Abstract
Background
Recruitment of large, diverse populations into genetic studies remains challenging. Potential strategies to overcome limitations include leveraging electronic health data and minimizing patient burden. We sought to describe the overall participation rate and identify characteristics associated with participation in a genetic substudy of patients with type 2 diabetes mellitus, in which patients were identified via electronic hospital data and asked to participate by providing DNA samples by mail.
Methods
During a phone interview, participants (n = 455) were asked to take part in a genetic substudy. Subjects verbally consenting were mailed saliva collection kits and written consent forms. We examined demographic and clinical variables associated with verbal consent and DNA kit return using logistic regression.
Results
Overall, 90% (n = 410) verbally consented to the genetic substudy during interviews. However, of those consenting, only 70% returned the DNA kit (n = 287). Among those consenting, after covariate adjustment, male sex (odds ratio [OR], 1.70; 95% confidence interval [CI], 1.09–2.65), African American race (OR, 0.61; 95% CI, 0.39–0.95), hemoglobin A1c (HbA1c) (OR, 0.87; 95% CI, 0.75–1.00), and physical activity (OR, 0.58; 95% CI, 0.37–0.91) were significantly associated with DNA kit return.
Conclusions
To our knowledge, we are the first to demonstrate an inverse association between HbA1c and participation in genetic research, potentially indicating a compliance-related trait needing further exploration. The DNA kit return rate being notably lower than the verbal consent rate suggests that the greater convenience of a telephone/mail-in process did not drastically enhance full participation. Direct comparison to in-person donation may be warranted.
Keywords: genetics, type 2 diabetes, race, recruitment
In the burgeoning epidemic of type 2 diabetes mellitus (DM), unraveling the complex link between genetic susceptibility, lifestyle, and environmental factors is critical to understanding its pathogenesis and optimizing treatment regimens. However, case-control studies to date have failed to explain most of the expected genetic contribution to the risk of type 2 DM.1 Thus, to uncover genetic loci exerting important subgroup effects in type 2 DM, there is a need for prospective epidemiological studies that are designed to minimize bias.2 Recruitment of large, diverse populations into genetic studies, however, remains challenging. Barriers to participation in genetic studies may differ from other recruitment settings. It has been shown that the general public is more skeptical toward genetic studies than other types of medical research.3 Because of potentially complex associations between personal characteristics related to adherence, outcomes, and genetic distributions, differential rate of participation may introduce biases in estimates of genetic relationships.4
Participation rates for genetic studies range from 21% to 99%.5 In contrast to data on the general public’s opinion on theoretical participation in genetic studies, the data on correlates of actual participation are sparse and sometimes conflicting.5,6 Furthermore, despite ongoing interest in conducting patientbased genetic research, there are limited descriptions of who actually participates, particularly among patient populations, including individuals with type 2 DM. Growing interest has focused on using electronic medical records (EMRs), administrative databases, and novel or pragmatic patient contact schemes for recruitment in genetic studies.7,8 Little, however, is known about the utility of such approaches in selecting a well-balanced and diverse genetic cohort.
To address this paucity of data, we conducted a descriptive study to identify sociodemographic and clinical characteristics associated with participation in a genetic substudy of insulin-treated patients with type 2 DM, who were identified via hospital administrative claims data, contacted only by mail and telephone, and asked to participate by providing DNA samples by mail.
MATERIALS AND METHODS
Study Population
The goal of the overall study was to examine health outcomes among patients with type 2 DM undergoing insulin treatment. Potential participants were identified by searching the automated claims data (corporate data store) of the Henry Ford Health System (HFHS), which provides medical care to between 20% and 30% of the metropolitan Detroit population. Patients who were identified as having type 2 DM (International Classification of Diseases, Ninth Revision [ICD-9] codes 250.xx, 357.2x, 362.0x, 366.41)9 and who were treated with insulin for at least 1 month, were older than 40 years, and had a hemoglobin A1c (HbA1c) measure within 3 months before starting insulin treatment were identified. Patients who initiated insulin between January 1, 2005, and October 31, 2009, were eligible. All patients were members of the Health Alliance Plan, a nonprofit health maintenance organization part of the HFHS.
Patients part of a clinical trial related to diabetes and women pregnant at the time of insulin initiation were excluded. A total of 897 potential study participants were identified and sent an initial mailing, which introduced the study. Trained interviewers followed up the letters with a phone call explaining the purpose of the study and inviting the patient to participate.
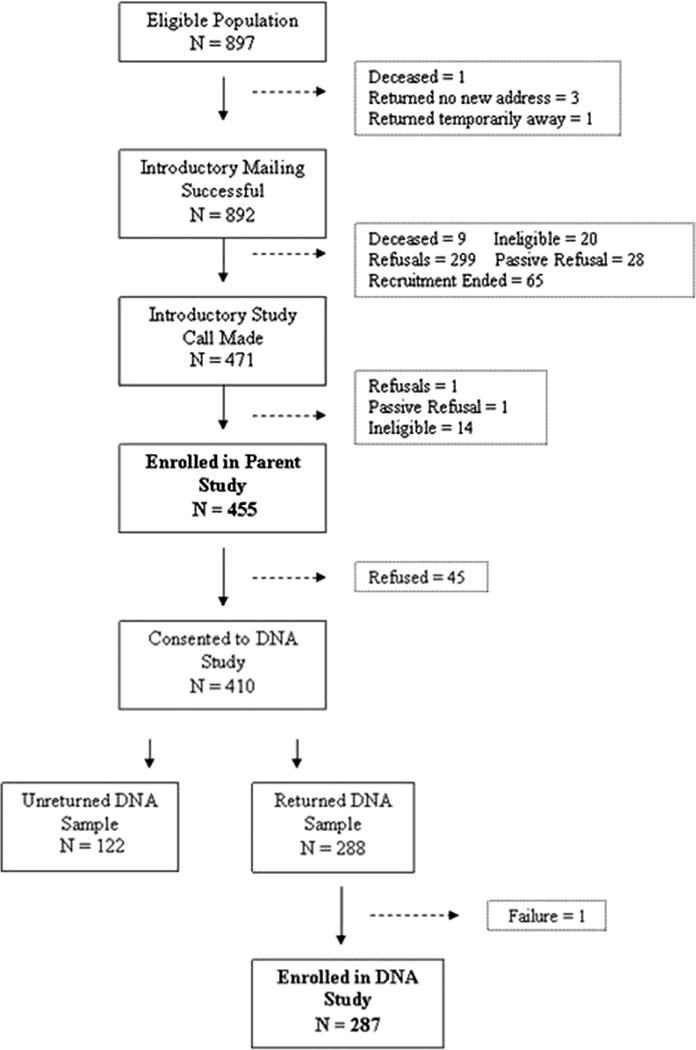
Study recruitment spanned from October 2009 to March 2010. A total of 471 individuals agreed to participate in the study. During the interview, eligibility was confirmed by asking the participants if they had ever taken insulin. Fourteen people were found to be ineligible based on insulin status. Two people prematurely ended the interview process and were excluded. A total of 455 individuals receiving the introductory letter successfully completed the interview for a response rate of 53.0% (455/858 eligible individuals) (Fig. 1). For agreeing to be in the study and completing the interview, participants were mailed a $25 incentive.
FIGURE 1.
Flow diagram of the study population.
After completing the phone interview for the primary study, participants were then asked if they wanted to participate in an optional genetic substudy. The interviewer explained the purpose of the genetic substudy as well as the method of DNA collection (via saliva sample with collection kit provided by mail); 410 individuals agreed to participate and were mailed a DNA kit. Participants were told the DNA kit would include a $2 bill, an amount previously found useful for encouraging participants to open study-related mail.10 The packet also included instructions on how to complete the DNA kit and an informed consent document that was to be returned with the kit. Upon returning the DNA kit, participants were mailed an additional $20 incentive.
Two hundred eighty-seven participants successfully completed and returned the DNA kit; 122 samples were not returned, despite a reminder phone call, and 1 sample was completed incorrectly (Fig. 1). The study protocol was approved by the institutional review board at HFHS. Verbal informed consent was obtained for the phone interview, and written informed consent was obtained for the genetic substudy; both consents included permission to obtain electronically available health data. Waiver of consent was also obtained to allow comparison of nonparticipants to participants on basic demographic and clinical variables.
Data Collection
During the interview, participants self-reported date of birth, sex, education, race, ethnicity, family history of disease, and lifestyle information. Family history of type 2 DM was defined as a first-degree relative with type 2 DM. Family history of premature cardiovascular disease (CVD) was defined as a first-degree relative being diagnosed with CVD younger than 55 years for men and younger than 65 years for women. Physical activity was defined as being physically active for at least 4 hours per week; participants reporting physical activity only in the summer were considered inactive.
Corporate data store was used to obtain additional data elements, including dates of diabetes diagnosis and insulin initiation, marital status, and low-density lipoprotein cholesterol and HbA1c values closest to and before the interview. Height and weight closest to and before the interview were obtained, and body mass index (BMI) calculated as weight (in kilograms)/height (in meters squared). Obesity was defined as BMI of 30 kg/m2 or greater. Comorbid conditions were identified using the following diagnosis codes: hypertension (ICD-9 codes 401.0, 401.1, 401.9), hypercholesterolemia (ICD-9 code 272.0), and depression (ICD-9 code 311). Hyperlipidemia was defined as a fasting low-density lipoprotein cholesterol of 100 mg/dL or greater or a history of hypercholesterolemia diagnosis. Missing/ambiguous responses to the interview or missing data from corporate data store were refined through EMR review.
Statistical Analysis
We evaluated if there were differences in patient characteristics across 3 comparison groups. First, we compared those who did and did not verbally consent to be in the genetic substudy. Second, among those consenting to the genetic substudy, we compared those who did and did not return a DNA kit by mail. Finally, we compared those returning the DNA kit to those participating in the parent study (whether they consented to the genetic substudy or not). All participant characteristics were compared using Fisher exact test for categorical variables and Wilcoxon rank sum test for continuous variables.
For the same 3 comparison groups described, we additionally examined differences in patient characteristics using logistic regression models. Because this was an exploratory study of the characteristics differing between these groups of participants, stepwise selection in which significance levels for entry and stay in the model were 0.2 and 0.15, respectively, was used to identify subsets of variables that best explained the outcomes of interest; all models were forced to include age and sex. As only a small number of participants identified as a race other than African American or white, we grouped those identifying as other with the white category for statistical modeling. Two-sided P < 0.05 was considered to indicate statistical significance.
RESULTS
We compared overall study participants to nonparticipants with respect to available demographic and clinical characteristics. Nonparticipants were not different than participants with respect to age, sex, or duration of diabetes (all P > 0.10). Participants and nonparticipants did significantly differ by race (P = 0.01); among those participating in the study, 42.9% were African American race compared with only 35.8% of those not participating. Findings were similar comparing those in the genetic substudy to those who did not participate in the parent study.
Characteristics of Those Verbally Consenting to Participate in the Genetic Substudy
The overall rate of consent to participate in the genetic substudy was 90% (n = 410). In univariate analysis, those consenting to be in the genetic substudy were slightly older and were less likely to be African American (Table 1; all P < 0.05). In the fully adjusted model (Table 3, model 1), after adjusting for age and sex, only African American race was marginally associated with consent to participate in the genetic substudy (P = 0.072); compared with non–African American participants, African American participants were 44% less likely to consent to participate in the genetic substudy.
TABLE 1.
Descriptive Characteristics of the Study Population (n = 455), by Consent or Not to Genetic Substudy
| Characteristic | Consent to Genetic Substudy |
Did Not Consent to Genetic Substudy |
P* |
|---|---|---|---|
| n | 410 | 45 | |
| Age at interview, y | 65.2 ± 10.2 | 62.2 ± 10.8 | 0.043 |
| Male | 213 (52.0%) | 19 (42.2%) | 0.272 |
| Education level | 0.902 | ||
| <High-school | 56 (13.7%) | 5 (11.1%) | |
| High-school | 117 (28.5%) | 14 (31.1%) | |
| >High-school | 237 (57.8%) | 26 (57.8%) | |
| Marital status | 0.426 | ||
| Married | 294 (71.7%) | 28 (62.2%) | |
| Divorced/legally separated | 30 (7.3%) | 4 (8.9%) | |
| Widowed | 22 (5.4%) | 2 (4.4%) | |
| Single | 64 (15.6%) | 11 (24.4%) | |
| Currently employed | 125 (30.5%) | 19 (42.2%) | 0.128 |
| Race | 0.002 | ||
| African American | 169 (41.2%) | 26 (57.8%) | |
| White | 233 (56.8%) | 15 (33.3%) | |
| Other | 8 (2.0%) | 4 (8.9%) | |
| Hispanic ethnicity | 15 (3.7%) | 0 | 0.382 |
| Family history type 2 DM | 266 (66.5%) | 28 (62.2%) | 0.619 |
| Family history CVD | 112 (27.3%) | 12 (26.7%) | 1.000 |
| Smoking status | 0.181 | ||
| Current | 51 (12.4%) | 6 (13.3%) | |
| Former | 202 (49.3%) | 16 (35.6%) | |
| Never | 157 (38.3%) | 23 (51.1%) | |
| Lives alone | 72 (17.6%) | 8 (17.8%) | 1.000 |
| Physically active | 231 (56.3%) | 29 (64.4%) | 0.343 |
| Duration of diabetes, y | 10.7 ± 4.3 | 11.3 ± 5.2 | 0.474 |
| BMI, kg/m2 | 34.5 ± 7.7 | 33.4 ± 8.0 | 0.271 |
| BMI ≥30 kg/m2 | 284 (69.4%) | 29 (64.4%) | 0.500 |
| HbA1c, % | 8.0 ± 1.6 | 8.0 ± 1.7 | 0.706 |
| Hypertension | 398 (97.1%) | 43 (95.6%) | 0.639 |
| Depression | 18 (4.4%) | 1 (2.2%) | 0.709 |
| Hyperlipidemia | 194 (47.3%) | 26 (57.8%) | 0.210 |
Data are mean ± SD or n (%).
P from comparison of those who did and did not consent to the genetic substudy.
TABLE 3.
Stepwise Logistic Regression Model Results Comparing Those Who Did and Did Not Consent to the Genetic Substudy (Model 1), Comparing Those Who Did and Did Not Return the DNA Kit, Among Those Consenting to Be in the Genetic Substudy (Model 2), and, Comparing Genetic Substudy Participants to Those Participating in the Parent Study Only (Model 3)
| Model 1 (Consent to Genetic Substudy) |
Model 2 (DNA Kit Return Among Consenting) |
Model 3 (DNA Kit Return to Parent Study) |
||||
|---|---|---|---|---|---|---|
| Characteristic | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| Age at interview (per 2 y) | 1.05 (0.99–1.12) | 0.106 | 1.03 (0.99–1.08) | 0.159 | 1.05 (1.01–1.10) | 0.017 |
| Male | 1.44 (0.77–2.69) | 0.256 | 1.70 (1.09–2.65) | 0.020 | 1.70 (1.14–2.55) | 0.009 |
| African American | 0.56 (0.30–1.05) | 0.072 | 0.61 (0.39–0.95) | 0.030 | 0.54 (0.36–0.80) | 0.002 |
| HbA1c | NS | — | 0.87 (0.75–1.00) | 0.043 | NS | — |
| Physically active | NS | — | 0.58 (0.37–0.91) | 0.019 | 0.60 (0.40–0.90) | 0.013 |
CI indicates confidence interval; NS, not selected in stepwise logistic regression procedure; OR, odds ratio.
Characteristics of Those Returning a DNA Kit Among Those Who Consented to Genetic Substudy
Of those who consented to be in the genetic substudy, 70% returned the DNA kit (n = 287). Age, race, sex, employment status, physical activity, and HbA1c were associated with DNA kit return (all P < 0.05) (Table 2). In the fully adjusted model (Table 3, model 2), sex (P = 0.020), race (P = 0.030), HbA1c (P = 0.043), and physical activity (P = 0.019) were each associated with DNA kit return. Compared with women, men were 1.7 times more likely to return the DNA kit, whereas compared with non–African Americans, African American race was associated with a 39% lower odds of returning the DNA kit, and those who were physically active were 42% less likely to return the DNA kit compared with those who were not physically active. For every 1-unit increase in HbA1c, there was a corresponding 13% decreased odds of returning the DNA kit.
TABLE 2.
Comparison of Those Who Did and Did Not Return the DNA Kit, Among Those Consenting to Be in the Genetic Substudy and Comparison of Those Who Did Return the DNA Kit to Those Participating in the Parent Study Only
| Characteristic | Returned DNA Kit |
Did Not Return DNA Kit |
Parent Study Only Participant* |
P† | P‡ |
|---|---|---|---|---|---|
| n | 287 | 123 | 168 | ||
| Age at interview, y | 65.9 ± 10.1 | 63.5 ± 10.3 | 63.1 ± 10.4 | 0.028 | 0.004 |
| Male | 159 (55.4%) | 54 (43.9%) | 73 (43.5%) | 0.040 | 0.015 |
| Education level | 0.885 | 0.861 | |||
| <High-school | 39 (13.6%) | 17 (13.8%) | 22 (13.1%) | ||
| High-school | 80 (27.9%) | 37 (30.1%) | 51 (30.4%) | ||
| >High-school | 168 (58.5%) | 69 (56.1%) | 95 (56.5%) | ||
| Marital status | 0.269 | 0.230 | |||
| Married | 212 (73.9%) | 82 (66.7%) | 110 (65.5%) | ||
| Divorced/legally separated | 19 (6.6%) | 11 (8.9%) | 15 (8.9%) | ||
| Widowed | 12 (4.2%) | 10 (8.1%) | 12 (7.1%) | ||
| Single | 44 (15.3%) | 20 (16.3%) | 31 (18.5%) | ||
| Currently employed | 78 (27.2%) | 47 (38.2%) | 66 (39.3%) | 0.035 | 0.009 |
| Race | 0.006 | 0.001 | |||
| African American | 104 (36.2%) | 65 (52.8%) | 91 (54.2%) | ||
| White | 177 (61.7%) | 56 (45.5%) | 71 (42.3%) | ||
| Other | 6 (2.1%) | 2 (1.6%) | 6 (3.6%) | ||
| Hispanic ethnicity | 10 (3.5%) | 5 (4.1%) | 5 (3.0%) | 0.778 | 1.000 |
| Family history type 2 DM | 183 (65.4%) | 83 (69.2%) | 111 (67.3%) | 0.490 | 0.756 |
| Family history CVD | 83 (28.9%) | 29 (23.6%) | 41 (24.4%) | 0.279 | 0.327 |
| Smoking status | 0.470 | 0.345 | |||
| Current | 32 (11.1%) | 19 (15.4%) | 25 (14.9%) | ||
| Former | 144 (50.2%) | 58 (47.2%) | 74 (44.0%) | ||
| Never | 111 (38.7%) | 46 (37.4%) | 69 (41.1%) | ||
| Lives alone | 49 (17.1%) | 23 (18.7%) | 31 (18.5%) | 0.674 | 0.704 |
| Physically active | 151 (52.6%) | 80 (65.0%) | 109 (64.9%) | 0.023 | 0.011 |
| Duration of diabetes, y | 10.5 ± 4.4 | 11.0 ± 4.3 | 11.1 ± 4.5 | 0.313 | 0.227 |
| BMI, kg/m2 | 34.1 ± 7.6 | 35.3 ± 8.0 | 34.8 ± 8.0 | 0.160 | 0.434 |
| BMI ≥30 kg/m2 | 194 (67.6%) | 90 (73.8%) | 119 (71.3%) | 0.242 | 0.462 |
| HbA1c, % | 7.9 ± 1.4 | 8.4 ± 1.8 | 8.3 ± 1.8 | 0.006 | 0.023 |
| Hypertension | 280 (97.6%) | 118 (95.9%) | 161 (95.8%) | 0.356 | 0.399 |
| Depression | 12 (4.2%) | 6 (4.9%) | 7 (4.2%) | 0.794 | 1.000 |
| Hyperlipidemia | 131 (45.6%) | 63 (51.2%) | 89 (53.0%) | 0.332 | 0.145 |
Data are mean ± SD or n (%).
Includes those who did and did not consent to the DNA study.
P from comparison, among those consenting, of those who did and did not return the DNA kit.
P from comparison of those returning the DNA kit and those who participated in the parent study only.
Characteristics of Genetic Substudy Participants Compared with Overall Study Participants
We additionally examined genetic substudy participants to those participating only in the parent study, regardless of consent to be in the genetic substudy. A total of 287 (63.1%) of the entire 455 study participants were included in the genetic substudy. Age, race, sex, employment status, physical activity, and HbA1c were each associated with genetic substudy participation in the full sample (all P < 0.05) (Table 2). In the fully adjustedmodel (Table 3, model 3), age (P = 0.017), sex (P = 0.009), race (P = 0.002), and physical activity (P = 0.013) were each associated with genetic substudy participation. For every 2-year increase in age, the odds of participating in the genetic substudy increased by 1.05. Compared with women, men were 1.7 times more likely to participate in the genetic substudy, whereas compared with non–African Americans, African American race was associated with a 46% lower odds of participating in the genetic substudy, and those who were physically active were 40% less likely to participate in the genetic substudy compared with those who were not physically active.
DISCUSSION
Our study extends the current literature regarding participation in genetic studies by identifying correlates of actual participation in a disease-specific population (type 2 DM) specifically recruited using electronic administrative data. Furthermore, we found evidence of a novel and disease state–specific clinical parameter potentially associated with participation, HbA1c.
In our study, participation loss occurred at multiple steps in this tiered-recruitment process, so that ultimately 63% of those in the parent study donated DNA. However, when considering the 90% of subjects who verbally consented to the genetic substudy (which was necessary to receive a kit), 70% provided written consent and returned the DNA kit. Although this proportion is somewhat lower than some previously described studies of patients who had already agreed to medical research (which reached 80%–90% participation5), some of these feature hospitalized patients, and ours had a higher consent and return rate than described in similarly designed studies.11,12 Given this and the known challenges in recruiting participants in genetic research, and the fact that our approach is broadly applicable to population-wide studies, our 70% genetic substudy participation rate could be viewed quite encouragingly.
We had a rather low overall participation rate in the parent study (53%; n = 455); however, this is comparable to other studies with published response rates that included patients with diabetes or adults from the general population.13,14 Our participants were similar to the identified eligible population for all compared characteristics except race. Even a relatively large nonresponse rate often has no or minimal impact on association estimates, with overaggressive recruitment strategies to force an increase in response rate sometimes causing error or bias in itself.14 Our study, among others, highlights the critical need for development of additional and novel methodological approaches to participant recruitment and retention in both population- and clinic-based studies.
We found similar demographic characteristics associated with participation in the genetic substudy as described elsewhere.4,5,8,15–17 In particular, we again demonstrate that African American participants were less likely to participate in the genetic substudy than non–African Americans. While underlying reasons for lower participation in genetic studies by racial and ethnic minorities are still being explored, data suggest it may be independent of education or socioeconomic status18–20 and that mistrust is a possible factor.21,22 It has also been suggested that African Americans may be more likely to associate the term ‘‘genetics’’ with dysfunctional families and upbringing, a stigmatizing phenomenon.23 A well-designed approach to address the ethical, cultural, and social concerns from the beginning of the recruitment process to the completion of participation may improve the willingness of minorities to become involved in genetic research. For example, a recent systematic review of strategies to increase participation of African Americans in genetics research suggests that initial face-to-face contact for recruitment, rather than the more formal approach of mailing letters used in the current study, may increase participation rates of African Americans.24
Some but not all studies25,26 show that age and educational level, regardless of race/ethnicity, determine participation; older or less educated individuals are often less willing to participate in genetic studies.16,19,27,28 In the current study, age, but not education, was associated with genetic substudy participation, with older individuals being more likely to participate. We did not explore the motivations for participation in our study; however, we share the same speculation as Wood et al.,8 who showed that in their study older respondents were more altruistic in their motivation to participate, whereas younger participants had concerns over time commitment, and middle-aged participants were concerned about privacy and security. Similarly, there are conflicting data on the influence of sex as a predictor of contribution in genetic studies, some associating female sex with nonconsent,4,19 whereas others showing no association with sex.27 In our study, female sex was associated with lower DNA kit return.
We also evaluated the impact of clinical and behavioral characteristics on participation in the genetic substudy. Poor health status is often correlated with lower participation rates.15,28 Depression was associated with participation in some studies,26,29 but not in our sample. On the other hand, increased physical activity was associated with less DNA kit return in our sample, in contrast to a previous study examining exercise capacity as a predictor, which showed no association.4 Our measure of physical activity, which was based on a single self-reported question on time spent in physical activity, however, may have inadequately captured activity levels. While genetic substudy participants and nonparticipants were balanced in most clinical factors, we did find some differences. To our knowledge, we are the first to report that higher HbA1c was related to lower participation in a genetic study. In a single previous study of the effect of lifestyle intervention for weight loss on the risk of serious cardiovascular events among overweight or obese type 2 DM patients (Look AHEAD [Action for Health in Diabetes] Study), no association was found between HbA1c and consent for genetic participation.4 In contrast to the current study, the Look AHEAD Study did not require participants to actively mail back their own DNA specimens. Hemoglobin A1c is considered by some to be a surrogate measure of type 2 DMtreatment adherence/compliance30 and thus may be a disease-specific marker of the underlying likelihood of fully complying with a study protocol. Ascertaining whether HbA1c represents poorer health status or a noncompliance proclivity, however, requires further investigation.
The 2 phases of recruitment and sample return to the genetic substudy should be further investigated. One could compare the written communication/phone interview method to in-person encounters, regular visits, online communications, regular reinforcement such as newsletters, or addressing specific concerns arising after initial agreement to participation. Individuals may be more likely to refuse once they are no longer engaged with a representative from the study.
There are several limitations of our work that should be considered. No adjustment for multiple comparisons was made given the exploratory nature of this study. As Kho et al.31 recently demonstrated, there can be difficulty in discerning between type 1 and type 2 DM using electronic administrative and EMR-based data, and thus we may have included patients with type I DM into our cohort. However, diabetes type was verified during telephone interview and with manual chart abstraction, thereby minimizing the likelihood of including type I DM patients in the present study. We have little to no information on why participants chose not to consent to the genetic substudy nor why they did not return the DNA kit if they originally consented, so we cannot be certain of the mechanisms of any associations. A few subjects volunteered to the interviewer that they reconsidered their participation after reading the written consent document. Language in the consent form has been reported previously as a barrier to participation in epidemiological studies, in general14 with lengthy, legal language often mentioning risks that may be unfounded.32 Developing appropriate consent documents that incorporate the feedback of research participants may improve the understanding of the information within the consent form and potentially could improve participation rates. In addition, lack of face-to-face contact in the current study, while reducing participant burden, may have prohibited the development of trust with the study/ researchers and thus reduced the number of participants willing to return the DNA kit.5,33
Our data suggest that a telephone recruitment/mail-in process may not enhance participation and that those with more severe disease (based on HbA1c) may be less likely to participate. Consistent with prior studies, we found underrepresentation of African Americans in this genetic substudy of patients with type 2 DM. Novel reinforcement tactics should be explored to further mitigate recruitment gaps, and direct comparison of mail to inperson donation would also be informative.
Acknowledgments
This study was supported by funding from Sanofi-Aventis and by a contract from EpiSource, LLC.
REFERENCES
- 1.McCarthy MI. Dorothy Hodgkin lecture 2010. From hype to hope? A journey through the genetics of type 2 diabetes. Diabet Med. 2011;28:132–140. doi: 10.1111/j.1464-5491.2010.03194.x. [DOI] [PubMed] [Google Scholar]
- 2.Rathmann W, Kowall B, Giani G. Type 2 diabetes: unravelling the interaction between genetic predisposition and lifestyle. Diabetologia. 2011;54:2217–2219. doi: 10.1007/s00125-011-2222-5. [DOI] [PubMed] [Google Scholar]
- 3.Matsui K, Kita Y, Ueshima H. Informed consent, participation in, and withdrawal from a population based cohort study involving genetic analysis. J Med Ethics. 2005;31:385–392. doi: 10.1136/jme.2004.009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Espeland MA, Dotson K, Jaramillo SA, et al. Consent for genetics studies among clinical trial participants: findings from Action for Health in Diabetes (Look AHEAD) Clin Trials. 2006;3:443–456. doi: 10.1177/1740774506070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanfear DE, Jones PG, Cresci S, et al. Factors influencing patient willingness to participate in genetic research after a myocardial infarction. Genome Med. 2011;3:39. doi: 10.1186/gm255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sterling R, Henderson GE, Corbie-Smith G. Public willingness to participate in and public opinions about genetic variation research: a review of the literature. Am J Public Health. 2006;96:1971–1978. doi: 10.2105/AJPH.2005.069286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kullo IJ, Fan J, Pathak J, et al. Leveraging informatics for genetic studies: use of the electronic medical record to enable a genome-wide association study of peripheral arterial disease. J Am Med Inform Assoc. 2010;17:568–574. doi: 10.1136/jamia.2010.004366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood F, Kowalczuk J, Elwyn G, et al. Achieving online consent to participation in large-scale gene-environment studies: a tangible destination. J Med Ethics. 2011;37:487–492. doi: 10.1136/jme.2010.040352. [DOI] [PubMed] [Google Scholar]
- 9.Rector TS, Wickstrom SL, Shah M, et al. Specificity and sensitivity of claims-based algorithms for identifying members of Medicare+Choice Health Plans that have chronic medical conditions. Health Serv Res. 2004;39:1839–1857. doi: 10.1111/j.1475-6773.2004.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexander GL, Divine GW, Couper MP, et al. Effect of incentives and mailing features on online health program enrollment. Am J Prev Med. 2008;34:382–388. doi: 10.1016/j.amepre.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kozlowski LT, Vogler GP, Vandenbergh DJ, et al. Using a telephone survey to acquire genetic and behavioral data related to cigarette smoking in “made-anonymous” and “registry” samples. Am J Epidemiol. 2002;156:68–77. doi: 10.1093/aje/kwf010. [DOI] [PubMed] [Google Scholar]
- 12.Bhutta MF, Hobson L, Lambie J, et al. Alternative recruitment strategies influence saliva sample return rates in community-based genetic association studies. Ann Hum Genet. 2013;77:244–250. doi: 10.1111/ahg.12009. [DOI] [PubMed] [Google Scholar]
- 13.Nelson K, Garcia RE, Brown J, et al. Do patient consent procedures affect participation rates in health services research? Med Care. 2002;40:283–288. doi: 10.1097/00005650-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Galea S, Tracy M. Participation rates in epidemiologic studies. Ann Epidemiol. 2007;17:643–653. doi: 10.1016/j.annepidem.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Henderson G, Garrett J, Bussey-Jones J, et al. Great expectations: views of genetic research participants regarding current and future genetic studies. Genet Med. 2008;10:193–200. doi: 10.1097/GIM.0b013e318164e4f5. [DOI] [PubMed] [Google Scholar]
- 16.McQuillan GM, Pan Q, Porter KS. Consent for genetic research in a general population: an update on the National Health and Nutrition Examination Survey experience. Genet Med. 2006;8:354–360. doi: 10.1097/01.gim.0000223552.70393.08. [DOI] [PubMed] [Google Scholar]
- 17.Arar NH, Hazuda H, Steinbach R, et al. Ethical issues associated with conducting genetic family studies of complex disease. Ann Epidemiol. 2005;15:712–719. doi: 10.1016/j.annepidem.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Furr LA. Perceptions of genetics research as harmful to society: differences among samples of African-Americans and European-Americans. Genet Test. 2002;6:25–30. doi: 10.1089/109065702760093889. [DOI] [PubMed] [Google Scholar]
- 19.McQuillan GM, Porter KS, Agelli M, et al. Consent for genetic research in a general population: the NHANES experience. Genet Med. 2003;5:35–42. doi: 10.1097/00125817-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Laskey SL, Williams J, Pierre-Louis J, et al. Attitudes of African American premedical students toward genetic testing and screening. Genet Med. 2003;5:49–54. doi: 10.1097/00125817-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Bussey-Jones J, Garrett J, Henderson G, et al. The role of race and trust in tissue/blood donation for genetic research. Genet Med. 2010;12:116–121. doi: 10.1097/GIM.0b013e3181cd6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shavers VL, Lynch CF, Burmeister LF. Racial differences in factors that influence the willingness to participate in medical research studies. Ann Epidemiol. 2002;12:248–256. doi: 10.1016/s1047-2797(01)00265-4. [DOI] [PubMed] [Google Scholar]
- 23.Schnittker J, Freese J, Powell B. Nature, nurture, neither, nor: black-white differences in beliefs about the causes and appropriate treatment of mental illness. Soc Forces. 2000;78:1101–1132. [Google Scholar]
- 24.Johnson VA, Powell-Young YM, Torres ER, et al. A systematic review of strategies that increase the recruitment and retention of African American adults in genetic and genomic studies. ABNF J. 2011;22:84–88. [PMC free article] [PubMed] [Google Scholar]
- 25.Le Marchand L, Lum-Jones A, Saltzman B, et al. Feasibility of collecting buccal cell DNA by mail in a cohort study. Cancer Epidemiol Biomarkers Prev. 2001;10:701–703. [PubMed] [Google Scholar]
- 26.Murphy EJ, Wickramaratne P, Weissman MM. Racial and ethnic differences in willingness to participate in psychiatric genetic research. Psychiatr Genet. 2009;19:186–194. doi: 10.1097/YPG.0b013e32832cec89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mezuk B, Eaton WW, Zandi P. Participant characteristics that influence consent for genetic research in a population-based survey: the Baltimore epidemiologic catchment area follow-up. Community Genet. 2008;11:171–178. doi: 10.1159/000113880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moorman PG, Skinner CS, Evans JP, et al. Racial differences in enrolment in a cancer genetics registry. Cancer Epidemiol Biomarkers Prev. 2004;13:1349–1354. [PubMed] [Google Scholar]
- 29.Cox LS, Bronars CA, Thomas JL, et al. Achieving high rates of consent for genetic testing among African American smokers. Nicotine Tob Res. 2007;9:711–716. doi: 10.1080/14622200701365228. [DOI] [PubMed] [Google Scholar]
- 30.O’Hea EL, Bodenlos JS, Moon S, et al. The multidimensional health locus of control scales: testing the factorial structure in sample of African American medical patients. Ethn Dis. 2009;19:192–198. [PubMed] [Google Scholar]
- 31.Kho AN, Hayes MG, Rasmussen-Torvik L, et al. Use of diverse electronic medical record systems to identify genetic risk for type 2 diabetes within a genome-wide association study. J Am Med Inform Assoc. 2012;19:212–218. doi: 10.1136/amiajnl-2011-000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamvas A, Madden KK, Nogee LM, et al. Informed consent for genetic research. Arch Pediatr Adolesc Med. 2004;158:551–555. doi: 10.1001/archpedi.158.6.551. [DOI] [PubMed] [Google Scholar]
- 33.Helgesson G. Are enrollment sites the key to optimizing participation in genetic studies? Genome Med. 2011;3:41. doi: 10.1186/gm257. [DOI] [PMC free article] [PubMed] [Google Scholar]