Abstract
Maternal obesity is associated with placental lipotoxicity, oxidative stress, and inflammation, where MAPK activity may play a central role. Accordingly, we have previously shown that placenta from obese women have increased activation of MAPK-JNK. Here, we performed RNA-sequencing on term placenta from twenty-two subjects who were dichotomized based on pre-pregnancy BMI into lean (BMI 19–24 kg/m2; n = 12) and obese groups (BMI, 32–43 kg/m2; n = 12). RNA-seq revealed 288 genes to be significantly different in placenta from obese women by ≥1.4-fold. GO analysis identified genes related to lipid metabolism, angiogenesis, hormone activity, and cytokine activity to be altered in placenta from obese women. Indicative of a lipotoxic environment, increased placental lipid and CIDEA protein were associated with decreased AMPK and increased activation of NF-κB(p65) in placenta from obese women. Furthermore, we observed a 25% decrease in total antioxidant capacity and increased nuclear FOXO4 localization in placenta from obese women that was significantly associated with JNK activation, suggesting that maternal obesity may also be associated with increased oxidative stress in placenta. Maternal obesity was also associated with decreased HIF-1α protein expression, suggesting a potential link between increased inflammation/oxidative stress and decreased angiogenic factors. Together, these findings indicate that maternal obesity leads to a lipotoxic placental environment that is associated with decreased regulators of angiogenesis and increased markers of inflammation and oxidative stress.
Keywords: Developmental programming, gestational obesity, vascular development, FOXO
INTRODUCTION
Currently over 60% of all pregnancies in the United States are in women who are overweight or obese at conception. Offspring of obese mothers are at an increased risk for being born large for gestational age (LGA) and for developing obesity, cardiovascular disease, and diabetes in adulthood [1–3]. As the interface between the maternal and fetal environment, the placenta plays a central role in how maternal obesity influences programming of offspring health. Studies have shown that maternal obesity is associated with augmented inflammation [4] and elevated proteins involved in nitrative [5] and oxidative stress [6] that may in turn contribute to impaired trophoblast invasion and differentiation (reviewed, [7]), vascular development and function [8, 9], and alterations in placental nutrient transport [10–12].
Using placental trophoblast cells (BeWo), we recently demonstrated that exposure to lipotoxic stimuli induces a pro-inflammatory response in placental cells that is regulated by JNK and early growth response protein-1 (EGR-1) [13]. Furthermore, our studies showed that JNK/EGR-1 signaling is activated in term placenta from obese women [13], suggesting that lipotoxicity may contribute to placental dysfunction associated with maternal obesity. Increased fatty acid uptake by trophoblasts has been demonstrated in animal and cell culture studies in response to obesogenic environments [14, 15], suggesting that maternal obesity may induce a lipotoxic milieu within the placenta. Among other effects, elevated lipids in turn can adversely affect mitochondria, leading to increased ROS production, oxidative stress and cellular dysfunction.
In this study, we tested the hypothesis that maternal obesity promotes a lipotoxic placental environment characterized by increased placental lipid, inflammation and oxidative stress. Additionally, we examined the placental transcriptome using RNA-seq and employed gene ontology (GO) analysis to identify broad functional categories that are altered in placenta from obese women. Consistent with our hypothesis, we identified pathways affected by inflammation, lipotoxicity, and oxidative stress to be significantly increased in placenta from obese women. Furthermore, RNA-seq analysis identified genes related to angiogenesis and hormone activity to be significantly decreased in placenta from obese women, suggesting that an obese maternal environment may adversely affect placental development and function.
METHODS
Collection of term placental samples
Placenta and umbilical cord blood (UCB) (mixed aterial and venous) were collected at the University of Arkansas for Medical Sciences (UAMS), after obtaining informed consent from mothers at term. The protocol was approved by the Institutional Review Board at UAMS (NCT01104454). Included in this study were non-smoking mothers without gestational diabetes or pre-eclampsia who had either vaginal or cesarean deliveries. Maternal clinical characteristics were obtained from medical records and presented in Table 1a [16]. Subjects were dichotomized based on self-reported pre-pregnancy BMI into lean (BMI 19–24 kg/m2; n = 12) and obese groups (BMI, 32–43 kg/m2; n = 12), P < 0.05.
Table 1.
Clinical Characteristics of Pregnancies for Placentas Studied
| a. Maternal Characteristics
| |||
|---|---|---|---|
| Lean | Obese | P value | |
| n | 12 | 12 | --- |
| Gravidity | UK | UK | --- |
| Parity (median, 25–75%) | 2, 0.5–3 | 2.5, 2–5 | 0.1 |
| Gestational age (weeks) | 39 ± 1.1 | 39.0 ± 1.0 | 0.9 |
| Maternal age (years) | 27.4 ± 6.6 | 31.7 ± 5.8 | 0.6 |
| Maternal Weight (lbs.) | 130.2 ± 4.0 | 208.2 ± 6.1* | <0.001 |
| Maternal Height (in) | 65.1 ± 0.7 | 65.2 ± 0.9 | 0.9 |
| Maternal BMI (kg/m2) | 22.1 ± 0.4 | 35.9 ± 0.9* | <0.001 |
| Race | UK | UK | --- |
| Ethnicity | UK | UK | --- |
| Prenatal Vitamin | Y = 8, N = 1, UK = 2 | Y = 8, N = 1, UK = 2 | --- |
| Drugs (cigarettes/alcohol) | N/A | N/A | --- |
| Previous prenatal admission(s) | UK | UK | --- |
| Blood pressures <140/90 mm Hg | Y =10, N = 2 | Y = 12, N = 0 | --- |
| Screened for diabetes | Y = 12 | Y = 12 | --- |
| Antibiotics in labor | UK | UK | --- |
| Beta strep status | UK | UK | --- |
| Antenatal steroids | UK | UK | --- |
| Magnesium sulfate | UK | UK | --- |
| Anesthesia | UK | UK | --- |
| Cervical ripening agent | UK | UK | --- |
| Induction | Y = 2, N = 8, UK = 2 | Y = 1, N = 10, UK = 1 | --- |
| C-section | Y = 6, N = 3, UK = 3 | Y = 7, N = 4, UK = 1 | --- |
|
b. Offspring and Umbilical Cord Measures
| |||
| Birth weight (g) | 3256.8 ± 100.3 | 3312.7 ± 128.6 | 0.7 |
| Placental weight (g) | 647.9 ± 46.4 | 645.9 ± 36.1 | 0.9 |
| Offspring Sex | F = 4, M = 8 | F = 3, M = 9 | --- |
| TG (mg/dl) | 10 ± 3.1 | 8.7 ± 1.9 | 0.7 |
| NEFA (mmol/l) | 0.2 ± 0.03 | 0.2 ± 0.02 | 0.6 |
| Cholesterol (mg/dl) | 78 ± 8.7 | 55.4 ± 5.5* | 0.03 |
| Glucose (mg/dl) | 57 ± 1.6 | 67 ± 2.8* | 0.01 |
| Insulin (pg/ml) | 148 ± 32 | 153 ± 27 | 0.9 |
| Leptin (pg/ml) | 4031 ± 822 | 7744 ± 1083* | 0.03 |
| IL-6 (pg/ml) | 1.6 ± 0.6 | 2.4 ± 0.8 | 0.4 |
| TNFa (pg/ml) | 14.7 ± 1.3 | 17 ± 1.2 | 0.2 |
Values are expressed as mean ± SE unless otherwise stated. Y = yes, N = no, UK = unknown, F = female, M = male, TG = triglyceride, and NEFA = non-esterified fatty acid
Umbilical cord plasma analysis
Colorimetric assays were used to measure UC plasma glucose, triglycerides (TAGs) (Fisher Scientific) and non-esterified free fatty acids (NEFA) (Wako Chemicals). Concentrations of plasma insulin and leptin were measured by ELISA (Millipore). Cytokines interleukin 6 (IL-6) and tumor necrosis factor alpha (TNFα) were measured in plasma using the Milliplex Map Human Cytokine Panel.
RNA-seq libraries Sequencing, alignment, and data analysis
Preparation of RNA-seq libraries, sequencing and data analyses were carried out as previously described. Total RNA was isolated from placenta (9-lean, 11-obese) using a combination of TRI reagent (Molecular Research Center) and RNeasy-mini columns, including on-column deoxyribonuclease digestion (Qiagen). RNA integrity was assessed using Experion RNA StdSens analysis kit (BioRad). cDNA libraries for RNA-seq were prepared using polyA-mRNA from each individual RNA sample (supplemental methods for details) [17]. Single-read 36-bp sequencing of libraries was performed using Illumina GAIIX. Alignment to the human genome (hg19) was carried out using Bowtie [18]. All aligned reads were exported in SAM format, and subsequent data analysis was performed in Avadis-NGS and SeqMonk software packages (details in supplemental methods).
Placental lipid and total antioxidant capacity (TAC) analysis
Lipids were extracted from 300 – 500 mg of placental villi with chloroform-methanol (2:1, vol/vol) [19], allowed to dry to completion under nitrogen gas, and weighed. Data were expressed as total extractable lipids/g tissue. TAC was determined using the Antioxidant Assay Kit (#709001, Cayman, Ann Arbor, MI) as per manufacturer’s instructions.
Immunoblotting
Total tissue lysates were prepared (12-lean, 12-obese) in RIPA buffer containing 1mM PMSF and protease inhibitor cocktail. Nuclear and cytoplasmic proteins were isolated using NE-PER reagents (Thermo Fisher Scientific) on a subset of samples (9-lean, 9-obese). Immunoblotting was carried out following standard procedures [13]. See Table S1 for antibody details. Immunoblots were quantified using Quantity One software (BioRad).
Oil-red-O staining and Immuno-histofluorochemistry
Oil-red-O in propylene glycol (Electron Microscopy Sources) was used to stain for neutral lipids in paraformaldehyde-fixed frozen sections from 6-lean and 6-obese placenta, following manufactures instructions. Vessel density was quantified in another set of sections following immunolabeling with primary antibodies against CD31 (#3528S, Cell Signaling). Immunoreactivity was visualized using secondary antibodies conjugated with Alexa-488 (Molecular Probes) and examined using an AxioVert 200 fluorescent microscope (Carl Zeiss).
Statistical analysis
Data are expressed as means ± SEM. Statistical differences between lean and obese groups were determined using two-tailed student’s t test. Correlations between protein levels were determined using the Pearson’s product-moment correlation coefficient (r). P ≤ 0.05 was considered statistically significant. Statistical analyses were performed using SigmaStat 3.3 software (Systat Software Inc).
RESULTS
Birth weight, placental weight, and UCB analysis
Birth weight and placental weight were not different in this cohort of women (Table 1b). There were no differences in UCB TG or NEFA content, however there was a 30% decrease (P = 0.03) in cholesterol in UCB compared to lean groups (Table 1b). Maternal obesity was also associated with significantly increased glucose levels (P = 0.01) and leptin (P = 0.03). Although there was not a significant difference in UCB IL-6 or TNFα levels, we observed a numerical increase of 50% and 15%, respectively, associated with maternal obesity (Table 1b).
RNA-seq analysis of term placenta from lean and obese women
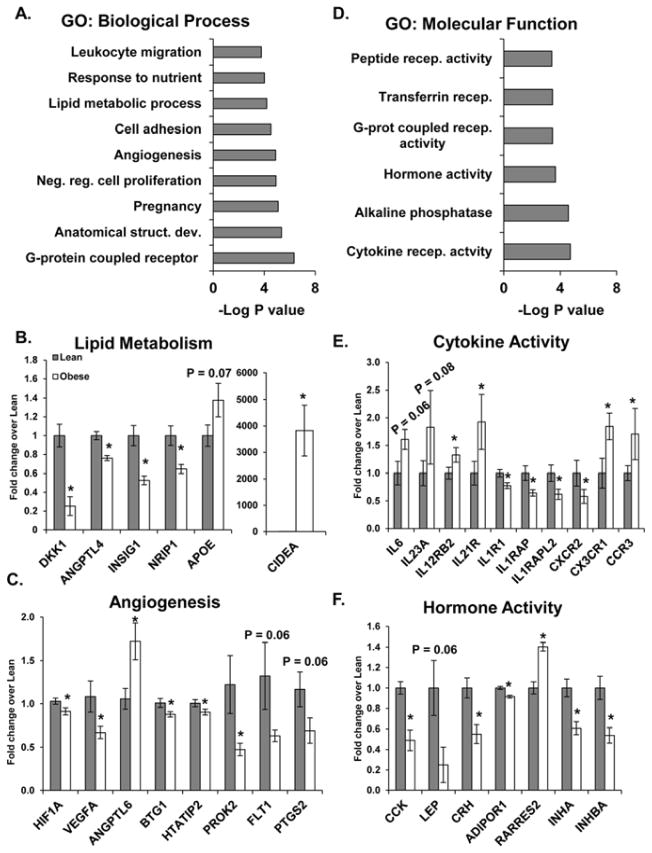
RNA-seq revealed 288 genes to be significantly different in placenta from obese women (±1.4-fold, P < 0.05). Hierarchical clustering of differentially expressed genes is presented in Figure S1A. Interactions of the gene ontology (GO) biological processes (development, stress response, immune response, differentiation, chromatin modification, and reproduction) significantly altered in placenta from obese women are depicted in Figure S1B. Some of the most significantly altered processes are shown in Figure 1A. Gene-set-enrichment-analysis (GSEA) of biological processes also identified genes involved in angiogenesis (HIF1A, VEGFA, ANGPTL6, BTG1, HTATIP2, PROK2, FLIT1 (P = 0.06), and PTGS2 (P = 0.06)) (Figure 1B) and lipid metabolism (DKK1, ANGPTL4, INSIG1, and NRIP1) (Figure 1C) to be significantly decreased (P < 0.05) in placenta from obese women. Maternal obesity was associated with a marked increase in lipid droplet-associated protein CIDEA (P < 0.001) mRNA.
Figure 1. The effects of maternal obesity on global gene expression in placenta.
Differentially expressed genes (±1.4-fold, P < 0.05) identified by RNA-seq in placenta (9-lean and 11-obese) were used for gene ontology (GO) analysis of biological processes (A) and molecular functions (D). B, C, E, and F: Fold change of RPKM values for genes identified by gene-set enrichment analysis of biological process (B and C) and molecular functions (E and F) showing genes involved in lipid metabolism (B), angiogenesis (C), hormone activity (E) and cytokine activity (F). Values are expressed as mean fold change ± SE, where * indicates statistical significance (P < 0.05).
GO analysis of molecular functions identified functions related to receptor signaling and hormone activity to be significantly altered in placenta from obese women (Figure 1D). Likewise, GSEA of molecular functions confirmed that genes involved in cytokine activity were significantly altered (Figure 1E), identifying a trending increase in pro-inflammatory interleukins (IL-6 (P = 0.06) and IL-23A (P = 0.08)) and a significant increase in interleukin and chemokine receptors (IL12RB2, IL21R, CX3CR1, and CCR3) associated with maternal obesity (P < 0.05). In contrast, a significant decrease in pro-inflammatory interleukin-1 receptor-1 (IL1R1, P < 0.01) and receptor accessory proteins (IL1RAP and IL1RAPL2, P < 0.02) was found in placenta from obese compared to lean women. GSEA of genes associated with hormone activity revealed a significant decrease in a number of hormones (CCK, Lep, CRH, INHA and INHBA) and the adiponectin receptor (ADIPOR1) in placenta from obese compared to lean women (P < 0.05).
Maternal obesity is associated with a lipotoxic environment
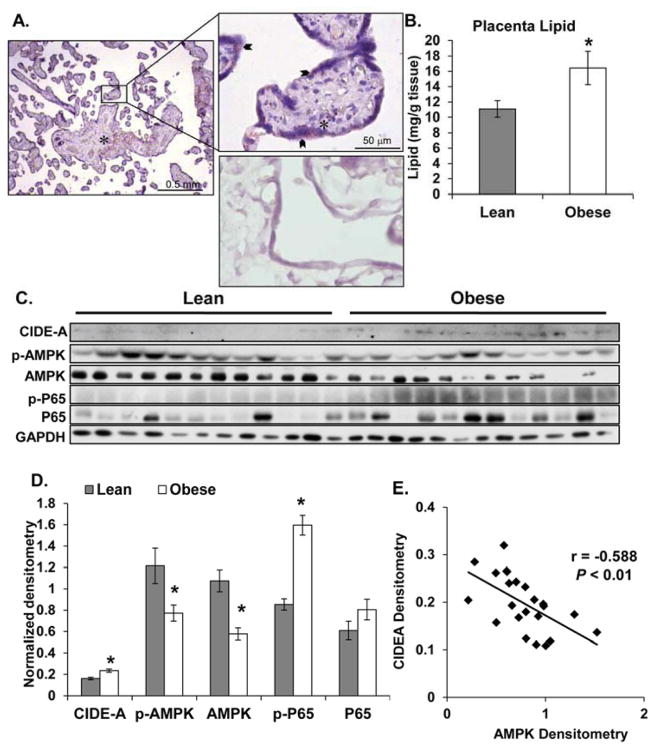
Lipid accumulation was observed in placenta from both lean and obese women and was mainly localized to the villi stroma, however lipid droplets were also present in the syncytium (Figure 2A). Placenta from obese women had 50% more lipid than placenta from lean women (P < 0.03, Figure 2B). A significant increase in CIDE-A (P < 0.001 Figure 2C,D) and a significant decreased in AMPK protein levels (P < 0.001 Figure 2C,D) was associated with maternal obesity. Furthermore, CIDE-A and AMPK levels were inversely correlated (r = −0.588, P < 0.01, Figure 2E). Finally, NF-κB activation (p-P65) was significantly increased in placenta from obese women compared to placenta from lean women (P < 0.001, Figure 2C,D).
Figure 2. The effects of maternal obesity on placental lipid and regulators of lipid metabolism.
A: Representative 10x and 40x images from 6-lean and 6-obese placental sections stained for neutral lipids. Staining of lipid in the villous stroma (asterisk) and syncytium (arrow). Negative control for Oil-Red-O staining (bottom panel, 40X). B: Quantification of placental lipid following lipid extraction and normalization to tissue weight (12-lean and 12-obese). C and D: Western blot (C) and densitometric analysis (D) of lipid metabolism and inflammatory regulators (CIDE-A, AMPK, and NF-κB(p65)). Values are expressed as mean ± SE. E: Correlation analysis between CIDE-A and AMPK densitometry values. r = Pearson’s correlation coefficient, where a negative value indicates an inverse relationship. Statistical significance was determined when P < 0.05 (*).
Decreased TAC and increased nuclear FOXO4 localization are associated with decreased angiogenic regulators in placenta from obese women
Maternal obesity was associated with a 25% decrease in placental TAC (P < 0.02, Figure 3A). Additionally, placenta from obese women showed a 75% increase in nuclear FOXO4 (P < 0.05 Figure 3B,D) when compared to placenta from lean women. Nuclear FOXO4 levels were also positively correlated with activated JNK (pJNK54/JNK54) in placenta tissue (r = 0.628, P = 0.01), Figure 3C. HIF-1α levels were significantly lower (P < 0.05) and VEGF-A monomer levels (21kd) were tending to decrease (P = 0.08) in placenta from obese women, when compared to lean (Figure 4A,B). However, placental vessel density did not differ between lean and obese groups (Figure 3B).
Figure 3. The effects of maternal obesity on TAC and FoxO4 cellular localization in placenta.
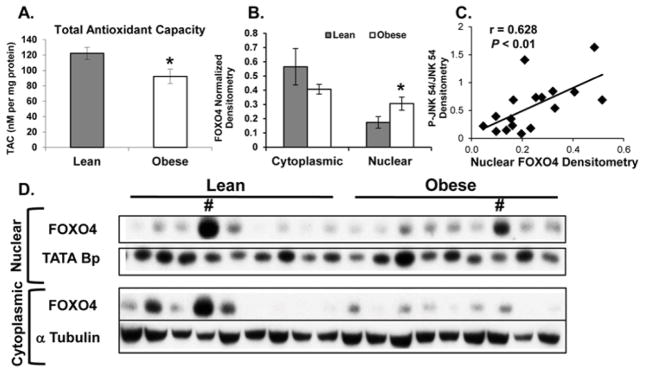
A: Total antioxidant capacity (TAC) analysis of term placenta from lean and obese women (12-lean and 12-obese). TAC was normalized to total protein and expressed as nM TAC per mg protein. B and D: Western blot (D) and densitometric analysis (B) of FoxO4 levels in the nuclear and cytoplasmic fractions isolated from 8-lean and 8-obese placenta. (#) Represents statistical outliers that were removed from the analysis. Values are expressed as means ± SE. C: Correlations between the densitometry values for placental activated JNK (obtained from our previous publication [13]) and nuclear FoxO4. r = Pearson’s correlation coefficient, where a positive value indicates a positive relationship. Statistical significance was determined when P < 0.05 (*).
Figure 4. The effects of maternal obesity on placental VEGF-A and HIF-1α and vessel density.
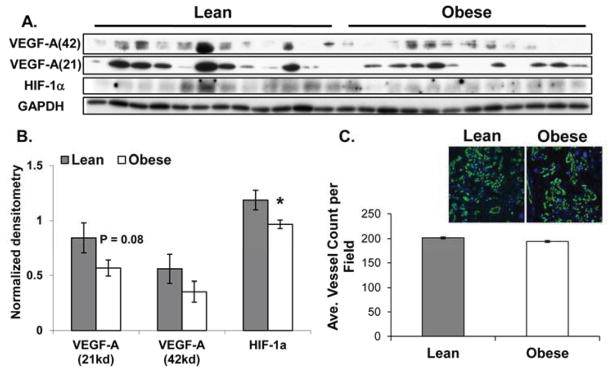
A and B: Western blot (A) and densitometric analysis (B) of VEGF-A and HIF-1α protein in term placenta from lean (n =12) and obese women (n = 12). C: Vessel density was determined as the number of vessels (stained with CD31) per field. Mean values were determined from 4 fields per section for 6-lean and 6-obese subjects. Values are expressed as means ± SE where * indicates statistical significance (P < 0.05).
DISCUSSION
Previous studies have shown that obesity during pregnancy promotes a maternal environment conducive to increased lipotoxicity, inflammation, and oxidative stress in the placenta [4–6]. Several noteworthy observations are evident from the current study. First, we present a genome-wide transcriptomic view of the effects of maternal obesity on the placenta, in the absence of gestational diabetes and other attendant complications. RNA-seq identified genes involved in lipid metabolism, angiogenesis, hormone activity, and inflammation to be significantly altered in placenta from obese women. Second, these studies provide evidence for increased lipids and decreased TAC in placenta from obese women, confirming the notion that maternal obesity is associated with a lipotoxic placental environment. Third, our findings identify key signaling pathways (increased JNK/FoxO4 signaling) and downstream mediators (HIF-1α and VEGF-A) that provide a link between maternal-obesity, placental inflammation/oxidative stress, and altered angiogenic factors. Together, these data suggest that maternal obesity is associated with a placental milieu (summarized in Figure 5) that is susceptible to increased cellular stress, which may in turn adversely affect placental development and function.
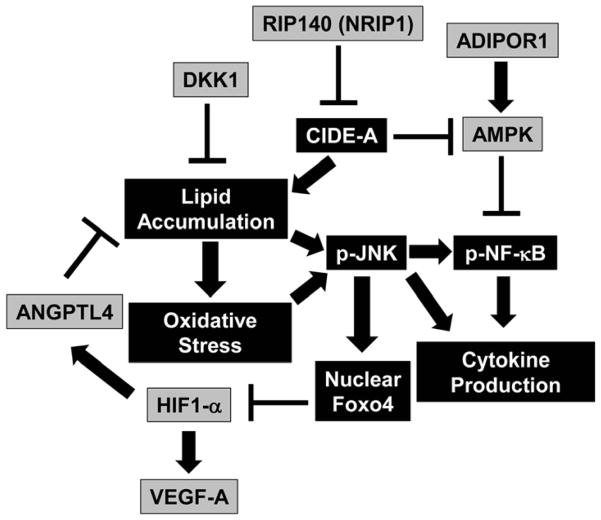
Figure 5. Proposed model of the effects of maternal obesity on placental lipotoxicity, oxidative stress, and inflammation.
Figure 5 summarizes the effects of maternal obesity on the placental environment. Boxes colored grey indicated decreased levels in obese placenta, whereas boxes colored black with white writing indicate increased levels. Arrows indicate a positive regulation and bar-headed lines show negative regulation. Interactions depicted are based on studies performed in various tissues (in some cases placenta) and have been previously published.
Lipotoxicity begins with lipid accumulation in non-adipose tissue. Similar to tissues such as liver and muscle, the placenta is also susceptible to obesity-associated lipid accretion [14, 15, 20]. Furthermore, maternal obesity has been associated with elevated LPL activity and fatty acid transporter levels [21]. We identified a number of gene expression changes that may contribute to increased placental lipid from obese women. Dickkopf homolog-1 (DKK1), an inhibitor of WNT signaling that can limit lipid accumulation in placental cells in vitro [14], was decreased in placenta from obese women. Similarly, lower DKK1 was associated with elevated placental lipid in obesity-prone rats [14]. Additionally, maternal obesity was associated with decreased angiopoietin-like protein 4 (ANGPTL4), a potent inhibitor of LPL activity [22], suggesting that lower placental ANGPTL4 may promote lipid uptake via increased LPL activity. Furthermore, a recent study found ANGPL4 to be negatively associated with elevated circulating maternal lipids and increased neonatal adiposity [23], suggesting that it may influence the fetal environment. Although we did not observe a significant difference in birth weights of offspring born to lean and obese women, it remains possible that maternal obesity-associated changes to the placental environment resulted in programming of offspring metabolism, as previously observed in a rat model of maternal obesity-induced metabolic programming [24, 25]. In further support of this concept, we observed elevated UCB leptin in offspring of obese women. Taken together with the observation that placental leptin gene expression was decreased in obese subjects, these findings presumably suggest greater expression of leptin in offspring tissues in the obese group, consistent with increased adiposity. Placental lipid accumulation may also result from impaired lipid export into fetal circulation. Accordingly, we found lower UCB cholesterol; however since there was no differences in UCB NEFA or TG the relevance of impaired lipid export remains unclear. In contrast to our findings, Dube et. al. found a significant increase in UC blood cholesterol associated with maternal obesity [21]. Differences in UCB collection may contribute to the discrepancy in our results. Additionally, the lack of LGA incidence may explain some differences in UC blood parameters between these findings and previous studies.
Impaired fatty acid oxidation and/or increased de novo lipogenesis is also likely to contribute to obesity-related lipotoxicity. In the placenta, increased CIDE-A and decreased AMPK suggest that maternal obesity may be associated with impaired placental fatty acid oxidation and increased de novo lipogenesis. CIDE-A regulates lipid droplet size [26] and plays a role in thermogenesis [27]. CIDE-A also negatively regulates AMPK protein levels through promoting ubiquitin-mediated degradation [28], indicating a potential mechanism for the decreased AMPK protein levels observed in this study. AMPK is a master regulator of cellular energy homeostasis; switching on catabolic processes and turning off anabolic processes (reviewed, [29]). Consistent with our findings, Jansson et. al. recently showed placental AMPK activity to be significantly decreased in obese women and negatively correlated with birth weight [30]. Although it is likely that decreased-AMPK/increased-mTOR signaling promotes fetal growth through increased amino acid transport [30], it is also possible that lower AMPK promotes lipotoxicity within placental cells [31]. Furthermore, AMPK activation is thought to be anti-inflammatory, suggesting that loss of AMPK may induce an environment more susceptible to pro-inflammatory stimuli.
It is relatively well established that maternal obesity is associated with a pro-inflammatory milieu during pregnancy and in the placenta [4, 32, 33]. Maternal obesity-associated placental inflammation has been characterized by increased infiltration of activated macrophages and elevated pro-inflammatory cytokines [4, 32, 34, 35]. Although the mechanisms responsible for increased cytokine expression in the placenta have not been fully elucidated, evidence exists supporting a central role for TLR/JNK/NF-κB signaling [13, 32]. Accordingly, a previous study in over-nourished sheep showed increased placental cytokine expression associated with elevated TLR-2/4 expression and activated JNK/NF-κB signaling [32]. Similarly, we previously identified JNK and EGR-1 as regulators of placental cytokine expression in response to lipotoxic insults [13]. Furthermore, our observations of maternal obesity-associated increases in placental NF-κB activation and our previous study showing increased JNK activation [13], suggest a role for both pathways in potentially regulating maternal obesity-associated placental inflammation.
FOXO transcription factors are activated under pro-inflammatory and oxidative environments via post-translational modifications by a variety of kinases including JNK [36, 37] causing nuclear translocation. Although FOXO1 and 3a have been shown to promote pro-inflammatory environments [38, 39], FOXO4 activation has been shown to be protective against inflammation and oxidative stress [40, 41]. In unrelated studies, we observed FOXO4 to be the highest expressed FOXO isoform in the placenta (unpublished data); therefore FOXO4 may serve as an adaptive mechanism for the placenta to respond to cellular stress. In Type 1 diabetic pregnancies, placental antioxidant defenses increase in parallel with increased oxidative stress, suggesting that in some cases the placenta can adapt to higher levels of oxidative stress [42]. In contrast, our findings suggest that placenta from obese women have an impaired capacity to deal with oxidative stress, despite the increase in nuclear FOXO4. Although our data support the notion that maternal obesity is associated with increased oxidative stress in the placenta, experiments specifically measuring oxidant-associated damage are needed to determine the oxidative status of placenta from obese women.
Both FOXO1 and FOXO3 have been shown to regulate adult angiogenesis [43] and although FOXO4 has not yet been implicated in placental or fetal vascular development, it has been shown to inhibit hypoxia-induced angiogenesis in vitro [44] through promoting HIF-1α degradation. Tzu-Ling and colleagues elegantly showed a mechanistic link between PI3K/Akt activation and increased angiogenesis through inhibition and nuclear exclusion of FOXO4 [44]. In the absence of Akt activation, nuclear FOXO4 indirectly inhibits HIF-1α protein stability, leading to increased protein degradation and subsequent decreases in the HIF-1α target gene, VEGFA [44]. Our data indicate that increased JNK activity in placenta from obese women may be responsible for increased nuclear FOXO4 translocation and suggest that an analogous mechanism may occur leading to lower HIF-1α levels and decreased angiogenic gene expression. Although vessel density in term placenta did not appear to be affected by maternal obesity, alterations in HIF-1α levels later in pregnancy might impact other HIF-1α targets such as those involved in placental glucose and ion transport (reviewed, [45]).
There are many strengths of this study; however are a few limitations exist. First, our study design did not allow for full analysis of maternal characteristics; including serum parameters or a true measurement of body composition. Additionally, increased blood pressure was recorded in the medical records for two of the lean participants, however based on the medical records it appears that for one patient this was an acute incidence. Careful examination of the data from these participants furthermore did not reveal any discrepancies. Finally, a number of the parameters measured showed trends but did not reach significance, suggesting that for some end-points our study was probably underpowered. It is possible that the heterogeneity of offspring sex contributed to some of the variability in the data. Future studies should stratify analysis based on fetal sex.
In conclusion, our data suggest that maternal obesity is associated with changes in the placenta transcriptome that correspond with increased placental lipid, oxidative stress, and inflammation. Therefore, these results provide evidence for maternal obesity-induced placental lipotoxicity that may be mediated through JNK signaling. A lipotoxic placental environment may impact placental development and function, as demonstrated by decreased angiogenic factors and altered UCB nutrients, leading to metabolic programming in the offspring.
Supplementary Material
RNA-seq analysis of the human placenta. A: Unsupervised clustering of differentially expressed genes in 9-lean vs. 11-obese pooled placenta (±1.5-fold change, P ≤ 0.05). B: CytoScape representation of BINGO generated GO terms showing the interactions and clustering of biological processes significantly enriched with maternal obesity.
Placental protein expression of JNK and p-JNK Western blot and densitometric analysis of Jun N-terminal kinase (JNK) and phosphorylated (Thr183/Tyr185) JNK protein expression in term placenta (12-lean and 12-obese). Analysis of placental JNK/p-JNK in a subset (11-lean and 11-obese) of these subjects was previously published [13].
Acknowledgments
These studies were supported in part by the USDA Agriculture Research Service CRIS 6251-51000-007-04S and National Institutes of Health-R01-DK084225 (K.S.). We gratefully acknowledge the members of the ACNC-Human Studies Core for their assistance in studies using human subjects. We also thank Dr. Curtis L. Lowrey, Jr. and members of the nursing staff at the UAMS Labor & Delivery department for their assistance in sample collection. Nursing support for these studies was provided in part by the UAMS Translational Research Institute funded by the National Institutes of Health Clinical and Translational Science Award (CTSA) program, grants UL1TR000039 and KL2TR000063.
Footnotes
DISCLOSURES
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cnattingius S, Villamor E, Lagerros YT, Wikstrom AK, Granath F. High birth weight and obesity--a vicious circle across generations. Int J Obes (Lond) 2012;36(10):1320–4. doi: 10.1038/ijo.2011.248. [DOI] [PubMed] [Google Scholar]
- 2.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32(6):1076–80. doi: 10.2337/dc08-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson SM, Sattar N, Freeman DJ, Walker JD, Lindsay RS. Inflammation and endothelial activation is evident at birth in offspring of mothers with type 1 diabetes. Diabetes. 2007;56(11):2697–704. doi: 10.2337/db07-0662. [DOI] [PubMed] [Google Scholar]
- 4.Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, Hauguel-de Mouzon S. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 2008;29(3):274–81. doi: 10.1016/j.placenta.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts VH, Smith J, McLea SA, Heizer AB, Richardson JL, Myatt L. Effect of increasing maternal body mass index on oxidative and nitrative stress in the human placenta. Placenta. 2009;30(2):169–75. doi: 10.1016/j.placenta.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliva K, Barker G, Riley C, Bailey MJ, Permezel M, Rice GE, Lappas M. The effect of pre-existing maternal obesity on the placental proteome: two-dimensional difference gel electrophoresis coupled with mass spectrometry. J Mol Endocrinol. 2012;48(2):139–49. doi: 10.1530/JME-11-0123. [DOI] [PubMed] [Google Scholar]
- 7.Williams PJ, Broughton Pipkin F. The genetics of pre-eclampsia and other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25(4):405–17. doi: 10.1016/j.bpobgyn.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kossenjans W, Eis A, Sahay R, Brockman D, Myatt L. Role of peroxynitrite in altered fetal-placental vascular reactivity in diabetes or preeclampsia. Am J Physiol Heart Circ Physiol. 2000;278(4):H1311–9. doi: 10.1152/ajpheart.2000.278.4.H1311. [DOI] [PubMed] [Google Scholar]
- 9.Hayes EK, Lechowicz A, Petrik JJ, Storozhuk Y, Paez-Parent S, Dai Q, Samjoo IA, Mansell M, Gruslin A, Holloway AC, Raha S. Adverse fetal and neonatal outcomes associated with a life-long high fat diet: role of altered development of the placental vasculature. PLoS One. 2012;7(3):e33370. doi: 10.1371/journal.pone.0033370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Araujo JR, Correia-Branco A, Pereira AC, Pinho MJ, Keating E, Martel F. Oxidative stress decreases uptake of neutral amino acids in a human placental cell line (BeWo cells) Reprod Toxicol. 2013;40:76–81. doi: 10.1016/j.reprotox.2013.06.073. [DOI] [PubMed] [Google Scholar]
- 11.Farley DM, Choi J, Dudley DJ, Li C, Jenkins SL, Myatt L, Nathanielsz PW. Placental amino acid transport and placental leptin resistance in pregnancies complicated by maternal obesity. Placenta. 2010;31(8):718–24. doi: 10.1016/j.placenta.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Varastehpour A, Radaelli T, Minium J, Ortega H, Herrera E, Catalano P, Hauguel-de Mouzon S. Activation of phospholipase A2 is associated with generation of placental lipid signals and fetal obesity. J Clin Endocrinol Metab. 2006;91(1):248–55. doi: 10.1210/jc.2005-0873. [DOI] [PubMed] [Google Scholar]
- 13.Saben J, Zhong Y, Gomez-Acevedo H, Thakali KM, Borengasser SJ, Andres A, Shankar K. Early Growth Response Protein-1 Mediates Lipotoxicity-Associated Placental Inflammation: Role in Maternal Obesity. Am J Physiol Endocrinol Metab. 2013 doi: 10.1152/ajpendo.00076.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strakovsky RS, Pan YX. A decrease in DKK1, a WNT inhibitor, contributes to placental lipid accumulation in an obesity-prone rat model. Biol Reprod. 2012;86(3):81. doi: 10.1095/biolreprod.111.094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heerwagen MJ, Stewart MS, de la Houssaye BA, Janssen RC, Friedman JE. Transgenic increase in N-3/n-6 Fatty Acid ratio reduces maternal obesity-associated inflammation and limits adverse developmental programming in mice. PLoS One. 2013;8(6):e67791. doi: 10.1371/journal.pone.0067791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson DM, Burton GJ. A technical note to improve the reporting of studies of the human placenta. Placenta. 2011;32(2):195–6. doi: 10.1016/j.placenta.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Shankar K, Zhong Y, Kang P, Blackburn ML, Soares MJ, Badger TM, Gomez-Acevedo H. RNA-seq analysis of the functional compartments within the rat placentation site. Endocrinology. 2012;153(4):1999–2011. doi: 10.1210/en.2011-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10(3):R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 20.Zhu MJ, Ma Y, Long NM, Du M, Ford SP. Maternal obesity markedly increases placental fatty acid transporter expression and fetal blood triglycerides at midgestation in the ewe. Am J Physiol Regul Integr Comp Physiol. 2010;299(5):R1224–31. doi: 10.1152/ajpregu.00309.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dube E, Gravel A, Martin C, Desparois G, Moussa I, Ethier-Chiasson M, Forest JC, Giguere Y, Masse A, Lafond J. Modulation of fatty acid transport and metabolism by maternal obesity in the human full-term placenta. Biol Reprod. 2012;87(1):14, 1–1. doi: 10.1095/biolreprod.111.098095. [DOI] [PubMed] [Google Scholar]
- 22.Lafferty MJ, Bradford KC, Erie DA, Neher SB. Angiopoietin-like Protein 4 Inhibition of Lipoprotein Lipase: EVIDENCE FOR REVERSIBLE COMPLEX FORMATION. J Biol Chem. 2013;288(40):28524–34. doi: 10.1074/jbc.M113.497602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ortega-Senovilla H, Schaefer-Graf U, Meitzner K, Abou-Dakn M, Herrera E. Decreased concentrations of the lipoprotein lipase inhibitor angiopoietin-like protein 4 and increased serum triacylglycerol are associated with increased neonatal fat mass in pregnant women with gestational diabetes mellitus. J Clin Endocrinol Metab. 2013;98(8):3430–7. doi: 10.1210/jc.2013-1614. [DOI] [PubMed] [Google Scholar]
- 24.Shankar K, Kang P, Harrell A, Zhong Y, Marecki JC, Ronis MJ, Badger TM. Maternal overweight programs insulin and adiponectin signaling in the offspring. Endocrinology. 2010;151(6):2577–89. doi: 10.1210/en.2010-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borengasser SJ, Lau F, Kang P, Blackburn ML, Ronis MJ, Badger TM, Shankar K. Maternal obesity during gestation impairs fatty acid oxidation and mitochondrial SIRT3 expression in rat offspring at weaning. PLoS One. 2011;6(8):e24068. doi: 10.1371/journal.pone.0024068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puri V, Ranjit S, Konda S, Nicoloro SM, Straubhaar J, Chawla A, Chouinard M, Lin C, Burkart A, Corvera S, Perugini RA, Czech MP. Cidea is associated with lipid droplets and insulin sensitivity in humans. Proc Natl Acad Sci U S A. 2008;105(22):7833–8. doi: 10.1073/pnas.0802063105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Z, Yon Toh S, Chen Z, Guo K, Ng CP, Ponniah S, Lin SC, Hong W, Li P. Cidea-deficient mice have lean phenotype and are resistant to obesity. Nat Genet. 2003;35(1):49–56. doi: 10.1038/ng1225. [DOI] [PubMed] [Google Scholar]
- 28.Qi J, Gong J, Zhao T, Zhao J, Lam P, Ye J, Li JZ, Wu J, Zhou HM, Li P. Downregulation of AMP-activated protein kinase by Cidea-mediated ubiquitination and degradation in brown adipose tissue. Embo J. 2008;27(11):1537–48. doi: 10.1038/emboj.2008.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25(18):1895–908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jansson N, Rosario FJ, Gaccioli F, Lager S, Jones HN, Roos S, Jansson T, Powell TL. Activation of placental mTOR signaling and amino acid transporters in obese women giving birth to large babies. J Clin Endocrinol Metab. 2013;98(1):105–13. doi: 10.1210/jc.2012-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cacicedo JM, Benjachareonwong S, Chou E, Yagihashi N, Ruderman NB, Ido Y. Activation of AMP-activated protein kinase prevents lipotoxicity in retinal pericytes. Invest Ophthalmol Vis Sci. 2011;52(6):3630–9. doi: 10.1167/iovs.10-5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu MJ, Du M, Nathanielsz PW, Ford SP. Maternal obesity up-regulates inflammatory signaling pathways and enhances cytokine expression in the mid-gestation sheep placenta. Placenta. 2010;31(5):387–91. doi: 10.1016/j.placenta.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Shankar K, Zhong Y, Kang P, Lau F, Blackburn ML, Chen JR, Borengasser SJ, Ronis MJ, Badger TM. Maternal obesity promotes a proinflammatory signature in rat uterus and blastocyst. Endocrinology. 2011;152(11):4158–70. doi: 10.1210/en.2010-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farley D, Tejero ME, Comuzzie AG, Higgins PB, Cox L, Werner SL, Jenkins SL, Li C, Choi J, Dick EJ, Jr, Hubbard GB, Frost P, Dudley DJ, Ballesteros B, Wu G, Nathanielsz PW, Schlabritz-Loutsevitch NE. Feto-placental adaptations to maternal obesity in the baboon. Placenta. 2009;30(9):752–60. doi: 10.1016/j.placenta.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts KA, Riley SC, Reynolds RM, Barr S, Evans M, Statham A, Hor K, Jabbour HN, Norman JE, Denison FC. Placental structure and inflammation in pregnancies associated with obesity. Placenta. 2011;32(3):247–54. doi: 10.1016/j.placenta.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 36.van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol. 2007;8(6):440–50. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- 37.Essers MA, Weijzen S, de Vries-Smits AM, Saarloos I, de Ruiter ND, Bos JL, Burgering BM. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. Embo J. 2004;23(24):4802–12. doi: 10.1038/sj.emboj.7600476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamo N, Ke B, Busuttil RW, Kupiec-Weglinski JW. PTEN-mediated Akt/beta-catenin/Foxo1 signaling regulates innate immune responses in mouse liver ischemia/reperfusion injury. Hepatology. 2013;57(1):289–98. doi: 10.1002/hep.25958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snoeks L, Weber CR, Turner JR, Bhattacharyya M, Wasland K, Savkovic SD. Tumor suppressor Foxo3a is involved in the regulation of lipopolysaccharide-induced interleukin-8 in intestinal HT-29 cells. Infect Immun. 2008;76(10):4677–85. doi: 10.1128/IAI.00227-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Araujo J, Breuer P, Dieringer S, Krauss S, Dorn S, Zimmermann K, Pfeifer A, Klockgether T, Wuellner U, Evert BO. FOXO4-dependent upregulation of superoxide dismutase-2 in response to oxidative stress is impaired in spinocerebellar ataxia type 3. Hum Mol Genet. 2011;20(15):2928–41. doi: 10.1093/hmg/ddr197. [DOI] [PubMed] [Google Scholar]
- 41.Zhou W, Cao Q, Peng Y, Zhang QJ, Castrillon DH, DePinho RA, Liu ZP. FoxO4 inhibits NF-kappaB and protects mice against colonic injury and inflammation. Gastroenterology. 2009;137(4):1403–14. doi: 10.1053/j.gastro.2009.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Araujo JR, Ramalho C, Correia-Branco A, Faria A, Ferraz T, Keating E, Martel F. A parallel increase in placental oxidative stress and antioxidant defenses occurs in pre-gestational type 1 but not gestational diabetes. Placenta. 2013 doi: 10.1016/j.placenta.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Potente M, Urbich C, Sasaki K, Hofmann WK, Heeschen C, Aicher A, Kollipara R, DePinho RA, Zeiher AM, Dimmeler S. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest. 2005;115(9):2382–92. doi: 10.1172/JCI23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang TT, Lasky LA. The forkhead transcription factor FOXO4 induces the down-regulation of hypoxia-inducible factor 1 alpha by a von Hippel-Lindau protein-independent mechanism. J Biol Chem. 2003;278(32):30125–35. doi: 10.1074/jbc.M302042200. [DOI] [PubMed] [Google Scholar]
- 45.Pringle KG, Kind KL, Sferruzzi-Perri AN, Thompson JG, Roberts CT. Beyond oxygen: complex regulation and activity of hypoxia inducible factors in pregnancy. Hum Reprod Update. 2010;16(4):415–31. doi: 10.1093/humupd/dmp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RNA-seq analysis of the human placenta. A: Unsupervised clustering of differentially expressed genes in 9-lean vs. 11-obese pooled placenta (±1.5-fold change, P ≤ 0.05). B: CytoScape representation of BINGO generated GO terms showing the interactions and clustering of biological processes significantly enriched with maternal obesity.
Placental protein expression of JNK and p-JNK Western blot and densitometric analysis of Jun N-terminal kinase (JNK) and phosphorylated (Thr183/Tyr185) JNK protein expression in term placenta (12-lean and 12-obese). Analysis of placental JNK/p-JNK in a subset (11-lean and 11-obese) of these subjects was previously published [13].