Abstract
Objectives
This study evaluated the effect of three antioxidizing agents on pull-out bond strengths of dentin treated with sodium hypochlorite.
Materials and Methods
Root canals of 75 single-rooted human teeth were prepared. Fifteen teeth were irrigated with normal saline for a negative control group, and the remaining 60 teeth (groups 2 - 5) with 2.5% NaOCl. The teeth in group 2 served as a positive control. Prior to post cementation, the root canals in groups 3 - 5 were irrigated with three antioxidizing agents including 10% rosmarinic acid (RA, Baridge essence), 10% hesperidin (HPN, Sigma), and 10% sodium ascorbate hydrogel (SA, AppliChem). Seventy-five spreaders (#55, taper .02, Produits Dentaires S.A) were coated with silica and silanized with the Rocatec system and ceramic bond. All the prepared spreaders were cemented with a self-adhesive resin cement (Bifix SE, Voco Gmbh) in the prepared canals. After storage in distilled water (24 h/37℃), the spreaders were pulled out in a universal testing machine at a crosshead speed of 1.0 mm/min. Pull-out strength values were analyzed by one-way ANOVA and Tukey's HSD test (α = 0.05).
Results
There were significant differences between study groups (p = 0.016). The highest pull-out strength was related to the SA group. The lowest strength was obtained in the positive control group.
Conclusions
Irrigation with NaOCl during canal preparation decreased bond strength of resin cement to root dentin. Amongst the antioxidants tested, SA had superior results in reversing the diminishing effect of NaOCl irrigation on the bond strength to root dentin.
Keywords: Antioxidant, Bond strength, Endodontically treated teeth, Resin cement
Introduction
Root-filled teeth can be reconstructed by direct or indirect restorative techniques. Teeth with extensive loss of tooth structure require intracanal posts for adequate retention of the restoration.1 Post retention is essential for the long-term survival of the restored tooth.2
Clinical studies have shown successful results with the use of carbon-, glass-, and quartz fiber-reinforced resin posts (FRC) with a success rate range of 95 - 97%.3,4 However, there are some reports about failures, including failure of the cement-FRC-core complex with debonding of the resin cement from dentin.5 In other words, debonding of the resin cement from root dentin is rather common. According to previous studies, hybrid layer decomposition has a deleterious effect on the resin bond to dentin, resulting in the failure of the adhesive restoration.6 This disintegration, as a result of hydrolytic degradation of both the resin and collagen fibrils, exposes some collagen fibrils, making them available for the enzymatic degradation of the host's dentin matrix metalloproteinases (MMPs).7,8,9 The dentin MMPs are activated by the acidic nature of adhesive systems and resin cements.6
Different techniques have been proposed to prevent collagen fibril degradation and preserve resin-dentin bond integrity. One technique consists of inhibiting the proteolytic activity of sound and carious dentin because the organic matrix of dentin collagen might be degraded by MMPs found in dentin and saliva.6,10 Chlorhexidine (CHX), a potent non-specific MMP-inhibitor, prevents hybrid layer degradation.6,11
Another technique consists of the application of cross-linking agents to render collagen resistant to degradation. Cross-linking agents, such as tannic acid, decrease the enzymatic degradation rate of collagen and increase dentin mechanical properties.12 In addition, glutaraldehyde (GA) and grape seed extract (GSE), as cross-linking agents, increase collagen stability and bond strength.13,14 Epigallaocatechin gallate (EGCG), the most abundant catechin in green tea, decreases dentin demineralization, possibly by inhibiting MMPs.15,16
Some plant-derived antioxidants inhibit MMPs.17 A number of natural flavonoids, such as GSE and hesperidin (HPN), have been used on root caries to study their cross-linking effects on demineralized lesions in order to render collagen matrix more stable.18 Flavonoids are polyphenolic compounds in plant-derived food products. More than 4,000 flavonoids have been identified, each with unique structural properties.19
HPN, a citrus flavonoid, is an extract of citrus fruits, with medical benefits ranging from antioxidation to anti-inflammation to anti-carcinogenicity. In addition, HPN affects bone formation.18,20 Islam et al. applied HPN to tooth structures in an in vitro caries model and concluded that its cross-linking properties might inhibit degradation of collagen and demineralization of bovine root dentin.18
A recent study on HPN showed that it protects bovine dentin collagen against proteolytic activities.18 Moreover, HPN prevents demineralization of dentin by acid and has the capacity to help remineralize dentin.21,22 In another study, the effect of HPN on root dentin was confirmed in relation to its capacity to inhibit collagen degradation and arrest demineralization. Citrus flavonoids contain antioxidative, anti-inflammatory, anticarcinogenic, and hypoglycemic properties and preserve bone.23,24,25,26,27,28 HPN arrests demineralization and might enhance remineralization processes even when fluoride is absent.21,29 Incorporation of HPN into Clearfil SE primer in a recent study improved immediate µTBS and the mechanical properties of the resin-dentin interface.29
In addition, rosemary extract (rosmarinic acid, RA), which contains p-toluenesulfinic acid sodium salt, is a potent antioxidizing agent and MMP inhibitor.30,31,32,33 It improves compromised bond strength to NaOCl-treated dentin and might stabilize the resin-dentin interface due to its antioxidative and MMP-inhibiting capacities. Commercially, a product referred to as Accel (Sun Medical Co. Ltd., Kyoto, Japan), which contains p-toluenesulfinic acid sodium salt, has been marketed as a pretreatment agent for adhesive root canal sealers to decrease or eliminate the oxidative effect of irrigation with NaOCl.32 Moreover, p-toluenesulfinic acid sodium salt can accelerate polymerization of composite resin.33 Based on previous reports, application of Accel for 30 seconds improves bond strength to normal dentin and caries-affected dentin treated with NaOCl.34,35
Several recent studies have shown the effect of sodium ascorbate (SA) on improving the compromised bond strength after tooth bleaching.36,37,38,39 Furthermore, some studies have shown its efficacy in compensating for the compromised bond after application of sodium hypochlorite.36,37
An increased inherent cavity design factor, the C-factor, can impair bonding to root dentin because the higher C-factor increases polymerization shrinkage stress of resin materials.40,41,42 It appears application of a reducing agent to dentin prior to post cementation would prepare the dentin surface for penetration of the cement and formation of more numerous and longer resin tags. It might also increase fiber post retention by producing greater friction with root wall dentin.3
NaOCL, which is used during endodontic treatment, changes dentin structure. It acts as a dentin deproteinizing agent, improving adhesive wettability. On the other hand, its remnants and by-products result in a negative effect on the polymerization of adhesive systems. Thus, antioxidants such as SA have been demonstrated to reverse the negative effects of oxidants.
Therefore, the aim of this study was to evaluate the pull-out strength of modified metal spreaders cemented to NaOCl-treated root canal dentin after irrigation with three different antioxidizing/reducing agents and cementing with a one-step self-etching adhesive cement. The null hypothesis tested in this study was that application of RA, HPN, or SA does not influence the immediate pull-out bond strength to root dentin irrigated with NaOCl.
Materials and methods
Seventy-five freshly extracted sound human anterior teeth, with straight roots, were selected for the purpose of this study and stored in 0.2% thymol solution. The crown of each tooth was removed. Every canal was prepared up to file #60 and taper .02 with Flex Master instruments (Mani, Tochigi, Japan) at a working length of 1 mm short of the anatomic apex. The coronal parts of the roots were trimmed to produce a root canal length of 12 mm. The roots were embedded in acrylic resin.
All the root canals were irrigated with 2.5% NaOCl for 1 minute, except for group 1 samples (negative control group), which were irrigated with normal saline. In groups 2 - 5, normal saline was used for final irrigation to enhance similarity to clinical processes. All the root canals were dried with #55 and #60 paper points. Then all the canals were obturated with AH26 sealer (Densply, Konstanz, Germany) and gutta-percha (Suredent, Seongnam, South Korea). After 1 week of incubation at 37℃, the gutta-percha was removed up to 8 mm from inside all the canals using #2 and #3 Peeso reamers, respectively. The Peeso reamers were replaced for every 5 specimens.
The specimens in the negative control group (group 1) and the prepared root canals in the experimental groups were randomly divided into 4 groups (n = 15 each) and prepared as follows:
Groups 1 and 2 (negative and positive control groups). Subsequent to drying with paper points for 5 seconds, the prepared posts were cemented as explained in the next part.
Groups 3 - 5. After drying with paper points for 5 seconds, freshly prepared solutions of 10% RA (Baridgeessence, Kashan, Iran), 10% HPN (Sigma, Madrid, Spain), and 10% SA (Applichem, Damstadt, Germany) were used for 2 minutes and rinsed with distilled water for 30 seconds, respectively.
Stainless steel spreaders #55 with handles (Produits Dentaires S.A., Vevey, Switzerland) were used as posts in the canals. All the spreaders had a length of 10 mm for the tapered part and 8 mm of the length was later cemented into each prepared root canal. Immediately before insertion into the dried root canal, each spreader was rinsed with distilled water, cleaned with ethanol, and silica-coated and silanized with the Rocatec system, including ESPE Sil (3M ESPE, Seefeld, Germany), for 20 seconds. Subsequently, Ceramic Bond (Voco GmbH, Cuxhaven, Germany) was applied to the silanized post surfaces according to the manufacturer's instructions (Table 1).
Table 1.
Materials used in the study and the mode of their application according to the manufacturers' instructions
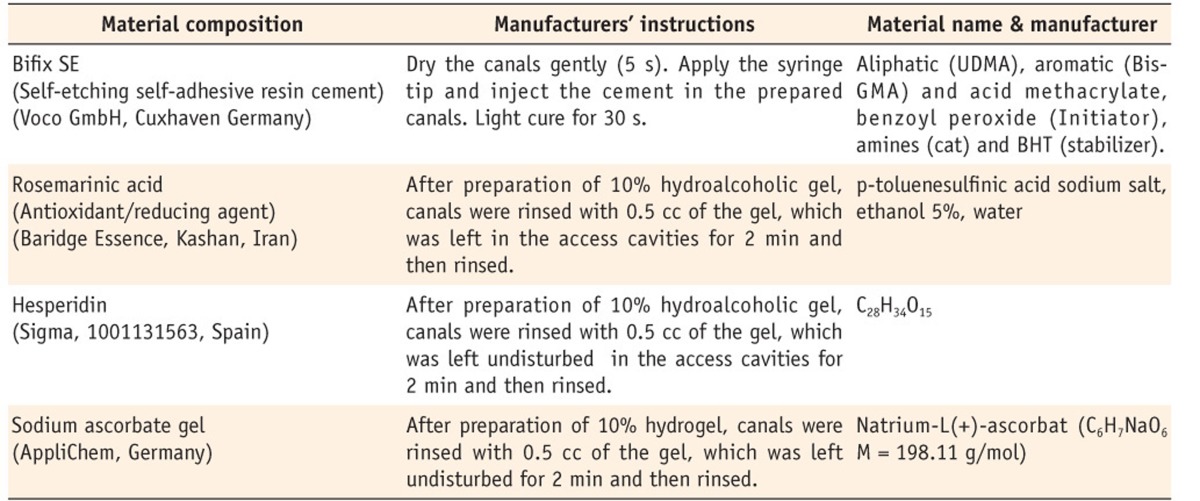
UDMA, urethane dimethacrylate; Bis-GMA, bisphenol A diglycidyl methacrylate; BHT, butylhydroxytoluene.
Then, all the pretreated canals were dried with air, and each silanized spreader was cemented using Bifix SE one-step self-etching cement (Voco, GmbH) according to the manufacturer's instructions. All the specimens were light-cured using a QTH light-curing unit (Optilux 501, Kerr Corp., Orange, CA, USA) at a light intensity of 500 mW/cm2.
All of the roots with cemented posts were stored in distilled water at 37℃ for 24 hours before the pull-out test in a universal testing machine (HC10, Dartec, Stourbridge, UK) at a crosshead speed of 1 mm/min. The data was analyzed with SPSS 15.0 (SPSS Inc., Chicago, IL, USA) using the Kolmogorov-Smirnov test, one-way ANOVA, and Tukey's HSD test (α = 0.05). Subsequent to the pull-out test, each specimen was visually examined under a stereomicroscope (MBC-10, St. Petersburg, Russia) at a magnification of ×20 to assess failure patterns. Three types of failure were classified, such as, 'adhesive-dentin' failure at the cement-root canal dentin interface, 'adhesive-spreader' failure between the cement and the spreader, and a 'mixed' fracture with a combination of cohesive and adhesive ones.
Moreover, two prepared root in each group were cross-sectioned for SEM analysis. The samples were dehydrated in ascending concentrations of ethanol (50, 70, 95, and 100%) for 1.5 hours and embedded in acrylic resin. The surfaces were polished with 600-, 800-, 1,000-, and 1,200-grit silicon carbide papers under running water. Between each polishing step, the specimens were put in an ultrasonic device for 15 minutes. The exposed interfaces were treated with 6 N hydrochloric acid for 35 seconds followed by a 10-minute immersion in 2.5% NaOCl. Subsequent to 15-minute ultrasonication, the specimens were dehydrated for 36 hours, affixed to an aluminum mounting stub, and sputter-coated with platinum-gold for analysis under SEM. Different magnifications were used to provide SEM images at a distance of 25 mm. An accelerating voltage of 20.0 kV was used for the analysis.
Results
The mean pull-out strength values (± standard deviations) for the study groups were 9.27 ± 3.19, 6.71 ± 2.56, 8.86 ± 3.02, 9.30 ± 2.49, and 10.03 ± 2.59 MPa, respectively, with significant differences in bond strength values between the groups (p = 0.016, Table 2). The highest pull-out bond strength value against root canal dentin was recorded in groups 1 and 5. There was a significant difference in the bond strength values between the two control groups (p = 0.025). Comparison of different antioxidants showed that groups 4 and 5, in which the canals were rinsed with HPN and SA, respectively, had the highest pull-out bond strength values, most similar to that of group 1 (the negative control group). There were no significant differences between RA and the positive control group (p > 0.05).
Table 2.
Pull-out bond strength of the specimens in the study groups (MPa)
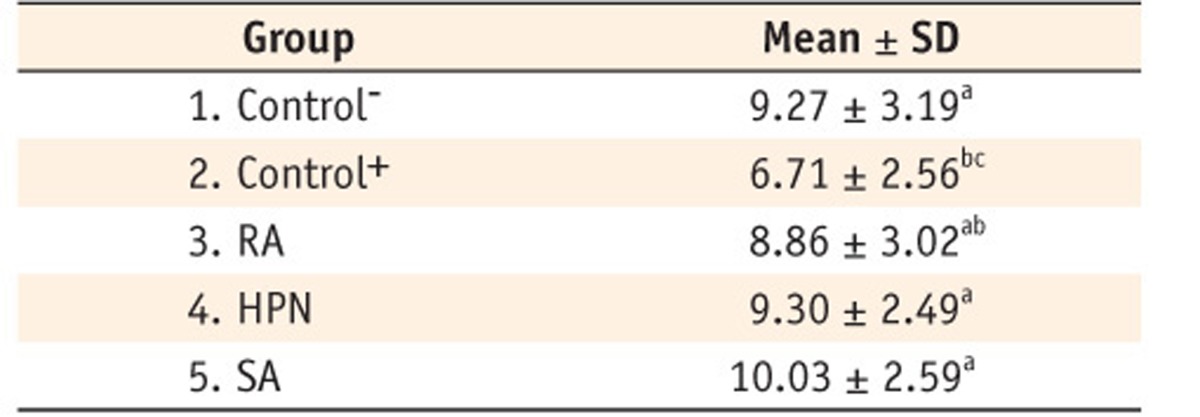
Groups with the same superscript are not statistically different (P > 0.05).
RA, rosemarinic acid; HPN, hesperidin; SA, sodium ascorbate gel; SD, standard deviation.
In terms of the fracture patterns, the 15 samples of the positive control group exhibited 5 mixed fractures and 8 adhesive failures between the cement and spreader. The most favorable fractures with regard to the sum of mixed and cohesive fractures were related to groups 1 and 5, respectively (Table 3). No adhesive fracture was observed between the spreader and cement (0%), with no failure of the spreader itself.
Table 3.
Distribution of different fracture modes in the study groups
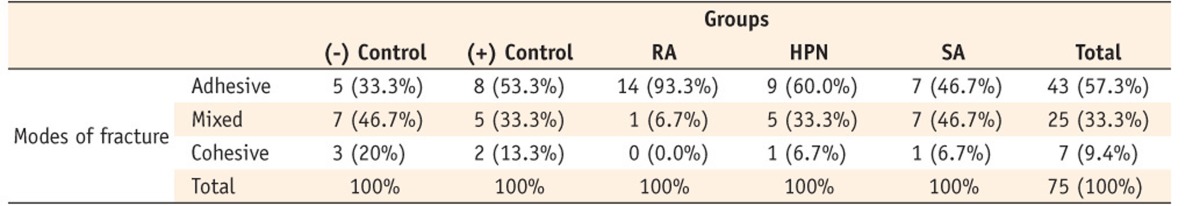
RA, rosemarinic acid; SA, sodium ascorbate; HPN, hesperidin.
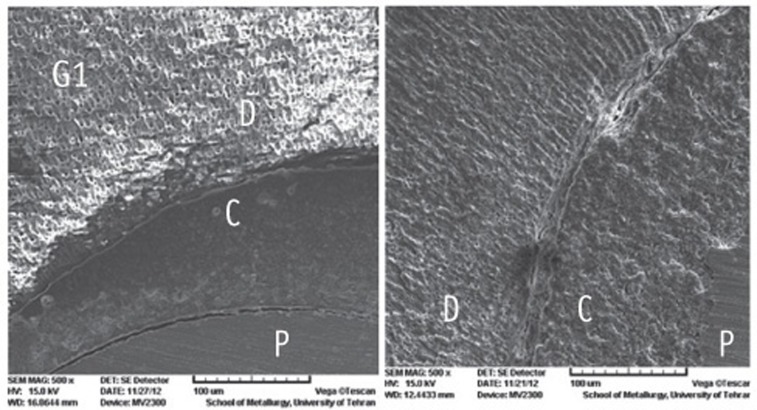
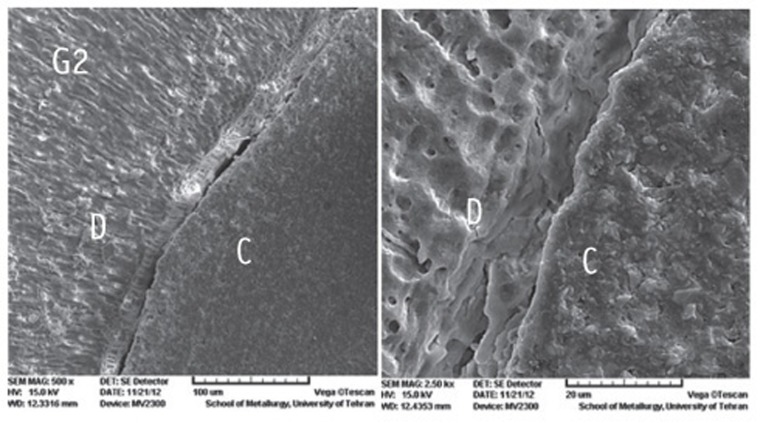
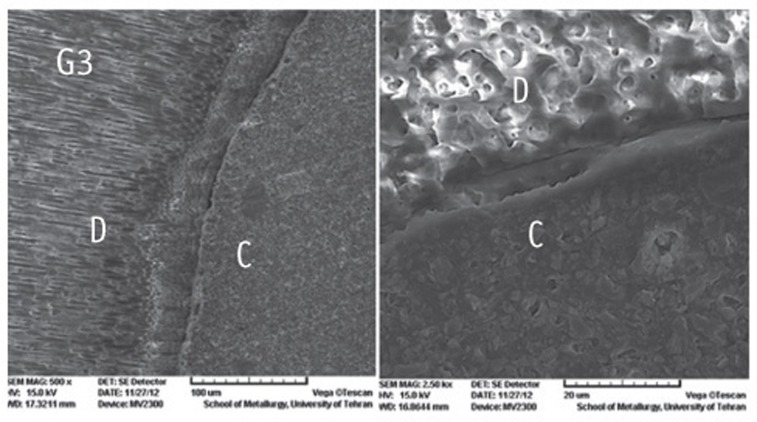
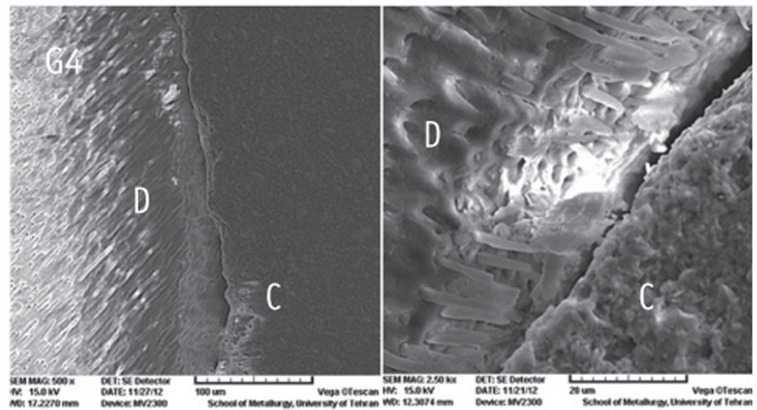
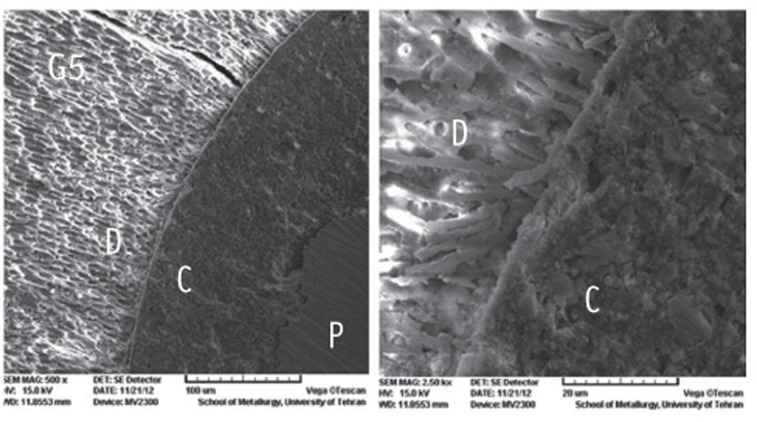
The SEM photomicrographs are shown in Figures 1,2,3,4,5. As it appears in the photomicrographs, the resin interface area seems to be wider in groups 2 and 3. There is a much better adaptation between the resin cement and root dentin in groups 1, 4, and 5. In group 5, the resin tags apparently are larger and more numerous.
Figure 1.
The SEM photomicrographs of group 1.
C, cement resin; D, dentin; P, post (Magnification: both ×500).
Figure 2.
The SEM photomicrographs of group 2.
C, cement resin; D, dentin; (Magnifications: ×500 and ×2,500, respectively).
Figure 3.
The SEM photomicrographs of group 3.
C, cement resin; D, dentin; (Magnifications: ×500 and ×2,500, respectively).
Figure 4.
The SEM photomicrographs of group 4.
C, cement resin; D, dentin (Magnifications: ×500 and ×2,500, respectively).
Figure 5.
The SEM photomicrographs of group 5.
C, cement resin; D, dentin; P, post (Magnifications: ×500 and ×2,500, respectively). As it appears in the photomicrographs, the resin interface area seems to be wider in groups 2 and 3. There is a much better adaptation between the resin cement and root dentin in groups 1, 4, and 5. In group 5, the resin tags apparently are larger and more numerous.
Discussion
In this study, the effects of three different antioxidizing/reducing agents on the pull-out strength of silanized spreaders luted into NaOCl-treated human roots were evaluated. The null hypothesis that application of antioxidizing/reducing agents would not alter the pull-out strength in comparison to the control group was not confirmed.
In this study, a recently introduced technique by Ebert et al. was used to test the pull-out strength of silica-coated and silanized steel spreaders in order to compare post retention provided by resin cement in the dentinal root after application of three different antioxidants.2 The results of this study confirmed the claim that this procedure eliminates the region of weakness in the post-cement system, which has been reported to sometimes be the most prevalent failure mode.2,43 Dowel failure might be avoided by using modified stainless steel spreaders as endodontic posts. Pretreatment of metal surfaces with tribochemical silica coating and silanization of spreaders with Rocatec is believed to produce desirable bond strength and eliminate fracture risk at the luting agent-spreader interface.
In this study, NaOCl was used for irrigation of the canals in groups 2 - 5. This irrigant is widely used during endodontic treatment due to its antibacterial effects and capacity to dissolve organic tissues.36,38 Based on the results, NaOCl decreases the bond strength between composite resins and dentin, which was in accordance with the previous studies.37,38 The remnants and by-products of NaOCl probably exert a detrimental effect on polymerization of dental adhesive systems.36 In addition, some previous studies have shown that these remnants compromise bond strength to NaOCl-treated dentin, which might be reversed by the application of 10% SA as an antioxidant for more than 60 seconds before the adhesive procedure because it can interact with NaOCl by-products to neutralize and reverse the oxidizing effect of the NaOCl-treated dentin surface.35,36,37,38 It should be mentioned that in this study, the antioxidant was used after one week subsequent to NaOCl irrigation during endodontic therapy. A one-week delay could somehow decrease the negative effect of NaOCl on the adhesion of resin cement.
In recent years, the use of antioxidants and reducing agents has been recommended in order to increase the longevity of the bond of resin materials to dentin and to decrease microleakage.18,29,36,37,38,44 In addition, in more recent reports, reducing agents have been introduced as polymerization-facilitating agents and cross-linkers.18,29,36 Some of these materials are derived from natural sources and their natural extracts have been used, but others have been synthetically produced.18
In the present study, a synthetic powder of SA was prepared and used in the form of 10% hydrogel. Some recent studies have recommended the use of SA in order to increase the bond strength to dentin and enamel and in order to neutralize the effects of NaOCl, which is used as a common irrigation solution during root canal treatment.36,37 In the present study, there were significant differences in the pull-out bond strength of the groups under study. The results in the SA group were the most similar to those of the negative control group. In the present study, HPN was synthetically produced, but RA extract was produced from the rosemary plant in the form of 10% hydroalcoholic extract. A recent study showed that use of HPN solution with a 5% concentration of alcohol is effective in increasing the bond strength of composite resin to dentin.29 Interestingly, in the present study in the RA and HPN groups, bubbles were observed on the surface of cement in samples with adhesive failure. Most probably, more care should be exercised in selecting the concentration of the antioxidant and the reducing agent because the use of higher concentrations of the antioxidant might result in bubbling and decreasing bond strength, making it difficult to rinse and remove the material from the area and maybe decreasing its effect as a cross-linker. This is probably the reason for differences between the results of the three studied antioxidants in the present study. However, it should be pointed out that an important limitation of this study might be the short-term bond strength evaluation. Considering the protective role of antioxidants on collagen fibers, their effect on the durability of the adhesive interface is an important issue and should be evaluated in future studies. In the present study, a self-etching self-adhesive cement was used. Recently, a new simplified one-step technique was introduced for cementation of posts, inlays and onlays, and fixed partial dentures, requiring no pretreatment of dentin or enamel.5 The organic matrix is composed of multi-functional phosphoric acidic methacrylates, which help form bonds with tooth structures. The basic fillers can undergo a cementation reaction with the functional monomers, which have acidic groups (Table 1). As a result of cementation reactions, the pH of the material increases during the setting reaction.5 The main setting reaction starts with the polymerization of free radicals by light or a redox system, similar to that with dual-cured composite resins. In addition, phosphoric acidic methacrylates in the monomer can react with the basic fillers and hydroxyapatite crystals of tooth hard structures. The reaction releases water molecules, accelerating the neutralization reaction.6,45,46,47
The mean pull-out strength in the study groups for Bifix SE cement was approximately 9 MPa. In a recent study, the pull-out strength of this cement was reported approximately 11 MPa, which is relatively consistent with the results of the control group in the present study.2 It has been reported that the bond strength of self-etching self-adhesive cements, which do not require a separate conditioning or priming step before bonding of the post or indirect restorations is less than the earlier generations of resin cements. Therefore, it has been recommended that the results of bond strength tests of other resin cements be evaluated with the use of the cross-linkers and antioxidants that have recently been introduced. Two previous studies have shown the impact of the antioxidative effect of SA in restoring the decreased bond strength after bleaching of enamel and dentin, depending on the type of the adhesive used, with much better results with the use of etch-and-rinse adhesives.48,49 It is not clear to what extent the chemical composition of the cement used is compatible with those of antioxidants.
In the present study, three different antioxidizing agents were used, two of which, HPN and RA, have also been known as natural cross-linking agents. Chemical analyses have demonstrated that some reducing agents, including HPN, CHX and GSE, protected and stabilized dentin collagen fibers.18,29 The effect of incorporating natural cross-linking agents into the structure of a self-etching primer on immediate bond strength and mechanical properties of the bonded interface was evaluated in a recent study.29 The results revealed that incorporation of HPN resulted in an increase in the immediate bond strength to dentin and the hardness and elastic modulus of the resin-dentin interface. Miguezl et al. reported that the tensile strength of dentin is significantly affected by the mechanical properties of the collagen matrix.50 HPN and proanthocyanidins are phenolic flavonoids, with a chroman ring. As a result, the chemical effect of HPN on collagen fibrils can be accounted for, similar to the cross-linking effect of proanthocyanidins. Recently, Islam et al. concluded that cross-linking agents with flavonoid groups, such as HPN and GSE, can enhance remineralization.29 Further studies are necessary to investigate the chemical reactions of HPN, RA, and SA with calcium and/or phosphate ions. The stabilized collagen matrix functions as a mechanical barrier, preventing diffusion of minerals, resisting demineralization, and promoting remineralization.18 However, the biochemical properties of HPN have not been adequately investigated to make it applicable to dentistry. Further studies are necessary to elucidate how HPN influences human tooth structure.
In the present study, the two HPN and RA materials were prepared in the form of hydroalcoholic preparations with the use of 5% ethanol because these two materials are not soluble in water, necessitating the use of a low concentration of alcohol. However, preparation of SA hydrogel does not require alcohol. The authors of this study observed that SA was more easily rinsed away from the surface and did not leave in place any chemical agent remnants after its antioxidative/reducing effects. Therefore, it seems it does not leave any residues to interfere with bonding. Of course, these are speculations that should be evaluated with more accurate studies on the properties of these substances during chemical reactions. The present study was a preliminary study on the effect of three antioxidative/reducing agents on the bond of resin cement to posts, which might exert an effect on the polymerization of resin cement as a cross-linker. Further, more accurate evaluations are necessary in the future.
In the present study, the most numerous cohesive and mixed failure modes were observed in groups 1, 4, and 5, which might reflect the effect of a stronger cross-linking effect for the two antioxidative/reducing agents. Moreover, the outcomes of SEM (Figures 1,2,3,4,5) were in accordance with the obtained bond strength and fracture modes. More studies are recommended to evaluate the precise effect of different concentrations of HPN, SA, and other useful reducing agents with different application durations on the resin-dentin bond and other possible uses of reducing agents in adhesive dentistry.
Conclusions
Within the limitations of this in vitro study, amongst the three antioxidants studied, use of 10% SA or HPN for 2 minutes did significantly restore the compromised resin cement bond strength to NaOCl-treated dentin. RA exhibited the least reversal effect with the same application time, compared to SA and HPN.
Acknowledgement
The authors gratefully acknowledge that this report is based on a thesis which was submitted to the School of Dentistry, Isfahan University of Medical Sciences, in partial fulfillment of the requirement for the DDS degree. This study was financially supported and approved by Isfahan University of Medical Sciences, Isfahan, Iran (#391356).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Morgano SM. Restoration of pulpless teeth: application of traditional principles in present and future contexts. J Prosthet Dent. 1996;75:375–380. doi: 10.1016/s0022-3913(96)90028-1. [DOI] [PubMed] [Google Scholar]
- 2.Ebert J, Leyer A, Günther O, Lohbauer U, Petschelt A, Frankenberger R, Roggendorf MJ. Bond strength of adhesive cements to root canal dentin tested with a novel pull-out approach. J Endod. 2011;37:1558–1561. doi: 10.1016/j.joen.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Glazer B. Restoration of endodontically treated teeth with carbon fibre posts-a prospective study. J Can Dent Assoc. 2000;66:613–618. [PubMed] [Google Scholar]
- 4.Malferrari S, Monaco C, Scotti R. Clinical evaluation of teeth restored with quartz fiber-reinforced epoxy resin posts. Int J Prosthodont. 2003;16:39–44. [PubMed] [Google Scholar]
- 5.Amaral M, Santini MF, Wandscher V, Amaral R, Valandro LF. An in vitro comparison of different cementation strategies on the pull-out strength of a glass fiber post. Oper Dent. 2009;34:443–451. doi: 10.2341/08-113. [DOI] [PubMed] [Google Scholar]
- 6.Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano Dorigo E. Dental adhesion review: aging and stability of the bonded interface. Dent Mater. 2008;24:90–101. doi: 10.1016/j.dental.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto M, Ohno H, Sano H, Kaga M, Oguchi H. In vitro degradation of resin-dentin bonds analyzed by microtensile bond test, scanning and transmission electron microscopy. Biomaterials. 2003;24:3795–3803. doi: 10.1016/s0142-9612(03)00262-x. [DOI] [PubMed] [Google Scholar]
- 8.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, Ito S. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83:216–221. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 9.Carrilho MR, Carvalho RM, de Goes MF, di Hipólito V, Geraldeli S, Tay FR, Pashley DH, Tjäderhane L. Chlorhexidine preserves dentin bond in vitro. J Dent Res. 2007;86:90–94. doi: 10.1177/154405910708600115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaussain-Miller C, Fioretti F, Goldberg M, Menashi S. The role of matrix metalloproteinases (MMPs) in human caries. J Dent Res. 2006;85:22–32. doi: 10.1177/154405910608500104. [DOI] [PubMed] [Google Scholar]
- 11.Hebling J, Pashley DH, Tjäderhane L, Tay FR. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J Dent Res. 2005;84:741–746. doi: 10.1177/154405910508400811. [DOI] [PubMed] [Google Scholar]
- 12.Bedran-Russo AK, Yoo KJ, Ema KC, Pashley DH. Mechanical properties of tannic-acid-treated dentin matrix. J Dent Res. 2009;88:807–811. doi: 10.1177/0022034509342556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macedo GV, Yamauchi M, Bedran-Russo AK. Effects of chemical cross-linkers on caries-affected dentin bonding. J Dent Res. 2009;88:1096–1100. doi: 10.1177/0022034509351001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Ammar A, Drummond JL, Bedran-Russo AK. The use of collagen cross-linking agents to enhance dentin bond strength. J Biomed Mater Res B Appl Biomater. 2009;91:419–424. doi: 10.1002/jbm.b.31417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang R, Zhou W, Jiang X. Reaction kinetics of degradation and epimerization of epigallocatechin gallate (EGCG) in aqueous system over a wide temperature range. J Agric Food Chem. 2008;56:2694–2701. doi: 10.1021/jf0730338. [DOI] [PubMed] [Google Scholar]
- 16.Magalhães AC, Wiegand A, Rios D, Hannas A, Attin T, Buzalaf MA. Chlorhexidine and green tea extract reduce dentin erosion and abrasion in situ. J Dent. 2009;37:994–998. doi: 10.1016/j.jdent.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Cho YH, Kim JH, Sim GS, Lee BC, Pyo HB, Park HD. Inhibitory effects of antioxidant constituents from Melothria heterophylla on matrix metalloproteinase-1 expression in UVA-irradiated human dermal fibroblasts. J Cosmet Sci. 2006;57:279–289. [PubMed] [Google Scholar]
- 18.Islam SM, Hiraishi N, Nassar M, Sono R, Otsuki M, Takatsura T, Yiu C, Tagami J. In vitro effect of hesperidin on root dentin collagen and de/re-mineralization. Dent Mater J. 2012;31:362–367. doi: 10.4012/dmj.2011-203. [DOI] [PubMed] [Google Scholar]
- 19.Guardia T, Rotelli AE, Juarez AO, Pelzer LE. Antiinflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco. 2001;56:683–687. doi: 10.1016/s0014-827x(01)01111-9. [DOI] [PubMed] [Google Scholar]
- 20.Trzeciakiewicz A, Habauzit V, Mercier S, Barron D, Urpi-Sarda M, Manach C, Offord E, Horcajada MN. Molecular mechanism of hesperetin-7-O-glucuronide, the main circulating metabolite of hesperidin, involved in osteoblast differentiation. J Agric Food Chem. 2010;58:668–675. doi: 10.1021/jf902680n. [DOI] [PubMed] [Google Scholar]
- 21.Nostro A, Cannatelli MA, Crisafi G, Musolino AD, Procopio F, Alonzo V. Modifications of hydrophobicity, in vitro adherence and cellular aggregation of Streptococcus mutans by Helichrysum italicum extract. Lett Appl Microbiol. 2004;38:423–427. doi: 10.1111/j.1472-765X.2004.01509.x. [DOI] [PubMed] [Google Scholar]
- 22.Hiraishi N, Sono R, Islam MS, Otsuki M, Tagami J, Takatsuka T. Effect of hesperidin in vitro on root dentine collagen and demineralization. J Dent. 2011;39:391–396. doi: 10.1016/j.jdent.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Hirata A, Murakami Y, Shoji M, Kadoma Y, Fujisawa S. Kinetics of radical-scavenging activity of hesperetin and hesperidin and their inhibitory activity on COX-2 expression. Anticancer Res. 2005;25:3367–3374. [PubMed] [Google Scholar]
- 24.Benavente-García O, Castillo J. Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity. J Agric Food Chem. 2008;56:6185–6205. doi: 10.1021/jf8006568. [DOI] [PubMed] [Google Scholar]
- 25.Miller EG, Peacock JJ, Bourland TC, Taylor SE, Wright JM, Patil BS, Miller EG. Inhibition of oral carcinogenesis by citrus flavonoids. Nutr Cancer. 2008;60:69–74. doi: 10.1080/01635580701616163. [DOI] [PubMed] [Google Scholar]
- 26.Wood N. Bound sugars in hepatic glycoproteins from male rats during dietary citrus bioflavonoid and/or ascorbic acid supplementation. J Med Food. 2005;8:512–517. doi: 10.1089/jmf.2005.8.512. [DOI] [PubMed] [Google Scholar]
- 27.Horcajada MN, Habauzit V, Trzeciakiewicz A, Morand C, Gil-Izquierdo A, Mardon J, Lebecque P, Davicco MJ, Chee WS, Coxam V, Offord E. Hesperidin inhibits ovariectomized-induced osteopenia and shows differential effects on bone mass and strength in young and adult intact rats. J Appl Physiol. 2008;104:648–654. doi: 10.1152/japplphysiol.00441.2007. [DOI] [PubMed] [Google Scholar]
- 28.Choi EM, Kim YH. Hesperetin attenuates the highly reducing sugar-triggered inhibition of osteoblast differentiation. Cell Biol Toxicol. 2008;24:225–231. doi: 10.1007/s10565-007-9031-0. [DOI] [PubMed] [Google Scholar]
- 29.Islam S, Hiraishi N, Nassar M, Yiu C, Otsuki M, Tagami J. Effect of natural cross-linkers incorporation in a self-etching primer on dentine bond strength. J Dent. 2012;40:1052–1059. doi: 10.1016/j.jdent.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 30.Lyubimova T, Caglio S, Gelfi C, Righetti PG, Rabilloud T. Photopolymerization of polyacrylamide gels with methylene blue. Electrophoresis. 1993;14:40–50. doi: 10.1002/elps.1150140108. [DOI] [PubMed] [Google Scholar]
- 31.Tirkeş S, Toppare L, Alkan S, Bakir U, Onen A, Yağci Y. Immobilization of glucose oxidase in polypyrrole/polytetrahydrofuran graft copolymers. Int J Biol Macromol. 2002;30:81–87. doi: 10.1016/s0141-8130(02)00011-9. [DOI] [PubMed] [Google Scholar]
- 32.Hernández-Hernández E, Ponce-Alquicira E, Jaramillo-Flores ME, Guerrero Legarreta I. Antioxidant effect rosemary (Rosmarinus officinalis L.) and oregano (Origanum vulgare L.) extracts on TBARS and colour of model raw pork batters. Meat Sci. 2009;81:410–417. doi: 10.1016/j.meatsci.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Apak R, Güçlü K, Ozyürek M, Bektas Oğlu B, Bener M. Cupric ion reducing antioxidant capacity assay for food antioxidants: vitamins, polyphenolics, and flavonoids in food extracts. Methods Mol Biol. 2008;477:163–193. doi: 10.1007/978-1-60327-517-0_14. [DOI] [PubMed] [Google Scholar]
- 34.Bowen RL. Adhesive bonding of various materials to hard tooth tissues. IV. Bonding to dentin, enamel, and fluorapatite improved by the use of a surface-active comonomer. J Dent Res. 1965;44:906–911. doi: 10.1177/00220345650440052601. [DOI] [PubMed] [Google Scholar]
- 35.Taniguchi G, Nakajima M, Hosaka K, Iwamoto N, Ikeda M, Foxton RM, Tagami J. Improving the effect of NaOCl pretreatment on bonding to caries-affected dentin using self-etch adhesives. J Dent. 2009;37:769–775. doi: 10.1016/j.jdent.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Prasansuttiporn T, Nakajima M, Kunawarote S, Foxton RM, Tagami J. Effect of reducing agents on bond strength to NaOCl-treated dentin. Dent Mater. 2011;27:229–234. doi: 10.1016/j.dental.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 37.da Cunha LF, Furuse AY, Mondelli RF, Mondelli J. Compromised bond strength after root dentin deproteinization reversed with ascorbic acid. J Endod. 2010;36:130–134. doi: 10.1016/j.joen.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Weston CH, Ito S, Wadgaonkar B, Pashley DH. Effects of time and concentration of sodium ascorbate on reversal of NaOCl-induced reduction in bond strengths. J Endod. 2007;33:879–881. doi: 10.1016/j.joen.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Moreira DM, de Andrade Feitosa JP, Line SR, Zaia AA. Effects of reducing agents on birefringence dentin collagen after use of different endodontic auxiliary chemical substances. J Endod. 2011;37:1406–1411. doi: 10.1016/j.joen.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 40.May LG, Salvia AC, Souza RO, Michida SM, Valera MC, Takahashi FE, Bottino MA. Effect of sodium ascorbate and the time lapse before cementation after internal bleaching on bond strength between dentin and ceramic. J Prosthodont. 2010;19:374–380. doi: 10.1111/j.1532-849X.2010.00576.x. [DOI] [PubMed] [Google Scholar]
- 41.Bouillaguet S, Troesch S, Wataha JC, Krejci I, Meyer JM, Pashley DH. Microtensile bond strength between adhesive cements and root canal dentin. Dent Mater. 2003;19:199–205. doi: 10.1016/s0109-5641(02)00030-1. [DOI] [PubMed] [Google Scholar]
- 42.Feilzer AJ, De Gee AJ, Davidson CL. Increased wall-to-wall curing contraction in thin bonded resin layers. J Dent Res. 1989;68:48–50. doi: 10.1177/00220345890680010701. [DOI] [PubMed] [Google Scholar]
- 43.Kremeier K, Fasen L, Klaiber B, Hofmann N. Influence of endodontic post type (glass fiber, quartz fiber or gold) and luting material on push-out bond strength to dentin in vitro. Dent Mater. 2008;24:660–666. doi: 10.1016/j.dental.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 44.Park JY, Kwon TY, Kim YK. Effective application duration of sodium ascorbate antioxidant in reducing microleakage of bonded composite restoration in intracoronally-bleached teeth. Restor Dent Endod. 2013;38:43–47. doi: 10.5395/rde.2013.38.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hikita K, Van Meerbeek B, De Munck J, Ikeda T, Van Landuyt K, Maida T, Lambrechts P, Peumans M. Bonding effectiveness of adhesive luting agents to enamel and dentin. Dent Mater. 2007;23:71–80. doi: 10.1016/j.dental.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 46.Kim DS, Park SH, Choi GW, Choi KK. The effect of bonding resin on bond strength of dual-cure resin cements. J Korean Acad Conserv Dent. 2007;32:426–436. [Google Scholar]
- 47.Kim SR, Yum J, Park JK, Hur B, Kim HC. Comparison of push-out bond strength of post according to cement application methods. J Korean Acad Conserv Dent. 2010;35:479–485. [Google Scholar]
- 48.Khoroushi M, Saneie T. Post-bleaching application of an antioxidant on dentin bond strength of three dental adhesives. Dent Res J (Isfahan) 2012;9:46–53. doi: 10.4103/1735-3327.92943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khoroushi M, Aghelinejad S. Effect of postbleaching application of an antioxidant on enamel bond strength of three different adhesives. Med Oral Patol Oral Cir Bucal. 2011;16:e990–e996. doi: 10.4317/medoral.17127. [DOI] [PubMed] [Google Scholar]
- 50.Miguez PA, Pereira PN, Atsawasuwan P, Yamauchi M. Collagen cross-linking and ultimate tensile strength in dentin. J Dent Res. 2004;83:807–810. doi: 10.1177/154405910408301014. [DOI] [PubMed] [Google Scholar]