent manner [106]. The authors found that preventing the TRPC6 degradation reduced infarct size and activating TRPC6 channels prevented neuronal death. It was concluded that the TRPC6 channel mediated moderate Ca2+ influx but not Ca2+ overload, which is beneficial for cell survival via activation of the cAMP response element–binding protein (CREB).
Collectively, the paradigm for the role of TRP channels in ischemic stroke is not very clear because of TRP family member diversity, no specific antagonists for distinguishing each member, unrevealed expression patterns in the brain and paradoxical action on intracellular Ca2+ increase. Much more work needs to be done to delineate the significance of TRP channel family members in stroke injury.
KCNQ2/3 channels and apoptosis
In addition to the discussion earlier about NMDA and AMPA receptors, the pro-apoptotic K+ efflux and intracellular K+ loss are most likely mediated via over-activation of selective and non-selective K+ channels [29, 30]. For instance, ischemia associated membrane depolarization activates the delayed rectifier K+ channels such as the Kv2.1 channel, and increased intracellular Ca2+ can activate Ca2+-activated K+ channels [29, 107, 108]. All of these commonly seen events can result in excessive K+ efflux and intracellular K+ depletion [29]. Since our report in 1997 [28], increasing types of K+ channels have been identified as mediators in the pro-apoptotic K+ efflux [29, 36, 109, 110].
Among many K+ channels that may participate in AVD, KCNQ channels, specifically the KCNQ1 channel, in cariomyocytes was shown to mediate the regulatory volume decrease [111]. Based on the expression pattern and the non-inactivating kinetics of the KCNQ channels, we examined the hypothesis that KCNQ2/3 channels, the molecular basis for the M-type K+ current, may contribute to apoptosis in CNS neurons [112]. KCNQ2/3 channel openers N-ethylmaleimide (NEM) and flupirtine caused dose-dependent K+ efflux, intracellular K+ depletion, caspase activation and cell death in hippocampal neurons. The NEM-induced cell death was antagonized by co-applied KCNQ channel inhibitor XE991, or by elevated extracellular K+ concentration. More specifically, expression of KCNQ2 or KCNQ2/3 channels in Chinese hamster ovary (CHO) cells initiated caspase-3 activation. NEM increased the expression of extracellular signal-regulated protein kinases 1 and 2 (ERK1/2), induced mitochondria membrane depolarization, cytochrome c release, formation of the apoptosome complex, and apoptosis-inducing factor (AIF) translocation into the nucleus. All of these events were attenuated by blocking KCNQ2/3 channels [99].
In a study on several Kv7 channels, Gamper et al. showed that oxidative stress markedly enhanced the currents carried by Kv7.2, Kv7.3, Kv7.2/7.3, Kv7.4, and Kv7.5 (corresponding to KCNQ2, KCNQ3, KCNQ2/3, KCNQ4, and KCNQ5, respectively) channels expressed in CHO cells [99]. They further showed that blocking KCNQ channels during a 30-min OGD worsened the outcome of the insult, suggesting a protective action of these channels. This protection from upregulated K+ channel activity during the acute phase of excitotoxicity is well-known. It is due to a hyperpolarization effect that reduces excitability and Ca2+ influx [113]. On the other hand, this protective effect would disappear if persistent channel activation leads to disruption of the K+ homeostasis. The consequences after a longer duration of KCNQ channel activation, however, were not tested in this report.
Na+/K+-ATPase, K+ homeostasis and hybrid cell death
Under physiological conditions when sufficient energy supply is available, up-regulation of K+-permeable channels/receptors may not cause detrimental consequences because K+ efflux can be well balanced by activated Na+/K+-ATPase. Given that energy depletion occurs in ischemia and the key role for Na+/K+-ATPase in ionic homeostasis especially in K+ homeostasis, a Na+/K+-ATPase failure plays a critical role in ischemic brain damage. Indeed, ATP depletion and dysfunction of the Na+ pump are indispensable event in ischemic pathology. Besides its dependence on a supply of energy, our data suggest that Na+/K+-ATPase activity is also highly sensitive to block by ROS production [15]. To better understand the consequence of the Na+ pump failure, we tested an in vitro model of cultured cortical neurons using ouabain as the selective Na+/K+-ATPase inhibitor [99]. Ouabain caused cell swelling as shown by previous investigations after an acute exposure to ouabain [99]. Somewhat unexpected at the time, several hours after initial cell swelling there was marked and enduring cell shrinkage, accompanied by intracellular K+ depletion, cytochrome c release, and caspase-3 activation [114]. Electron microscopic examination revealed the concurrent existence of ultrastructural features of apoptosis and necrosis in the same cells. This mixed cell death was named hybrid death, characterized by concurrent necrotic and apoptotic components in a given cell. According to recent investigations and the ultrastructural features of injured cells, such as the appearance of multiple vacuoles in the cytoplasm, ischemia-induced hybrid cell death may additionally include autophagic cell injury [115–118].
It is apparent that cells in the ischemic brain face insults which are far more complex than the controlled condition in any cytotoxicity experiment in vitro. Thus, it is not surprising that morphological and cellular evidence from in vivo studies often does not support the existence of typical or pure apoptotic alterations that can be seen in cultured cells [119, 120]. In adult rats subjected to focal barrel cortex ischemia, we specifically examined the phenotypes of cell death. Early cell death was detected several hours after ischemia while delayed cell death in the peri-infarct region and secondary cell death in the ventrobasal (VB) thalamus was seen 2–3 days later [99]. TUNEL positive neurons were found in these two regions, but with striking morphological differences, designated as type I and type II TUNEL positive cells. The type I TUNEL positive cells in the ischemic cortex underwent necrotic changes. The type II TUNEL positive cells in the thalamus and the cortex penumbra region underwent a hybrid death, featuring concurrent apoptotic and necrotic alterations in individual cells. This included marked caspase-3 activation, nuclear condensation/fragmentation, swollen cytoplasm, damaged organelles, and deteriorated membranes. Similar mixed cell death was also evident after ischemic stroke in the developing brain of neonatal rats [99]. The concept of mixed or hybrid cell death has gradually gained popularity with varies modifications such as the identification of necroptosis [2, 42, 117, 121–132]. More recent evidence suggests that autophagy may also contribute as a component of hybrid cell death after ischemia [133, 134]. Future investigations are necessary to better understand the interplay between different cell death mechanisms and the implication in the treatment of stroke.
Concluding Remarks
During the acute stage of cerebral ischemia, excessive Na+ and Ca2+ influx and cell swelling mediated by multiple receptors/channels is the earliest evidence of excitotoxicity in neuronal and non-neuronal cells. Recently identified cation channels activated by acidosis and ATP depletion in ischemic conditions have provided new targets for neuroprotective treatments of stroke. However, an efficient treatment for stroke may rely on a combination therapy targeting multiple receptors/ion channels and regulatory mechanisms. In addition to focusing on apoptosis, the existence of hybrid cell death, identified after Na+/K+-ATPase blockade in vitro or energy depletion in vivo, suggests that a more comprehensive approach for ischemic stroke therapy should target the parallel pathways which are involved in the hybrid cell death and promote Na+/K+-ATPase function.
Acknowledgments
This work was supported by NIH grant NS057255, the American Heart Association (AHA) Grant-in-Aid Award GRNT12060222, and AHA Postdoctoral Fellowship (POST12080252).
Footnotes
Compliance with Ethics Requirements
Conflict of Interest:
The two authors receive research grants from NIH and AHA as listed below.
Shan Ping Yu: NIH grant NS057255, the American Heart Association (AHA) Grant-in-Aid Award GRNT12060222.
Mingke Song: AHA Postdoctoral Fellowship (POST12080252).
This is a review article that does not contain any studies with human or animal subjects. The authors assume that all original studies cited in this review followed institutional and national guidelines for the care and use of laboratory animals and guidelines for clinical trials on patients. However, the authors are not responsibility for any violation of the guidelines in the original investigations.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yap E, Tan WL, Ng I, Ng YK. Combinatorial-approached neuroprotection using pan-caspase inhibitor and poly (ADP-ribose) polymerase (PARP) inhibitor following experimental stroke in rats; is there additional benefit? Brain Res. 2008;1195:130–8. doi: 10.1016/j.brainres.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 3.Baille V, Clarke PG, Brochier G, Dorandeu F, Verna JM, Four E, Lallement G, Carpentier P. Soman-induced convulsions: the neuropathology revisited. Toxicology. 2005;215:1–24. doi: 10.1016/j.tox.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 4.Gladstone DJ, Black SE, Hakim AM Heart, and Stroke Foundation of Ontario Centre of Excellence in Stroke, R. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–36. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- 5.Ginsberg MD. Current status of neuroprotection for cerebral ischemia: synoptic overview. Stroke. 2009;40:S111–4. doi: 10.1161/STROKEAHA.108.528877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minnerup J, Sutherland BA, Buchan AM, Kleinschnitz C. Neuroprotection for stroke: current status and future perspectives. Int J Mol Sci. 2012;13:11753–72. doi: 10.3390/ijms130911753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutherland BA, Minnerup J, Balami JS, Arba F, Buchan AM, Kleinschnitz C. Neuroprotection for ischaemic stroke: translation from the bench to the bedside. Int J Stroke. 2012;7:407–18. doi: 10.1111/j.1747-4949.2012.00770.x. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg MP, Ransom BR. New light on white matter. Stroke. 2003;34:330–2. doi: 10.1161/01.str.0000054048.22626.b9. [DOI] [PubMed] [Google Scholar]
- 9.Arakawa S, Wright PM, Koga M, Phan TG, Reutens DC, Lim I, Gunawan MR, Ma H, Perera N, Ly J, Zavala J, Fitt G, Donnan GA. Ischemic thresholds for gray and white matter: a diffusion and perfusion magnetic resonance study. Stroke. 2006;37:1211–6. doi: 10.1161/01.STR.0000217258.63925.6b. [DOI] [PubMed] [Google Scholar]
- 10.Pantoni L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke. 1996;27:1641–6. doi: 10.1161/01.str.27.9.1641. discussion 1647. [DOI] [PubMed] [Google Scholar]
- 11.Di Lisa F. Mitochondrial contribution in the progression of cardiac ischemic injury. IUBMB Life. 2001;52:255–61. doi: 10.1080/15216540152846073. [DOI] [PubMed] [Google Scholar]
- 12.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 13.Xing C, Arai K, Lo EH, Hommel M. Pathophysiologic cascades in ischemic stroke. Int J Stroke. 2012;7:378–85. doi: 10.1111/j.1747-4949.2012.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10:861–72. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- 15.Ramana MV, Nandikar M, Gurav RV, Johny Kumar T, Sanjappa M. Murdannia saddlepeakensis (Commelinaceae) - a new species from Andaman and Nicobar Islands, India. PhytoKeys. 2013:9–15. doi: 10.3897/phytokeys.20.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bkaily G, Jaalouk D, Sader S, Shbaklo H, Pothier P, Jacques D, D'Orleans-Juste P, Cragoe EJ, Jr, Bose R. Taurine indirectly increases [Ca]i by inducing Ca2+ influx through the Na(+)-Ca2+ exchanger. Mol Cell Biochem. 1998;188:187–97. [PubMed] [Google Scholar]
- 17.Caceres JP, Palazzi S, Palazzi JL, Llusa M, MS, SV Dissection of intercostal nerves by means of assisted video thoracoscopy: experimental study. J Brachial Plex Peripher Nerve Inj. 2013;8:3. doi: 10.1186/1749-7221-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nukada H, Dyck PJ. Acute ischemia causes axonal stasis, swelling, attenuation, and secondary demyelination. Ann Neurol. 1987;22:311–8. doi: 10.1002/ana.410220306. [DOI] [PubMed] [Google Scholar]
- 19.Yu SP, Choi DW. Na(+)-Ca2+ exchange currents in cortical neurons: concomitant forward and reverse operation and effect of glutamate. Eur J Neurosci. 1997;9:1273–81. doi: 10.1111/j.1460-9568.1997.tb01482.x. [DOI] [PubMed] [Google Scholar]
- 20.Cuomo O, Gala R, Pignataro G, Boscia F, Secondo A, Scorziello A, Pannaccione A, Viggiano D, Adornetto A, Molinaro P, Li XF, Lytton J, Di Renzo G, Annunziato L. A critical role for the potassium-dependent sodium-calcium exchanger NCKX2 in protection against focal ischemic brain damage. J Neurosci. 2008;28:2053–63. doi: 10.1523/JNEUROSCI.4912-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molinaro P, Cuomo O, Pignataro G, Boscia F, Sirabella R, Pannaccione A, Secondo A, Scorziello A, Adornetto A, Gala R, Viggiano D, Sokolow S, Herchuelz A, Schurmans S, Di Renzo G, Annunziato L. Targeted disruption of Na+/Ca2+ exchanger 3 (NCX3) gene leads to a worsening of ischemic brain damage. J Neurosci. 2008;28:1179–84. doi: 10.1523/JNEUROSCI.4671-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeffs GJ, Meloni BP, Sokolow S, Herchuelz A, Schurmans S, Knuckey NW. NCX3 knockout mice exhibit increased hippocampal CA1 and CA2 neuronal damage compared to wild-type mice following global cerebral ischemia. Exp Neurol. 2008;210:268–73. doi: 10.1016/j.expneurol.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Jeffs GJ, Meloni BP, Bakker AJ, Knuckey NW. The role of the Na(+)/Ca(2+) exchanger (NCX) in neurons following ischaemia. J Clin Neurosci. 2007;14:507–14. doi: 10.1016/j.jocn.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Gomez-Angelats M, Bortner CD, Cidlowski JA. Cell volume regulation in immune cell apoptosis. Cell Tissue Res. 2000;301:33–42. doi: 10.1007/s004410000216. [DOI] [PubMed] [Google Scholar]
- 25.Nunez R, Sancho-Martinez SM, Novoa JM, Lopez-Hernandez FJ. Apoptotic volume decrease as a geometric determinant for cell dismantling into apoptotic bodies. Cell Death Differ. 17:1665–71. doi: 10.1038/cdd.2010.96. [DOI] [PubMed] [Google Scholar]
- 26.Bortner CD, Cidlowski JA. A necessary role for cell shrinkage in apoptosis. Biochem Pharmacol. 1998;56:1549–59. doi: 10.1016/s0006-2952(98)00225-1. [DOI] [PubMed] [Google Scholar]
- 27.Ranjbar E, Shams J, Sabetkasaei M, MMS, Rashidkhani B, Mostafavi A, Bornak E, Nasrollahzadeh J. Effects of zinc supplementation on efficacy of antidepressant therapy, inflammatory cytokines, and brain-derived neurotrophic factor in patients with major depression. Nutr Neurosci. 2013 doi: 10.1179/1476830513Y.0000000066. [DOI] [PubMed] [Google Scholar]
- 28.Rich A, Gordon S, Brown C, Gibbons SJ, Schaefer K, Hennig G, Farrugia G. Kit signaling is required for development of coordinated motility patterns in zebrafish gastrointestinal tract. Zebrafish. 2013;10:154–60. doi: 10.1089/zeb.2012.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Oliveira Otto MC, Nettleton JA, Lemaitre RN, LMS, Kromhout D, Rich SS, MYT, Jacobs DR, Mozaffarian D. Biomarkers of dairy Fatty acids and risk of cardiovascular disease in the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2013;2:e000092. doi: 10.1161/JAHA.113.000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Remillard CV, Yuan JX. Activation of K+ channels: an essential pathway in programmed cell death. Am J Physiol Lung Cell Mol Physiol. 2004;286:L49–67. doi: 10.1152/ajplung.00041.2003. [DOI] [PubMed] [Google Scholar]
- 31.Xie Y, Zacharias E, Hoff P, Tegtmeier F. Ion channel involvement in anoxic depolarization induced by cardiac arrest in rat brain. J Cereb Blood Flow Metab. 1995;15:587–94. doi: 10.1038/jcbfm.1995.72. [DOI] [PubMed] [Google Scholar]
- 32.Somjen GG. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol Rev. 2001;81:1065–96. doi: 10.1152/physrev.2001.81.3.1065. [DOI] [PubMed] [Google Scholar]
- 33.Nedergaard M, Hansen AJ. Characterization of cortical depolarizations evoked in focal cerebral ischemia. J Cereb Blood Flow Metab. 1993;13:568–74. doi: 10.1038/jcbfm.1993.74. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Lee TH, Davidson C, Lazarus C, Wetsel WC, Ellinwood EH. Reversal of cocaine-induced behavioral sensitization and associated phosphorylation of the NR2B and GluR1 subunits of the NMDA and AMPA receptors. Neuropsychopharmacology. 2007;32:377–87. doi: 10.1038/sj.npp.1301101. [DOI] [PubMed] [Google Scholar]
- 35.GN, PVA, ARS, RR, MS, RS Role of TSH on Urinary Calcium Excretion In Post Menopausal Women of South Indian Population. J Clin Diagn Res. 2013;7:1099–101. doi: 10.7860/JCDR/2013/5290.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burg ED, Remillard CV, Yuan JX. Potassium channels in the regulation of pulmonary artery smooth muscle cell proliferation and apoptosis: pharmacotherapeutic implications. Br J Pharmacol. 2008;153 (Suppl 1):S99–S111. doi: 10.1038/sj.bjp.0707635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hughes FM, Jr, Cidlowski JA. Potassium is a critical regulator of apoptotic enzymes in vitro and in vivo. Adv Enzyme Regul. 1999;39:157–71. doi: 10.1016/s0065-2571(98)00010-7. [DOI] [PubMed] [Google Scholar]
- 38.Xiao AY, Homma M, Wang XQ, Wang X, Yu SP. Role of K(+) efflux in apoptosis induced by AMPA and kainate in mouse cortical neurons. Neuroscience. 2001;108:61–7. doi: 10.1016/s0306-4522(01)00394-3. [DOI] [PubMed] [Google Scholar]
- 39.Choi DW. Possible mechanisms limiting N-methyl-D-aspartate receptor overactivation and the therapeutic efficacy of N-methyl-D-aspartate antagonists. Stroke. 1990;21:III20–2. [PubMed] [Google Scholar]
- 40.Kwak S, Weiss JH. Calcium-permeable AMPA channels in neurodegenerative disease and ischemia. Curr Opin Neurobiol. 2006;16:281–7. doi: 10.1016/j.conb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Stanika RI, Pivovarova NB, Brantner CA, Watts CA, Winters CA, Andrews SB. Coupling diverse routes of calcium entry to mitochondrial dysfunction and glutamate excitotoxicity. Proc Natl Acad Sci U S A. 2009;106:9854–9. doi: 10.1073/pnas.0903546106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodriguez IA, Fernandez-Segura E, Ceballos G, Arrebola F, del Carmen Sanchez-Quevedo M, Campos A. Hybrid cell death induced by exposure to 2-hydroxyethyl methacrylate (HEMA): an ultrastructural and X-ray microanalytical study. J Adhes Dent. 2008;10:105–11. [PubMed] [Google Scholar]
- 43.Matute C. Characteristics of acute and chronic kainate excitotoxic damage to the optic nerve. Proc Natl Acad Sci U S A. 1998;95:10229–34. doi: 10.1073/pnas.95.17.10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li S, Stys PK. Mechanisms of ionotropic glutamate receptor-mediated excitotoxicity in isolated spinal cord white matter. J Neurosci. 2000;20:1190–8. doi: 10.1523/JNEUROSCI.20-03-01190.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Domercq M, Etxebarria E, Perez-Samartin A, Matute C. Excitotoxic oligodendrocyte death and axonal damage induced by glutamate transporter inhibition. Glia. 2005;52:36–46. doi: 10.1002/glia.20221. [DOI] [PubMed] [Google Scholar]
- 46.Tekkok SB, Goldberg MP. Ampa/kainate receptor activation mediates hypoxic oligodendrocyte death and axonal injury in cerebral white matter. J Neurosci. 2001;21:4237–48. doi: 10.1523/JNEUROSCI.21-12-04237.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshioka A, Hardy M, Younkin DP, Grinspan JB, Stern JL, Pleasure D. Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptors mediate excitotoxicity in the oligodendroglial lineage. J Neurochem. 1995;64:2442–8. doi: 10.1046/j.1471-4159.1995.64062442.x. [DOI] [PubMed] [Google Scholar]
- 48.Sanchez-Gomez MV, Matute C. AMPA and kainate receptors each mediate excitotoxicity in oligodendroglial cultures. Neurobiol Dis. 1999;6:475–85. doi: 10.1006/nbdi.1999.0264. [DOI] [PubMed] [Google Scholar]
- 49.McDonald JW, Althomsons SP, Hyrc KL, Choi DW, Goldberg MP. Oligodendrocytes from forebrain are highly vulnerable to AMPA/kainate receptor-mediated excitotoxicity. Nat Med. 1998;4:291–7. doi: 10.1038/nm0398-291. [DOI] [PubMed] [Google Scholar]
- 50.Follett PL, Rosenberg PA, Volpe JJ, Jensen FE. NBQX attenuates excitotoxic injury in developing white matter. J Neurosci. 2000;20:9235–41. doi: 10.1523/JNEUROSCI.20-24-09235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ouardouz M, Coderre E, Basak A, Chen A, Zamponi GW, Hameed S, Rehak R, Yin X, Trapp BD, Stys PK. Glutamate receptors on myelinated spinal cord axons: I. GluR6 kainate receptors. Ann Neurol. 2009;65:151–9. doi: 10.1002/ana.21533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ouardouz M, Coderre E, Zamponi GW, Hameed S, Yin X, Trapp BD, Stys PK. Glutamate receptors on myelinated spinal cord axons: II. AMPA and GluR5 receptors. Ann Neurol. 2009;65:160–6. doi: 10.1002/ana.21539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berger T, Walz W, Schnitzer J, Kettenmann H. GABA- and glutamate-activated currents in glial cells of the mouse corpus callosum slice. J Neurosci Res. 1992;31:21–7. doi: 10.1002/jnr.490310104. [DOI] [PubMed] [Google Scholar]
- 54.Patneau DK, Wright PW, Winters C, Mayer ML, Gallo V. Glial cells of the oligodendrocyte lineage express both kainate- and AMPA-preferring subtypes of glutamate receptor. Neuron. 1994;12:357–71. doi: 10.1016/0896-6273(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 55.Liu HN, Almazan G. Glutamate induces c-fos proto-oncogene expression and inhibits proliferation in oligodendrocyte progenitors: receptor characterization. Eur J Neurosci. 1995;7:2355–63. doi: 10.1111/j.1460-9568.1995.tb01032.x. [DOI] [PubMed] [Google Scholar]
- 56.Karadottir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–6. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438:1167–71. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- 58.Micu I, Jiang Q, Coderre E, Ridsdale A, Zhang L, Woulfe J, Yin X, Trapp BD, McRory JE, Rehak R, Zamponi GW, Wang W, Stys PK. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature. 2006;439:988–92. doi: 10.1038/nature04474. [DOI] [PubMed] [Google Scholar]
- 59.Besancon E, Guo S, Lok J, Tymianski M, Lo EH. Beyond NMDA and AMPA glutamate receptors: emerging mechanisms for ionic imbalance and cell death in stroke. Trends Pharmacol Sci. 2008;29:268–75. doi: 10.1016/j.tips.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 60.Price MP, Snyder PM, Welsh MJ. Cloning and expression of a novel human brain Na+ channel. J Biol Chem. 1996;271:7879–82. doi: 10.1074/jbc.271.14.7879. [DOI] [PubMed] [Google Scholar]
- 61.Chen CC, England S, Akopian AN, Wood JN. A sensory neuron-specific, proton-gated ion channel. Proc Natl Acad Sci U S A. 1998;95:10240–5. doi: 10.1073/pnas.95.17.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Price MP, Lewin GR, McIlwrath SL, Cheng C, Xie J, Heppenstall PA, Stucky CL, Mannsfeldt AG, Brennan TJ, Drummond HA, Qiao J, Benson CJ, Tarr DE, Hrstka RF, Yang B, Williamson RA, Welsh MJ. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature. 2000;407:1007–11. doi: 10.1038/35039512. [DOI] [PubMed] [Google Scholar]
- 63.Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH, Jr, Welsh MJ. The acid-activated ion channel ASIC contributes to synaptic plasticity learning and memory. Neuron. 2002;34:463–77. doi: 10.1016/s0896-6273(02)00661-x. [DOI] [PubMed] [Google Scholar]
- 64.Wemmie JA, Askwith CC, Lamani E, Cassell MD, Freeman JH, Jr, Welsh MJ. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J Neurosci. 2003;23 :5496–502. doi: 10.1523/JNEUROSCI.23-13-05496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nedergaard M, Kraig RP, Tanabe J, Pulsinelli WA. Dynamics of interstitial and intracellular pH in evolving brain infarct. Am J Physiol. 1991;260:R581–8. doi: 10.1152/ajpregu.1991.260.3.R581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wemmie JA, Taugher RJ, Kreple CJ. Acid-sensing ion channels in pain and disease. Nat Rev Neurosci. 2013;14:461–471. doi: 10.1038/nrn3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vergo S, Craner MJ, Etzensperger R, Attfield K, Friese MA, Newcombe J, Esiri M, Fugger L. Acid-sensing ion channel 1 is involved in both axonal injury and demyelination in multiple sclerosis and its animal model. Brain. 2011;134:571–84. doi: 10.1093/brain/awq337. [DOI] [PubMed] [Google Scholar]
- 68.Marmarou A. Intracellular acidosis in human and experimental brain injury. J Neurotrauma. 1992;9 (Suppl 2):S551–62. [PubMed] [Google Scholar]
- 69.Li M, Inoue K, Branigan D, Kratzer E, Hansen JC, Chen JW, Simon RP, Xiong ZG. Acid-sensing ion channels in acidosis-induced injury of human brain neurons. J Cereb Blood Flow Metab. 2010;30:1247–60. doi: 10.1038/jcbfm.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29:578–86. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 71.Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–98. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 72.Gao J, Duan B, Wang DG, Deng XH, Zhang GY, Xu L, Xu TL. Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron. 2005;48:635–46. doi: 10.1016/j.neuron.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 73.Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci. 2001;4:869–70. doi: 10.1038/nn0901-869. [DOI] [PubMed] [Google Scholar]
- 74.Wang WZ, Chu XP, Li MH, Seeds J, Simon RP, Xiong ZG. Modulation of acid-sensing ion channel currents, acid-induced increase of intracellular Ca2+, and acidosis-mediated neuronal injury by intracellular pH. J Biol Chem. 2006;281:29369–78. doi: 10.1074/jbc.M605122200. [DOI] [PubMed] [Google Scholar]
- 75.Pignataro G, Simon RP, Xiong ZG. Prolonged activation of ASIC1a and the time window for neuroprotection in cerebral ischaemia. Brain. 2007;130:151–8. doi: 10.1093/brain/awl325. [DOI] [PubMed] [Google Scholar]
- 76.Gu L, Yang Y, Sun Y, Zheng X. Puerarin inhibits acid-sensing ion channels and protects against neuron death induced by acidosis. Planta Med. 2010;76 :583–8. doi: 10.1055/s-0029-1240583. [DOI] [PubMed] [Google Scholar]
- 77.Chang Y, Hsieh CY, Peng ZA, Yen TL, Hsiao G, Chou DS, Chen CM, Sheu JR. Neuroprotective mechanisms of puerarin in middle cerebral artery occlusion-induced brain infarction in rats. J Biomed Sci. 2009;16:9. doi: 10.1186/1423-0127-16-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Y, Zhou L, Zhang X, Bai J, Shi M, Zhao G. Ginsenoside-Rd attenuates TRPM7 and ASIC1a but promotes ASIC2a expression in rats after focal cerebral ischemia. Neurol Sci. 2012;33:1125–31. doi: 10.1007/s10072-011-0916-6. [DOI] [PubMed] [Google Scholar]
- 79.Yifeng M, Bin W, Weiqiao Z, Yongming Q, Bing L, Xiaojie L. Neuroprotective effect of sophocarpine against transient focal cerebral ischemia via down-regulation of the acid-sensing ion channel 1 in rats. Brain Res. 2011;1382:245–51. doi: 10.1016/j.brainres.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 80.Bennett MV, Barrio LC, Bargiello TA, Spray DC, Hertzberg E, Saez JC. Gap junctions: new tools, new answers, new questions. Neuron. 1991;6 :305–20. doi: 10.1016/0896-6273(91)90241-q. [DOI] [PubMed] [Google Scholar]
- 81.Evans WH, De Vuyst E, Leybaert L. The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochem J. 2006;397:1–14. doi: 10.1042/BJ20060175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hansen KA, Torborg CL, Elstrott J, Feller MB. Expression and function of the neuronal gap junction protein connexin 36 in developing mammalian retina. J Comp Neurol. 2005;493:309–20. doi: 10.1002/cne.20759. [DOI] [PubMed] [Google Scholar]
- 83.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A. 2003;100:13644–9. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ray A, Zoidl G, Weickert S, Wahle P, Dermietzel R. Site-specific and developmental expression of pannexin1 in the mouse nervous system. Eur J Neurosci. 2005;21:3277–90. doi: 10.1111/j.1460-9568.2005.04139.x. [DOI] [PubMed] [Google Scholar]
- 85.Zoidl G, Petrasch-Parwez E, Ray A, Meier C, Bunse S, Habbes HW, Dahl G, Dermietzel R. Localization of the pannexin1 protein at postsynaptic sites in the cerebral cortex and hippocampus. Neuroscience. 2007;146:9–16. doi: 10.1016/j.neuroscience.2007.01.061. [DOI] [PubMed] [Google Scholar]
- 86.Vogt A, Hormuzdi SG, Monyer H. Pannexin1 and Pannexin2 expression in the developing and mature rat brain. Brain Res Mol Brain Res. 2005;141:113–20. doi: 10.1016/j.molbrainres.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 87.Orellana JA, Martinez AD, Retamal MA. Gap junction channels and hemichannels in the CNS: Regulation by signaling molecules. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 88.Kimelberg HK, Sankar P, O'Connor ER, Jalonen T, Goderie SK. Functional consequences of astrocytic swelling. Prog Brain Res. 1992;94:57–68. doi: 10.1016/s0079-6123(08)61739-2. [DOI] [PubMed] [Google Scholar]
- 89.Wu Z, Xu H, He Y, Yang G, Liao C, Gao W, Liang M, He X. Antisense oligodeoxynucleotides targeting connexin43 reduce cerebral astrocytosis and edema in a rat model of traumatic brain injury. Neurol Res. 2013;35 :255–62. doi: 10.1179/1743132813Y.0000000165. [DOI] [PubMed] [Google Scholar]
- 90.Thompson RJ, Zhou N, MacVicar BA. Ischemia opens neuronal gap junction hemichannels. Science. 2006;312:924–7. doi: 10.1126/science.1126241. [DOI] [PubMed] [Google Scholar]
- 91.Takeuchi H, Jin S, Suzuki H, Doi Y, Liang J, Kawanokuchi J, Mizuno T, Sawada M, Suzumura A. Blockade of microglial glutamate release protects against ischemic brain injury. Exp Neurol. 2008;214:144–6. doi: 10.1016/j.expneurol.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 92.de Pina-Benabou MH, Szostak V, Kyrozis A, Rempe D, Uziel D, Urban-Maldonado M, Benabou S, Spray DC, Federoff HJ, Stanton PK, Rozental R. Blockade of gap junctions in vivo provides neuroprotection after perinatal global ischemia. Stroke. 2005;36:2232–7. doi: 10.1161/01.STR.0000182239.75969.d8. [DOI] [PubMed] [Google Scholar]
- 93.Bargiotas P, Krenz A, Hormuzdi SG, Ridder DA, Herb A, Barakat W, Penuela S, von Engelhardt J, Monyer H, Schwaninger M. Pannexins in ischemia-induced neurodegeneration. Proc Natl Acad Sci U S A. 2011;108:20772–7. doi: 10.1073/pnas.1018262108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Owsianik G, D'Hoedt D, Voets T, Nilius B. Structure-function relationship of the TRP channel superfamily. Rev Physiol Biochem Pharmacol. 2006;156:61–90. [PubMed] [Google Scholar]
- 95.Latorre R. Perspectives on TRP channel structure and the TRPA1 puzzle. J Gen Physiol. 2009;133:227–9. doi: 10.1085/jgp.200910199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Minke B. Drosophila mutant with a transducer defect. Biophys Struct Mech. 1977;3 :59–64. doi: 10.1007/BF00536455. [DOI] [PubMed] [Google Scholar]
- 97.Abramowitz J, Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 2009;23:297–328. doi: 10.1096/fj.08-119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–77. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- 99.!!! INVALID CITATION !!!
- 100.Butenko O, Dzamba D, Benesova J, Honsa P, Benfenati V, Rusnakova V, Ferroni S, Anderova M. The increased activity of TRPV4 channel in the astrocytes of the adult rat hippocampus after cerebral hypoxia/ischemia. PLoS One. 2012;7:e39959. doi: 10.1371/journal.pone.0039959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ding X, He Z, Zhou K, Cheng J, Yao H, Lu D, Cai R, Jin Y, Dong B, Xu Y, Wang Y. Essential role of TRPC6 channels in G2/M phase transition and development of human glioma. J Natl Cancer Inst. 2010;102:1052–68. doi: 10.1093/jnci/djq217. [DOI] [PubMed] [Google Scholar]
- 102.Tai Y, Feng S, Ge R, Du W, Zhang X, He Z, Wang Y. TRPC6 channels promote dendritic growth via the CaMKIV-CREB pathway. J Cell Sci. 2008;121:2301–7. doi: 10.1242/jcs.026906. [DOI] [PubMed] [Google Scholar]
- 103.Chigurupati S, Venkataraman R, Barrera D, Naganathan A, Madan M, Paul L, Pattisapu JV, Kyriazis GA, Sugaya K, Bushnev S, Lathia JD, Rich JN, Chan SL. Receptor channel TRPC6 is a key mediator of Notch-driven glioblastoma growth and invasiveness. Cancer Res. 2010;70:418–27. doi: 10.1158/0008-5472.CAN-09-2654. [DOI] [PubMed] [Google Scholar]
- 104.Phelan KD, Shwe UT, Abramowitz J, Wu H, Rhee SW, Howell MD, Gottschall PE, Freichel M, Flockerzi V, Birnbaumer L, Zheng F. Canonical transient receptor channel 5 (TRPC5) and TRPC1/4 contribute to seizure and excitotoxicity by distinct cellular mechanisms. Mol Pharmacol. 2013;83:429–38. doi: 10.1124/mol.112.082271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jia Y, Zhou J, Tai Y, Wang Y. TRPC channels promote cerebellar granule neuron survival. Nat Neurosci. 2007;10:559–67. doi: 10.1038/nn1870. [DOI] [PubMed] [Google Scholar]
- 106.Du W, Huang J, Yao H, Zhou K, Duan B, Wang Y. Inhibition of TRPC6 degradation suppresses ischemic brain damage in rats. J Clin Invest. 2010;120:3480–92. doi: 10.1172/JCI43165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pal S, Hartnett KA, Nerbonne JM, Levitan ES, Aizenman E. Mediation of neuronal apoptosis by Kv2.1-encoded potassium channels. J Neurosci. 2003;23:4798–802. doi: 10.1523/JNEUROSCI.23-12-04798.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Redman PT, He K, Hartnett KA, Jefferson BS, Hu L, Rosenberg PA, Levitan ES, Aizenman E. Apoptotic surge of potassium currents is mediated by p38 phosphorylation of Kv2.1. Proc Natl Acad Sci U S A. 2007;104:3568–73. doi: 10.1073/pnas.0610159104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jehle J, Schweizer PA, Katus HA, Thomas D. Novel roles for hERG K(+) channels in cell proliferation and apoptosis. Cell Death Dis. 2:e193. doi: 10.1038/cddis.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jessica Chen M, Sepramaniam S, Armugam A, Shyan Choy M, Manikandan J, Melendez AJ, Jeyaseelan K, Sang Cheung N. Water and ion channels: crucial in the initiation and progression of apoptosis in central nervous system? Curr Neuropharmacol. 2008;6:102–16. doi: 10.2174/157015908784533879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Calloe K, Nielsen MS, Grunnet M, Schmitt N, Jorgensen NK. KCNQ channels are involved in the regulatory volume decrease response in primary neonatal rat cardiomyocytes. Biochim Biophys Acta. 2007;1773:764–73. doi: 10.1016/j.bbamcr.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 112.Zhou X, Wei J, Song M, Francis K, Yu SP. Novel role of KCNQ2/3 channels in regulating neuronal cell viability. Cell Death Differ. 18:493–505. doi: 10.1038/cdd.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Weston AH, Edwards G. Recent progress in potassium channel opener pharmacology. Biochem Pharmacol. 1992;43:47–54. doi: 10.1016/0006-2952(92)90659-7. [DOI] [PubMed] [Google Scholar]
- 114.Gensini GF, Simone I, Pantoni L, Inzitari D. Large trials in the secondary prevention of stroke. Ann Ital Med Int. 1996;11 (Suppl 2):102S–109S. [PubMed] [Google Scholar]
- 115.Wei N, Yu SP, Gu XH, Chen DD, Whalin MK, Xu GL, Liu XF, Wei L. The Involvement of Autophagy Pathway in Exaggerated Ischemic Brain Damage in Diabetic Mice. CNS Neurosci Ther. 2013 doi: 10.1111/cns.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yacobi-Sharon K, Namdar Y, Arama E. Alternative germ cell death pathway in Drosophila involves HtrA2/Omi, lysosomes, and a caspase-9 counterpart. Dev Cell. 2013;25:29–42. doi: 10.1016/j.devcel.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 117.Chaabane W, User SD, El-Gazzah M, Jaksik R, Sajjadi E, Rzeszowska-Wolny J, Los MJ. Autophagy, apoptosis, mitoptosis and necrosis: interdependence between those pathways and effects on cancer. Arch Immunol Ther Exp (Warsz) 2013;61:43–58. doi: 10.1007/s00005-012-0205-y. [DOI] [PubMed] [Google Scholar]
- 118.Puyal J, Ginet V, Grishchuk Y, Truttmann AC, Clarke PG. Neuronal autophagy as a mediator of life and death: contrasting roles in chronic neurodegenerative and acute neural disorders. Neuroscientist. 2012;18:224–36. doi: 10.1177/1073858411404948. [DOI] [PubMed] [Google Scholar]
- 119.Pantoni L, Moretti M, Inzitari D. The first Italian report on "Binswanger's disease". Ital J Neurol Sci. 1996;17:367–70. doi: 10.1007/BF01999900. [DOI] [PubMed] [Google Scholar]
- 120.Pantoni L, Garcia JH, Brown GG. Vascular pathology in three cases of progressive cognitive deterioration. J Neurol Sci. 1996;135:131–9. doi: 10.1016/0022-510x(95)00273-5. [DOI] [PubMed] [Google Scholar]
- 121.Linkermann A, Hackl MJ, Kunzendorf U, Walczak H, Krautwald S, Jevnikar AM. Necroptosis in Immunity and Ischemia-Reperfusion Injury. Am J Transplant. 2013 doi: 10.1111/ajt.12448. [DOI] [PubMed] [Google Scholar]
- 122.Tischner D, Manzl C, Soratroi C, Villunger A, Krumschnabel G. Necrosis-like death can engage multiple pro-apoptotic Bcl-2 protein family members. Apoptosis. 2012;17:1197–209. doi: 10.1007/s10495-012-0756-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gong J, Kumar SA, Graham G, Kumar AP. FLIP: Molecular Switch Between Apoptosis and Necroptosis. Mol Carcinog. 2013 doi: 10.1002/mc.22027. [DOI] [PubMed] [Google Scholar]
- 124.Bonnet MC, Bagot M, Bensussan A. Apoptotic and necroptotic cell death in cutaneous inflammation. Eur J Dermatol. 2013 doi: 10.1684/ejd.2013.1977. [DOI] [PubMed] [Google Scholar]
- 125.Chan FK. Fueling the flames: Mammalian programmed necrosis in inflammatory diseases. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dickens LS, Powley IR, Hughes MA, MacFarlane M. The 'complexities' of life and death: death receptor signalling platforms. Exp Cell Res. 2012;318:1269–77. doi: 10.1016/j.yexcr.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 127.Oerlemans MI, Koudstaal S, Chamuleau SA, de Kleijn DP, Doevendans PA, Sluijter JP. Targeting cell death in the reperfused heart: pharmacological approaches for cardioprotection. Int J Cardiol. 2013;165:410–22. doi: 10.1016/j.ijcard.2012.03.055. [DOI] [PubMed] [Google Scholar]
- 128.Han J, Zhong CQ, Zhang DW. Programmed necrosis: backup to and competitor with apoptosis in the immune system. Nat Immunol. 2011;12:1143–9. doi: 10.1038/ni.2159. [DOI] [PubMed] [Google Scholar]
- 129.Vanlangenakker N, Vanden Berghe T, Vandenabeele P. Many stimuli pull the necrotic trigger, an overview. Cell Death Differ. 2012;19:75–86. doi: 10.1038/cdd.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Galluzzi L, Vanden Berghe T, Vanlangenakker N, Buettner S, Eisenberg T, Vandenabeele P, Madeo F, Kroemer G. Programmed necrosis from molecules to health and disease. Int Rev Cell Mol Biol. 2011;289:1–35. doi: 10.1016/B978-0-12-386039-2.00001-8. [DOI] [PubMed] [Google Scholar]
- 131.Smith CC, Yellon DM. Necroptosis, necrostatins and tissue injury. J Cell Mol Med. 2011;15:1797–806. doi: 10.1111/j.1582-4934.2011.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev. 2007;54:34–66. doi: 10.1016/j.brainresrev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 133.Webster KA. Mitochondrial membrane permeabilization and cell death during myocardial infarction: roles of calcium and reactive oxygen species. Future Cardiol. 2012;8:863–84. doi: 10.2217/fca.12.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xu M, Zhang HL. Death and survival of neuronal and astrocytic cells in ischemic brain injury: a role of autophagy. Acta Pharmacol Sin. 2011;32:1089–99. doi: 10.1038/aps.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]