Abstract
The medial preoptic area of the adult sheep contains an ovine sexually dimorphic nucleus (oSDN) that is larger and has more neurons in males than in females. In the lamb fetus, the nascent oSDN occupies the central division of the medial preoptic nucleus (MPNc) and consists of a cluster of cells that is organized by the action of testosterone during gestational days 60 to 90 of a 147 day term pregnancy. The current study sought to determine whether programmed cell death contributes to the emergence of the oSDN. Male and female lamb fetuses were euthanized at different ages spanning the period during which the oSDN is organized. The expression of the pro- and anti-apoptotic genes bcl-2 and bax, respectively, was measured by quantitative RT-PCR to assess possible sex differences in neuron vulnerability to programmed cell death. The appearance of DNA-fragmentation was detected by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and used to estimate the occurrence of apoptotic cell death. We found that bcl-2 and bax mRNA expression in the medial preoptic area of the developing lamb fetus decreased during the last half of the 147-day gestation. The ratio of bcl-2/bax gene expression was highest at gestational day 85 but was equivalent between males and females. TUNEL staining in the MPNc was very low and although it decreased significantly with age, it was not significantly different between sexes. These results using two different methods to assess cell death indicate that a sex difference in the incidence of cell death is not a primary mechanism leading to sexual differentiation of the oSDN.
Keywords: sexual difference, sexual differentiation, TUNEL, bcl2, bax, ovine sexually dimorphic nucleus, medial preoptic nucleus, cell death, sheep, fetus
1. Introduction
A sexually dimorphic nucleus (SDN) exists in the central division of the medial preoptic nucleus (MPNc) of sheep and can be identified as a distinctive cluster of cells that express cytochrome P450 aromatase (CYP 19) mRNA (Roselli, Larkin, Resko, Stellflug, and Stormshak, 2004). This nucleus, which is referred to as the ovine sexually dimorphic nucleus (oSDN), is larger and contains more neurons in males than in females and has been associated with gender-typical sexual partner preferences in sheep (Roselli, Larkin, Resko, Stellflug, and Stormshak, 2004). The oSDN volume becomes sexually dimorphic in fetal life by gestational day 135 of a 147 day term sheep pregnancy (Roselli, Stadelman, Reeve, Bishop, and Stormshak, 2007). The sex difference appears to be due to the organizational effects of testosterone since treatment of females with testosterone from gestational days 60 – 90 eliminates the sex difference in the oSDN while testosterone manipulations in adulthood do not (Roselli, Estill, Stadelman, Meaker, and Stormshak, 2011;Roselli, Estill, Stadelman, and Stormshak, 2009). However, the cellular mechanism whereby testosterone controls the development of the oSDN is not yet established. The current study sought to determine whether programmed cell death contributes to the emergence of sex differences in the oSDN.
Several mammals possess sexually dimorphic preoptic nuclei (SDN-POA) including: rats, gerbils, guinea pigs, hyenas, ferrets, monkeys, and humans (Allen, Hines, Shryne, and Gorski, 1989;Byne, 1998;Commins and Yahr, 1984;Fenstemaker, Zup, Frank, Glickman, and Forger, 1999;Gorski, Gordon, Shryne, and Southam, 1978;Gorski, Harlan, Jacobson, Shryne, and Southam, 1980;Hines, Davis, Coquelin, Goy, and Gorski, 1995;Swaab and Fliers, 1985;Tobet, Zahniser, and Baum, 1986). Of these, the rat SDN-POA is the best studied. The SDN-POA is 3–5 times larger in male than in female rats and depends on the actions of estrogenic compounds derived by the aromatization of testosterone (Dohler, Srivastava, Shryne, Jarzab, Sipos, and Gorski, 1984). The hormone-induced masculinization of the SDN-POA is due, at least in part, to differential cell death during postnatal development (Davis, Popper, and Gorski, 1996;Dodson and Gorski, 1993). Female rats have a higher rate of cell death than males between postnatal days 7 and 10 and by the second week of life have fewer cells in the SDN-POA than do males (Chung, Swaab, and De Vries, 2000;Davis, Popper, and Gorski, 1996;Dodson and Gorski, 1993;Tsukahara, Kakeyama, and Toyofuku, 2006). Treating female rat pups with testosterone or estradiol around the time of birth reduces postnatal apoptosis in the SDN-POA (Arai, Sekine, and Murakami, 1996;Chung, Swaab, and De Vries, 2000;Davis, Popper, and Gorski, 1996;Yang, Chen, Hsieh, Jin, Hsu, and Hsu, 2004), and masculinizes its volume in adulthood.
Short-lived changes in cell and nuclear morphology that presage cell death are dramatic and defining characteristics of apoptosis. They are accompanied by DNA fragmentation and can be quantified by labeling the terminal end of nucleic acids using the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay (Gavrieli, Sherman, and Ben-Sasson, 1992). Apoptosis in the developing brain is regulated by proteins of the B cell lymphoma 2 (Bcl-2) family (Merry and Korsmeyer, 1997). Bcl-2 is anti-apoptotic and promotes cell survival, whereas Bcl-2–associated X protein (Bax) promotes apoptosis by forming heterodimers with Bcl-2 in vivo to neutralize its actions (White, Keller-Peck, Knudson, Korsmeyer, and Snider, 1998). The ratio of Bcl-2 to Bax determines the relative vulnerability of cells to succumb to apoptosis (Oltval, Milliman, and Korsmeyer, 1993). Higher levels of Bcl-2 and lower levels of Bax protein are present in the SDN-POA of male rats than of female rats during the postnatal critical period (Tsukahara, Kakeyama, and Toyofuku, 2006). Moreover, treatment with estradiol not only masculinizes the volume of the SDN-POA, but also reverses the sex difference in the Bcl-2/Bax ratio (Tsukahara, Hojo, Kuroda, and Fujimaki, 2008). The precise cellular mediators promoting neuronal survival in males are not yet known, although several proteins have been implicated including NMDA receptor signaling molecules and neurotrophic proteins such as RNA binding motif protein 3 and alpha-tubulin (Hsu, Shao, Tsai, Shih, Lee, and Hsu, 2005;Hsu, Yang, Shih, Hsieh, Chen, and Hsu, 2001;Tsukahara, 2009;Tsukahara, Hojo, Kuroda, and Fujimaki, 2008)
Despite strong evidence of a role for apoptosis in the rat model, modulation of cell death does not appear to contribute to the formation of the SDN in the male ferret (Park, Tobet, and Baum, 1998). More recently, the role of cell death as a universal mechanism underlying brain sex differences in rodents has been called into question. The use of genetically modified mice that either overexpress Bcl-2 or lack expression of Bax demonstrate that apoptotic mechanisms do not generate sex differences in all neuronal phenotypes within specific nuclei (De Vries, Jardon, Reza, Rosen, Immerman, and Forger, 2008;Semaan, Murray, Poling, Dhamija, Forger, and Kauffman, 2010;Zup, Carrier, Waters, Tabor, Bengston, Rosen, Simerly, and Forger, 2003). For example, these genetic manipulations did not alter the sex difference in the number of vasopressinergic neurons in the sexually dimorphic bed n. of the stria terminalis (De Vries, Jardon, Reza, Rosen, Immerman, and Forger, 2008). Thus, it remains an open question to what extent apoptosis/cell survival is involved in the determination of brain sex differences in other species such as the sheep.
In contrast to most models of brain sexual differentiation, the sheep is a long-gestation species in which brain sexual differentiation occurs largely prior to birth over an extended period of fetal growth and thus more closely mimics human brain development. The current study found that a reduction in programmed cell death during the critical period does not contribute to the emergence of the male-typical oSDN in sheep.
2. Results
2.1 Relative expression of pro- and anti-apoptotic genes
Analysis of bcl-2 mRNA expression by 2-way ANOVA revealed significant main effects of age (F[3,23] =19.6; P <0.001) and sex (F[1,23] = 25.4; P <0.001. The expression of bcl-2 mRNA was greater in females than in males and decreased significantly with age in both sexes across the four fetal ages studied (Fig. 1A). Bax mRNA expression exhibited a significant main effect of age (F[3,23] = 73.3; P <0.001) and a significant age x sex interaction (F[3,23] = 3.6; P <0.05). Bax expression was significantly higher at gestational day 65 than at all other ages and significantly lower at gestational day 85 than at all other ages (Fig. 1B). Although no main effect of sex was observed (F[1,23] = 0.3; P >0.05), post hoc analysis revealed that bax mRNA was greater in males than in females at gestational day 65 accounting for the interaction. Fig. 1C shows the ratio of bcl-2 to bax expression across the fetal ages studied. There were no sex differences in bcl-2 to bax ratio (F[1,23] = 2.5; P >0.05), but there was a main effect of age on the ratio bcl-2 to bax (F[2,23] = 49.9; P <0.001). In both sexes, the ratio was significantly higher at gestational day 85 than at all other ages (P<0.05).
Figure 1.
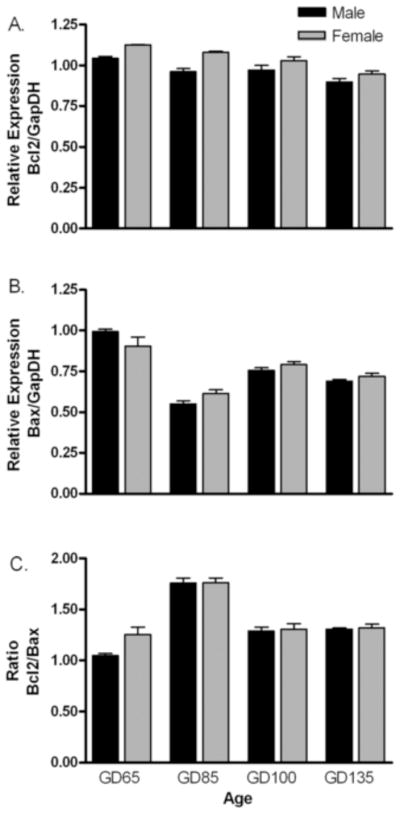
Developmental expression of bcl-2 (A), bax (B) and bcl-2/bax ratio (C) in fetal lamb MPOA. Expression of target gene is normalized to GAPDH mRNA expression in the same sample. Data are mean ± SE fold differences relative to mean expression of gestational day 65 male fetuses (n = 3–4 animals per group). Two-way ANOVA revealed significant age effects in all 3 measures, but only bcl-2 mRNA expression exhibited a significant main effect of sex (females > males).
2.2 Incidence of Apoptotic Cells
The cross sectional area of the MPNc significantly increased with gestational age from 0.13 to 0.25 mm2 (F[4,29] = 4.9; P <0.05), but there were no sex differences (F[1,29] = 0.3; P >0.05). Cell density significantly decreased from 1.5 × 104 to 0.5 × 104 cells/mm2 (F[4,29] = 86; P <0.001) without exhibiting significant sex differences (F[1,29] = 0.45; P >0.05). Very few TUNEL-positive cells were evident in the MPNc during any of the 5 fetal ages that were examined. This translated into a low percent TUNEL staining i.e., ≤0.3%. An example of positive TUNEL labeling is illustrated in Fig. 2. The percent TUNEL staining (Fig. 3) did not significantly differ by sex (F[1,29] = 0.03; P <0.05) or by age (F[4,25] = 2.5; P <0.05).
Figure 2.
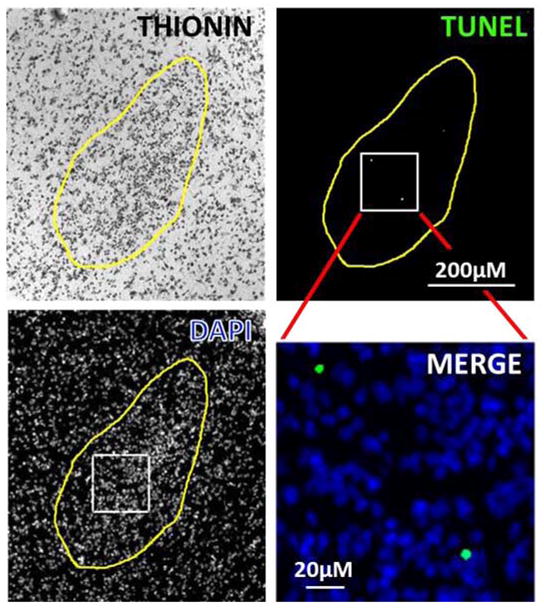
Example of staining of cells in the MPNc of an 86-day-old female lamb fetus. Composite figure shows thionin, DAPI, TUNEL and an enlarged inset of the merged DAPI and TUNEL staining.
Figure 3.
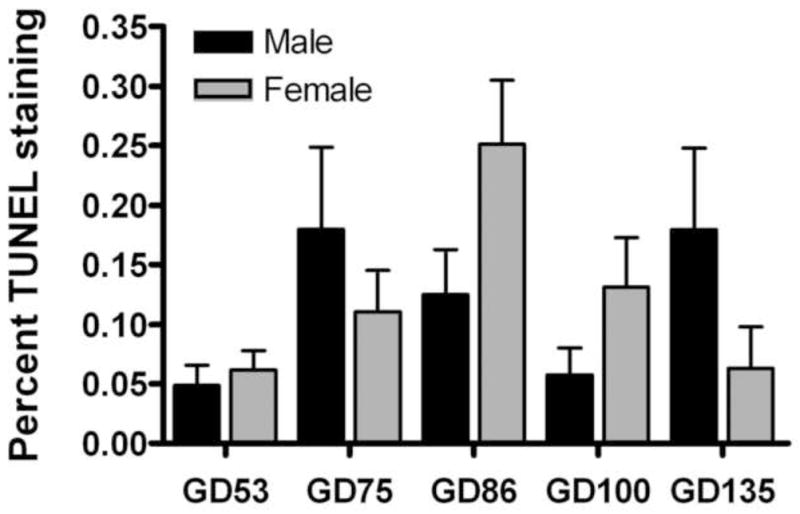
Development profile of the percentage TUNEL staining in the MPNc of lamb fetuses. Data are mean ± SE (n = 3–5 animals per group). Analysis of the data by two-way ANOVA revealed no significant main effects of age or sex.
Discussion
The results of this study indicate prenatal modulation of cell death does not play a primary role in generating a larger oSDN in male than in female sheep. We have used two different approaches that correlate with cell death. The results of both assays indicate that there is no sex differences in the incidence of apoptosis in the fetal lamb MPNc at any gestational age examined. We found evidence that bcl-2 and bax mRNA were expressed in the MPOA of the developing lamb fetus and appeared to decrease during the last half of the 147-day gestation. There was a significantly greater bcl2 expression in females over all ages and bax was significantly higher in males on GD 65. These results are opposite from those expected if the intrinsic cell death pathway mediates sexual differentiation of the oSDN. When the data were expressed as the ratio of anti-apoptotic bcl-2 to pro-apoptotic bax expression, an index of the relative vulnerability of cells to succumb to the cell death process (Mu, He, Anderson, Trojanowski, and Springer, 1996), there were no statistically significant sex or age differences to suggest that males are less susceptible than females to programmed cell death. Likewise, TUNEL revealed a low incidence of apoptotic cell death within the MPNc where the oSDN is differentiating, and there was no evidence of a significant sex difference in this measure.
The critical period during which testosterone organizes the MPNc in sheep extends from gestational days 60 to 90, while the oSDN is clearly discernible by gestational day 135 by Nissl-staining and aromatase mRNA expression (Roselli, Estill, Stadelman, Meaker, and Stormshak, 2011). In the present study, there was a trend for cell death to be elevated in the MPNc on GD 85 in females, but no statistically significant sex differences were evident at any of the gestational ages that were sampled. Thus, although we do not rule out a role for cell death in the development of the oSDN, it does not appear to play a principal role in establishing the volumetric sex difference. Our results differ from previous studies in rats that found the incidence of apoptosis was higher in the MPNc and principal nucleus of the bed n. of stria terminalis of postnatal females than of males (Chung, Swaab, and De Vries, 2000;Davis, Popper, and Gorski, 1996). Gonadal steroids appear to control the occurrence of cell death in the rat MPNc because testosterone or estradiol treatments reduce apoptosis in neonatal females and castrated males (Arai, Sekine, and Murakami, 1996;Chung, Swaab, and De Vries, 2000;Davis, Popper, and Gorski, 1996). Higher levels of bcl-2 protein together with lower levels of bax protein and activated caspase-3 are expressed in the MPNc of neonatal male rats than of females (Hsu, Shao, Tsai, Shih, Lee, and Hsu, 2005;Tsukahara, Kakeyama, and Toyofuku, 2006). In addition, postnatal treatment of female rats with estradiol increases bcl-2 and decreases bax protein expression demonstrating that gonadal steroid-regulated apoptosis during development, at least in part, occurs through the mitochondrial pathway (Tsukahara, Hojo, Kuroda, and Fujimaki, 2008). These results provide evidence that the control of neuron number by postnatal apoptosis is one process that could be responsible for the emergence of differences in nuclear volume and cell number in the sexually dimorphic areas of the adult rodent brain.
The low incidence of TUNEL staining across the fetal ages examined in the present study is not completely unexpected and probably does not account for the lack of a sex difference. In rats where the SDN volume is 5-fold larger in males than in females, only ~1.5% of cells in this region are apoptotic during the neonatal critical period (Davis, Popper, and Gorski, 1996). Similarly low levels of apoptosis have been reported in other sexually dimorphic nuclei (Holman, Collado, Skepper, and Rice, 1996;Murakami and Arai, 1989;Park, Tobet, and Baum, 1998). The in vivo time course for elimination of apoptotic cells has been estimated to take about 72 h (Hu, Yuri, Ozawa, Lu, and Kawata, 1997), so it is possible that our sampling schedule is insufficiently comprehensive to capture the critical period for apoptosis in females. It is also possible that sex differences in the incidence of cell death are regionally localized within the nucleus or exist within the migratory pathway for cells that populate it, either of which would have been missed by the limits of the sampling procedure employed. For these reasons, the current study cannot totally exclude the possibility that the modulation of cell death plays some role in the sexual differentiation of the oSDN. Thus, future studies using more frequent sampling should be performed to fully assess the degree to which apoptosis is involved in oSDN formation.
Other possible mechanisms for generating sex-related brain volume differences include: differential neurogenesis, cell migration, or differentiation of phenotype (Forger, 2006). Neurons comprising the SDN-POA of rats originate from the subependymal lining of the 3rd ventricle (Jacobson, Davis, and Gorski, 1985). Studies that examined birthdate of SDN-POA neurons found similar numbers generated prenatally in male and female rats (Jacobson, Davis, and Gorski, 1985;Orikasa, Kondo, Usui, and Sakuma, 2010). Studies in rats and mice support the idea that migration plays a major role in the establishment of the SDN-POA (Hamada and Sakuma, 2010;Orikasa, Kondo, and Sakuma, 2007;Orikasa and Sakuma, 2010). Migration into the SDN-POA is faster in male than in female mice suggesting a way that more cells could reach their final destination in males than in females and ultimately generate a larger nucleus (Tobet, Knoll, Hartshorn, Aurand, Stratton, Kumar, Searcy, and McClellan, 2009). Recent evidence indicates that stem cell activity in the rostral end of the 3rd ventricle may continue to play a role in the postnatal development and structural maintenance of the SDN-POA in rats (He, Ferguson, Cui, Greenfield, and Paule, 2013).
In ferrets, a species like sheep in that a sexually dimorphic medial preoptic nucleus develops prior to birth (Cherry, Basham, Weaver, Krohmer, and Baum, 1990), Park et al. (Park, Baum, Paredes, and Tobet, 1996;Park, Tobet, and Baum, 1998) found no sex difference in neurogenesis, migration or cell death and concluded that the specification of a distinctive neuronal phenotype i.e., soma size, area of dendritic fields or capacity to produce a specific molecule may be responsible for establishing volumetric sex differences in brain. The role of cell death and migration has also been challenged by recent studies in mice (Gilmore, Varnum, and Forger, 2012), which show that blocking bax-dependent developmental cell death through targeted deletion of the bax gene does not prevent sex differences from developing in the number of calbindin-immunoreactive neurons in the SDN. Mice overexpressing bcl-2 or carrying null mutation for bax also retained sex differences in the number of vasopressin and calbindin neurons in the bed n. of the stria terminalis, and kisspeptin in the anteroventral periventricular preoptic nucleus, while overall number of neurons expressing these neuropeptides was unchanged (De Vries, Jardon, Reza, Rosen, Immerman, and Forger, 2008;Gilmore, Varnum, and Forger, 2012;Semaan, Murray, Poling, Dhamija, Forger, and Kauffman, 2010). Together these studies suggest that the differentiation of cellular phenotypes may be a more likely mechanism for establishing brain sex differences than hormonal regulation of cell death.
In summary, we propose that the volumetric sex difference found in the sheep MPNc may not rely on differences in the incidence cell death, but may instead be the result of testosterone’s action on the differentiation of neuronal phenotype. The sheep model has not been as extensively studied as rodents and ferrets. However, by extrapolating from these other models, it seems unlikely that prenatal testosterone controls neurogenesis or migration to produce the oSDN. On the other hand, testosterone is an established regulator of dendritic growth (Cherry, Tobet, DeVoogd, and Baum, 1992;Mu, He, Anderson, Trojanowski, and Springer, 1996), and may thereby increase male oSDN volume by increasing neuropil volume. More research is needed to identify whether MPNc neurons in sheep exhibit a distinctive sex-related cellular phenotype in terms of soma area or dendritic fields, and whether regulation of these parameters by prenatal testosterone can account for the volumetric sex differences observed in the adult oSDN. A phenotypic signature of cells in the sheep oSDN is that they express aromatase and androgen receptors (Roselli, Larkin, Resko, Stellflug, and Stormshak, 2004;Scott, Clarke, Rao, and Tilbrook, 2004); raising the possibility that fetal exposure to testosterone acts to determine the potential for aromatase and androgen receptor gene expression in a subset of neurons that migrate into preoptic area and determine the boundaries of the MPNc during development.
3. Experimental procedures
3.1. Animals
Polypay ewes (2–3 yr old) were bred and maintained under standard husbandry conditions at the AAALAC-approved Sheep Research Facility at Oregon State University in Corvallis, OR. The gestational stage was defined as the time after mating (gestational day 1 = 24 h after mating). Lamb fetuses of various gestational ages ± 2 days (i.e., gestational days 53, 65, 75, 85, 100, and 135) were delivered surgically as described previously (Roselli, Estill, Stadelman, Meaker, and Stormshak, 2011). Three to five fetuses of each sex were studied at each age. All procedures complied with the principles and procedures of the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Oregon Health and Science University and Oregon State University.
3.2. Tissue collection and preparation
Brains were removed rapidly from the fetal skulls and split mid-sagittaly. The medial preoptic area (MPOA), a region encompassing the MPNc, was dissected from the left half of the brain and used for gene expression analysis. A larger diencephalic block of tissue that extended from the rostral aspect of the optic chiasm to the mammillary bodies was dissected from the right half of the brain and processed for analysis of TUNEL staining. The tissue blocks were immersion fixed in 4% paraformaldehyde overnight and then cryoprotected with 20% sucrose in 0.1M Sorenson’s buffer for 3–4days at 4°C, and then they were frozen in OCT (Tissue Tek) and stored at −80°C. Four parallel series of coronal sections were cut through the tissue on a cryostat (20 μm thick) and mounted onto Superfrost microscope slides (Fisher Scientific Co., Pittsburgh, PA, USA). The slides were desiccated under vacuum overnight and stored at −80°C. For GD53 fetuses a bilateral diencephalic block was used. Diencephalons from GD65 fetuses were damaged during processing and a separate set of GD75 tissues were used instead for histological analysis.
3.3. RNA extraction and real time reverse transcriptase-polymerase chain reaction (RT-PCR) quantification
Total RNA was extracted from fresh frozen MPOA according to manufacturer protocol using the Trizol® Reagent (Invitrogen, Life Technologies; Grand Island, NY). RNA concentration and quality was assayed using the Nanodrop 1000 spectrophotometer (Thermo Fisher Scientific) and stored at a working concentration of 62.5 ng/μl at −80°C. RNA samples (500ng) were converted to cDNA by reverse transcription using the First Strand Superscript III Kit (Invitrogen) according to the manufacturer’s instructions. The resulting cDNA samples were stored at −20°C until real-time (RT)-PCR was performed.
Primer pairs for bcl-2, bax and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) genes were designed using the Clone Manager Professional Suite Software 8.0 (Sci-Ed Software; Cary, NC). Primers were designed against Ovis aries (domestic sheep) sequences in the NCBI Genbank (Table 1). Sequences span intron/exon boundaries. In cases where intron sequence of Ovis aries was not reported, the positions of intron sequences were extrapolated based on homology to reported bovine sequences.
Table 1.
Ovine bcl-2, bax and GAPDH primer sequences used in RT-PCR
| Sense Primer | Antisense Primer | Accession # | Size (bp) | |
|---|---|---|---|---|
| Bax | CCGACGGCAACTTCAACTGG | GATCAACTCGGGCACCTTGG | AF163774.1 | 98 |
| Bcl-2 | GGATGACCGAGTACCTGAAC | GGCCATACAGCTCCACAAAG | DQ152929.1 | 84 |
| GAPDH | TCATCCATGACCACTTTG | AGTAGAAGCAGGGATGATGT | NM_001190390.1 | 145 |
Conventional PCR was performed using the Platinum® Taq DNA Polymerase (Invitrogen) with primer pairs to confirm the specificity of amplification by validating a product with the predicted size. RT-PCR was used to analyze bax, bcl-2, and GAPDH gene expression. GAPDH RNA was used as an internal control because it was shown previously not to be developmentally regulated and therefore valid to use as an housekeeping gene (Roselli and Stormshak, 2011). All reactions were run in an ABI Fast 7500 Thermal Cycler (Applied Biosystems, Life Technologies). Triplicate samples were amplified in a PowerSYBR Green Master Mix (Invitrogen) using a polymerase activation step at 95°C for 10 min followed by 45 cycles of 95°C for 15 s and 60°C for 60s. Primer efficiencies (>90%) and melting curves were validated at 300 nM, using the standard curve method, which assays threshold fluorescence of cDNA dilutions (1:2, 1:8, 1:32, 1:128, and 1:512). Control reactions were run at the same time to verify the primers were specific for amplified product and that the RNA was not contaminated with genomic DNA. Quantification of gene expression was performed by the relative standard curve method (Larionov, Krause, and Miller, 2005), normalized against the reference gene GAPDH, and reported as the fold difference relative to the mean expression level in MPOA of 65-day-old male fetuses.
3.4. TUNEL assay
Thionin-stained brain sections were used to identify the central division of the MPNc, a region of dense cluster of darkly staining cells, which corresponds to the oSDN in fetal and adult sheep (Roselli, Larkin, Resko, Stellflug, and Stormshak, 2004;Roselli, Stadelman, Reeve, Bishop, and Stormshak, 2007). For each subject, one to three 20-μm sections that were serially adjacent to thionin-stained sections and contained the MPNc were analyzed for the presence of apoptotic cells by TUNEL using the Apoptag Plus Fluorescein In Situ Apoptosis Detection Kit S7111(Chemicon/Millpore, Billerica, MA) according to instructions provided by the manufacturer. Positive control tissues from a normal female rodent mammary gland provided by the Apoptag Kit were included in each round of staining. Tissues were coverslipped using Prolong Gold antifade reagent with DAPI (Invitrogen) as a nuclear stain.
4.5. Image acquisition and analysis
Brain sections were observed under a Zeiss florescence microscope and photographed with an AxioCam camera (Carl Zeiss, Inc., Thornwood, NY). Image processing, area measurements and cell counts were performed with NIH Image J (Schneider, Rasband, and Eliceiri, 2012). The location of the MPNc was identified visually using anatomical landmarks established in the adult brain (Roselli, Larkin, Resko, Stellflug, and Stormshak, 2004) and the boundaries were outlined by hand. The trace was used to digitally measure the cross-sectional area of the MPNc and then applied to the fluorescein image for sampling. The number of apoptotic TUNEL events was counted in the thresholded fluorescein images within the boundaries of the MPNc. Threshold was set at 2 X background levels to distinguish positive staining from non-specific background. To distinguish between specific and non-specific TUNEL staining, TUNEL-positive DNA fragments i.e., objects were characterized as distinct, round and brightly stained objects that were easily distinguishable at 100 X magnification. These objects were localized within a group of DAPI stained nuclei. Since DAPI labels healthy cell nuclei, which excludes apoptotic cells, the percent TUNEL staining was calculated using the following formula: number of TUNEL events/(number of TUNEL events + number of cells) X 100 (Daniel and DeCoster, 2004).
4.6. Statistical analysis
Two-way analysis of variance (ANOVA) was used to determine the main effects of age and sex and the interaction between age and sex. Data was log transformed to correct for unequal variances. When significant interactions were detected, Fisher’s protected least significant difference tests were used to make post hoc comparisons among experimental groups. A probability of P < 0.05 was considered significant.
Highlights.
The oSDN of adult sheep is larger and has more neurons in males than in females.
This study assessed the role of cell death in formation of the oSDN.
Ratio of Bcl2/Bax mRNA and incidence of apoptosis were not different between sexes.
Differential cell death is not a primary mechanism in sexual differentiation of the oSDN.
Acknowledgments
We thank our work study students for helping with the care of our animals and OSU veterinary students for helping with surgical delivery of fetuses. This work was supported by NIH R01 OD011047 (to CER).
Abbreviations
- Bax
Bcl-2–associated X protein
- Bcl-2
B cell lymphoma 2
- CYP 19
cytochrome P450 aromatase
- DAPI
4′,6-diamidino-2-phenylindole
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- MPNc
central component of the medial preoptic nucleus
- MPOA
medial preoptic area
- oSDN
ovine sexually dimorphic nucleus
- RT-PCR
reverse transcriptase-polymerase chain reaction
- SDN-POA
sexually dimorphic preoptic nucleus
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen LS, Hines M, Shryne JE, Gorski RA. Two sexually dimorphic cell groups in the human brain. J Neurosci. 1989;9:497–506. doi: 10.1523/JNEUROSCI.09-02-00497.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai Y, Sekine Y, Murakami S. Estrogen and apoptosis in the developing sexually dimorphic preoptic area in female rats. Neurosci Res. 1996;25:403–407. doi: 10.1016/0168-0102(96)01070-x. [DOI] [PubMed] [Google Scholar]
- Byne W. The medial preoptic and anterior hypothalamic regions of the rhesus monkey: cytoarchitectonic comparison with the human and evidence for sexual dimorphism. Brain Res. 1998;793:346–350. doi: 10.1016/s0006-8993(98)00275-3. [DOI] [PubMed] [Google Scholar]
- Cherry JA, Basham ME, Weaver CE, Krohmer RW, Baum MJ. Ontogeny of the sexually dimorphic male nucleus in the preoptic/anterior hypothalamus of ferrets and its manipulation by gonadal steroids. J Neurobiol. 1990;21:844–857. doi: 10.1002/neu.480210603. [DOI] [PubMed] [Google Scholar]
- Cherry JA, Tobet SA, DeVoogd TJ, Baum MJ. Effects of sex and androgen treatment on dendritic dimensions of neurons in the sexually dimorphic preoptic/anterior hypothalamic area of male and female ferrets. J Comp Neurol. 1992;323:577–585. doi: 10.1002/cne.903230410. [DOI] [PubMed] [Google Scholar]
- Chung WC, Swaab DF, De Vries GJ. Apoptosis during sexual differentiation of the bed nucleus of the stria terminalis in the rat brain. J Neurobiol. 2000;43:234–243. [PubMed] [Google Scholar]
- Commins D, Yahr P. Adult testosterone levels influence the morphology of a sexually dimorphic area in the Mongolian gerbil brain. J Comp Neurol. 1984;224:132–140. doi: 10.1002/cne.902240112. [DOI] [PubMed] [Google Scholar]
- Daniel B, DeCoster MA. Quantification of sPLA2-induced early and late apoptosis changes in neuronal cell cultures using combined TUNEL and DAPI staining. Brain Res Protoc. 2004;13:144–150. doi: 10.1016/j.brainresprot.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Davis EC, Popper P, Gorski RA. The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Res. 1996;734:10–18. [PubMed] [Google Scholar]
- De Vries GJ, Jardon M, Reza M, Rosen GJ, Immerman E, Forger NG. Sexual differentiation of vasopressin innervation of the brain: cell death versus phenotypic differentiation. Endocrinology. 2008;149:4632–4637. doi: 10.1210/en.2008-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson RE, Gorski RA. Testosterone propionate administration prevents the loss of neurons within the central part of the medial preoptic nucleus. J Neurobiol. 1993;24:80–88. doi: 10.1002/neu.480240107. [DOI] [PubMed] [Google Scholar]
- Dohler KD, Srivastava SS, Shryne JE, Jarzab B, Sipos A, Gorski RA. Differentiation of the sexually dimorphic nucleus in the preoptic area of the rat brain is inhibited by postnatal treatment with an estrogen antagonist. Neuroendocrinology. 1984;38:297–301. doi: 10.1159/000123907. [DOI] [PubMed] [Google Scholar]
- Fenstemaker SB, Zup SL, Frank LG, Glickman SE, Forger NG. A sex difference in the hypothalamus of the spotted hyena. Nature Neurosci. 1999;2:943–945. doi: 10.1038/14728. [DOI] [PubMed] [Google Scholar]
- Forger NG. Cell death and sexual differentiation of the nervous system. Neuroscience. 2006;138:929–938. doi: 10.1016/j.neuroscience.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore RF, Varnum MM, Forger NG. Effects of blocking developmental cell death on sexually dimorphic calbindin cell groups in the preoptic area and bed nucleus of the stria terminalis. Biol Sex Differ. 2012;3:5. doi: 10.1186/2042-6410-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex difference within the medial preoptic area or the rat brain. Brain Res. 1978;148:333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- Gorski RA, Harlan RE, Jacobson CD, Shryne JE, Southam AM. Evidence for the existence of a sexually dimorphic nucleus in the preoptic area of the rat. J Comp Neurol. 1980;193:529–539. doi: 10.1002/cne.901930214. [DOI] [PubMed] [Google Scholar]
- Hamada T, Sakuma Y. Estrogen receptor {alpha} gene promoter 0/B usage in the rat sexually dimorphic nucleus of the preoptic area. Endocrinology. 2010;151:1923–1928. doi: 10.1210/en.2009-1022. [DOI] [PubMed] [Google Scholar]
- He Z, Ferguson SA, Cui L, Greenfield LJ, Paule MG. Role of neural stem cell activity in postweaning development of the sexually dimorphic nucleus of the preoptic area in rats. PLoS ONE. 2013;8:e54927. doi: 10.1371/journal.pone.0054927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M, Davis FC, Coquelin A, Goy RW, Gorski RA. Sexually dimorphic regions in the medial preoptic area and the bed nucleus of the stria terminalis of the guinea pig brain: a description and an investigation of their relationship to gonadal steroids in adulthood. J Neurosci. 1995;5:40–47. doi: 10.1523/JNEUROSCI.05-01-00040.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman SD, Collado P, Skepper JN, Rice A. Postnatal development of a sexually dimorphic, hypothalamic nucleus in gerbils: A stereological study of neuronal number and apoptosis. J Comp Neurol. 1996;376:315–325. doi: 10.1002/(SICI)1096-9861(19961209)376:2<315::AID-CNE12>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Hsu HK, Shao PL, Tsai KL, Shih HC, Lee TY, Hsu C. Gene regulation by NMDA receptor activation in the SDN-POA neurons of male rats during sexual development. J Mol Endocrinol. 2005;34:433–445. doi: 10.1677/jme.1.01601. [DOI] [PubMed] [Google Scholar]
- Hsu HK, Yang RC, Shih HC, Hsieh YL, Chen UY, Hsu C. Prenatal exposure of testosterone prevents SDN-POA neurons of postnatal male rats from apoptosis through NMDA receptor. J Neurophysiol. 2001;86:2374–2380. doi: 10.1152/jn.2001.86.5.2374. [DOI] [PubMed] [Google Scholar]
- Hu Z, Yuri K, Ozawa H, Lu H, Kawata M. The in vivo time course for elimination of adrenalectomy-induced apoptotic profiles from the granule cell layer of the rat hippocampus. J Neurosci. 1997;17:3981–3989. doi: 10.1523/JNEUROSCI.17-11-03981.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson CD, Davis FC, Gorski RA. Formation of the sexually dimorphic nucleus of the preoptic area: neural growth, migration and changes in cell number. Dev Brain Res. 1985;21:7–18. doi: 10.1016/0165-3806(85)90019-7. [DOI] [PubMed] [Google Scholar]
- Larionov A, Krause A, Miller W. A standard curve based method for relative real time PCR data processing. BMC Bioinformatics. 2005;6:62. doi: 10.1186/1471-2105-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merry DE, Korsmeyer SJ. Bcl-2 gene family in the nervous system. Annu Rev Neurosci. 1997;20:245–267. doi: 10.1146/annurev.neuro.20.1.245. [DOI] [PubMed] [Google Scholar]
- Mu X, He J, Anderson DW, Trojanowski JQ, Springer JE. Altered expression of bcl-2 and bax mRNA in amyotrophic lateral sclerosis spinal cord motor neurons. Ann Neurol. 1996;40:379–386. doi: 10.1002/ana.410400307. [DOI] [PubMed] [Google Scholar]
- Murakami S, Arai Y. Neuronal death in the developing sexually dimorphic periventricular nucleus of the preoptic area in the female rat: Effect of neonatal androgen treatment. Neurosci Lett. 1989;102:185–190. doi: 10.1016/0304-3940(89)90076-1. [DOI] [PubMed] [Google Scholar]
- Oltval ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Orikasa C, Kondo Y, Sakuma Y. Transient transcription of the somatostatin gene at the time of estrogen-dependent organization of the sexually dimorphic nucleus of the rat preoptic area. Endocrinology. 2007;148:1144–1149. doi: 10.1210/en.2006-1214. [DOI] [PubMed] [Google Scholar]
- Orikasa C, Kondo Y, Usui S, Sakuma Y. Similar numbers of neurons are generated in the male and female rat preoptic area in utero. Neurosci Res. 2010;68:9–14. doi: 10.1016/j.neures.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Orikasa C, Sakuma Y. Estrogen configures sexual dimorphism in the preoptic area of C57BL/6J and ddN strains of mice. J Comp Neurol. 2010;518:3618–3629. doi: 10.1002/cne.22419. [DOI] [PubMed] [Google Scholar]
- Park JJ, Baum MJ, Paredes RG, Tobet SA. Neurogenesis and cell migration into the sexually dimorphic preoptic area/anterior hypothalamus of the fetal ferret. J Neurobiol. 1996;30:315–328. doi: 10.1002/(SICI)1097-4695(199607)30:3<315::AID-NEU1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Park JJ, Tobet SA, Baum MJ. Cell death in the sexually dimorphic dorsal preoptic area/anterior hypothalamus of perinatal male and female ferrets. J Neurobiol. 1998;34:242–252. doi: 10.1002/(sici)1097-4695(19980215)34:3<242::aid-neu4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Estill C, Stadelman HL, Meaker M, Stormshak F. Separate critical periods exist for testosterone-induced differentiation of the brain and genitals in sheep. Endocrinology. 2011;152:2409–2415. doi: 10.1210/en.2010-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli CE, Estill CT, Stadelman HL, Stormshak F. The volume of the ovine sexually dimorphic nucleus of the preoptic area is independent of adult testosterone concentrations. Brain Res. 2009;1249:113–117. doi: 10.1016/j.brainres.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli CE, Larkin K, Resko JA, Stellflug JN, Stormshak F. The volume of a sexually dimorphic nucleus in the ovine medial preoptic area/anterior hypothalamus varies with sexual partner preference. Endocrinology. 2004;145:478–483. doi: 10.1210/en.2003-1098. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Stadelman H, Reeve R, Bishop CV, Stormshak F. The ovine sexually dimorphic nucleus of the medial preoptic area is organized prenatally by testosterone. Endocrinology. 2007;148:4450–4457. doi: 10.1210/en.2007-0454. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Stormshak F. Ontogeny of cytochrome P450 aromatase mRNA expression in the developing sheep brain. J Neuroendocrinol. 2011;24:443–452. doi: 10.1111/j.1365-2826.2011.02260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Meth. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CJ, Clarke IJ, Rao A, Tilbrook AJ. Sex differences in the distribution and abundance of androgen receptor mRNA-containing cells in the preoptic area and hypothalamus of the ram and ewe. J Neuroendocrinol. 2004;16:956–963. doi: 10.1111/j.1365-2826.2005.01261.x. [DOI] [PubMed] [Google Scholar]
- Semaan SJ, Murray EK, Poling MC, Dhamija S, Forger NG, Kauffman AS. BAX-dependent and BAX-independent regulation of Kiss1 neuron development in mice. Endocrinology. 2010;151:5807–5817. doi: 10.1210/en.2010-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaab DF, Fliers E. A sexually dimorphic nucleus in the human brain. Science. 1985;228:1112–1115. doi: 10.1126/science.3992248. [DOI] [PubMed] [Google Scholar]
- Tobet S, Knoll JG, Hartshorn C, Aurand M, Stratton M, Kumar P, Searcy B, McClellan K. Brain sex differences and hormone influences: A moving experience? J Neuroendocrinol. 2009;21:387–392. doi: 10.1111/j.1365-2826.2009.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobet SA, Zahniser DJ, Baum MJ. Sexual dimorphism in the preoptic/anterior hypothalamic area of ferrets: effects of adult exposure to sex steroids. Brain Res. 1986;364:249–257. doi: 10.1016/0006-8993(86)90837-1. [DOI] [PubMed] [Google Scholar]
- Tsukahara S. Sex differences and the roles of sex steroids in apoptosis of sexually dimorphic nuclei of the preoptic area in postnatal rats. J Neuroendocrinol. 2009;21:370–376. doi: 10.1111/j.1365-2826.2009.01855.x. [DOI] [PubMed] [Google Scholar]
- Tsukahara S, Hojo R, Kuroda Y, Fujimaki H. Estrogen modulates Bcl-2 family protein expression in the sexually dimorphic nucleus of the preoptic area of postnatal rats. Neurosci Lett. 2008;432:58–63. doi: 10.1016/j.neulet.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Tsukahara S, Kakeyama M, Toyofuku Y. Sex differences in the level of Bcl-2 family proteins and caspase-3 activation in the sexually dimorphic nuclei of the preoptic area in postnatal rats. J Neurobiol. 2006;66:1411–1419. doi: 10.1002/neu.20276. [DOI] [PubMed] [Google Scholar]
- White FA, Keller-Peck CR, Knudson CM, Korsmeyer SJ, Snider WD. Widespread elimination of naturally occurring neuronal death in Bax-deficient mice. J Neurosci. 1998;18:1428–1439. doi: 10.1523/JNEUROSCI.18-04-01428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SL, Chen YY, Hsieh YL, Jin SH, Hsu HK, Hsu C. Perinatal androgenization prevents age-related neuron loss in the sexually dimorphic nucleus of the preoptic area in female rats. Dev Neurosci. 2004;26:54–60. doi: 10.1159/000080712. [DOI] [PubMed] [Google Scholar]
- Zup SL, Carrier H, Waters EM, Tabor A, Bengston L, Rosen GJ, Simerly RB, Forger NG. Overexpression of Bcl-2 reduces sex differences in neuron number in the brain and spinal cord. J Neurosci. 2003;23:2357–2362. doi: 10.1523/JNEUROSCI.23-06-02357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


