Abstract
Cells transiently adapt to hypoxia by globally decreasing protein translation. However, specific proteins needed to respond to hypoxia evade this translational repression. The mechanisms of this phenomenon remain unclear. We screened for and identified small molecules that selectively decrease HIF-2a translation in an mTOR independent manner, by enhancing the binding of Iron Regulatory Protein 1 (IRP1) to a recently reported Iron-Responsive Element (IRE) within the 5’-untranslated region (UTR) of the HIF-2a message. Knocking down the expression of IRP1 by shRNA abolished the effect of the compounds. Hypoxia de-represses HIF-2a translation by disrupting the IRP1- HIF-2a IRE interaction. Thus, this chemical genetic analysis describes a molecular mechanism by which translation of the HIF-2a message is maintained during conditions of cellular hypoxia through inhibition of IRP-1 dependent repression. It also provides the chemical tools for studying this phenomenon.
INTRODUCTION
The tumor promoting Hypoxia Inducible Factor (HIF) is a central regulator of the cellular response to hypoxia (Semenza, 2000). HIF is a heterodimeric transcription factor consisting of hypoxia-regulated (HIF-a) and a constitutively expressed (HIF-1b) subunits (Semenza, 2000). There are two transactivating HIF-a isoforms, HIF-1a and HIF-2a, whose activity is tightly regulated by oxygen (Gordan and Simon, 2007; Raval et al., 2005). In well-oxygenated cells, the tumor suppressor protein pVHL targets HIF-a for ubiquitination and proteasomal degradation (Maxwell et al., 1999; Ohh et al., 2000). This interaction requires hydroxylation of HIF-a at conserved proline residues by iron-dependent prolylhydroxylases, termed EGLN1, 2 and 3 (Epstein et al., 2001; Ivan et al., 2001; Jaakkola et al., 2001). Hypoxia inhibits EGLN activity and disrupts the HIF-pVHL interaction. Stabilized HIF-a subunits enter the nucleus, heterodimerize with HIF-1b and bind to DNA sequences termed Hypoxia Response Elements (HREs) to transactivate genes that encode for secreted growth and pro-angiogenic factors (Maxwell et al., 2001). HIF’s transcriptional activity is similarly attenuated by Factor Inhibiting HIF (FIH), a second iron-dependent enzyme that hydroxylates a conserved asparagine residue in the transactivation domain of HIF (Bruick and McKnight, 2001; Lando et al., 2002a; Lando et al., 2002b; Mahon et al., 2001).
Part of the adaptive response to hypoxia in healthy cells is to conserve energy by diminishing global protein translation (Arsham et al., 2003; Bert et al., 2006; Lang et al., 2002; Liu et al., 2006; Schepens et al., 2005). This is at least in part mediated by Redd1, itself a HIF target gene, inhibiting mTOR via the tuberous sclerosis (TSC1/2) complex (DeYoung et al., 2008). However, specific messages that allow cells to cope with the hypoxic environment are spared this translational repression (Blais et al., 1994; Liu and Simon, 2004; Spicher et al., 1998; Thomas and Johannes, 2007; Wouters et al., 2005). The mechanisms for selectively supporting translation of certain messages in conditions of hypoxia are under investigation.
Here we devised a cell-based assay to screen for small molecule HIF inhibitor compounds in VHL-deficient RCC cells and identified four compounds that, in multiple cancer cell lines, selectively inhibited translation of the HIF-2a message in an mTOR independent manner. We found that the HIF-2a 5’-UTR is necessary and sufficient to confer compound sensitivity. Deletion analysis of the 5’-UTR revealed that the minimal region necessary and sufficient for compound efficacy mapped to a newly identified IRE within the 5’-UTR of the HIF-2a mRNA (Sanchez et al., 2007). Mutations within the conserved IRE motif abolished the effect of the compounds, as did knocking down the expression of the IRE binding protein, Iron Regulatory Protein 1 (IRP1). Electrophoretic mobility shift assays showed that the compounds directly promoted IRP1 binding to the HIF-2a IRE. Furthermore, we report that hypoxia de-repressed HIF-2a translation by impairing the IRP1/IRE interaction. These data explain how the HIF-2a message is translationally induced by hypoxia and provide chemical genetic tools to study this phenomenon.
RESULTS
High-throughput screen for small molecule HIF-2a inhibitors
Functionally validated HRE and control SV40 luciferase reporter constructs were stably introduced into VHL-deficient 786-O cells to generate 7H4 and 7SV lines, respectively (Figure S1, online). These lines were used to screen five commercial small molecule libraries (NCI Diversity Set, Chembridge, Maybridge, CEREP and Peakdale) as well as the ICCB Diversity-Oriented Synthesis Diversity Set 2 (DDS2) and serine-derived peptidomimetic (SDP1) collections, totaling 58,000 compounds. Eight compounds were identified that reproducibly decreased luciferase activity by greater than 80% when applied to 7H4 cells, while having little or no affect on the corresponding 7SV cells and worked on multiple RCC cell types when stably transfected with the same luciferase reporters (Figure S2).
Dose response curves of selected inhibitors
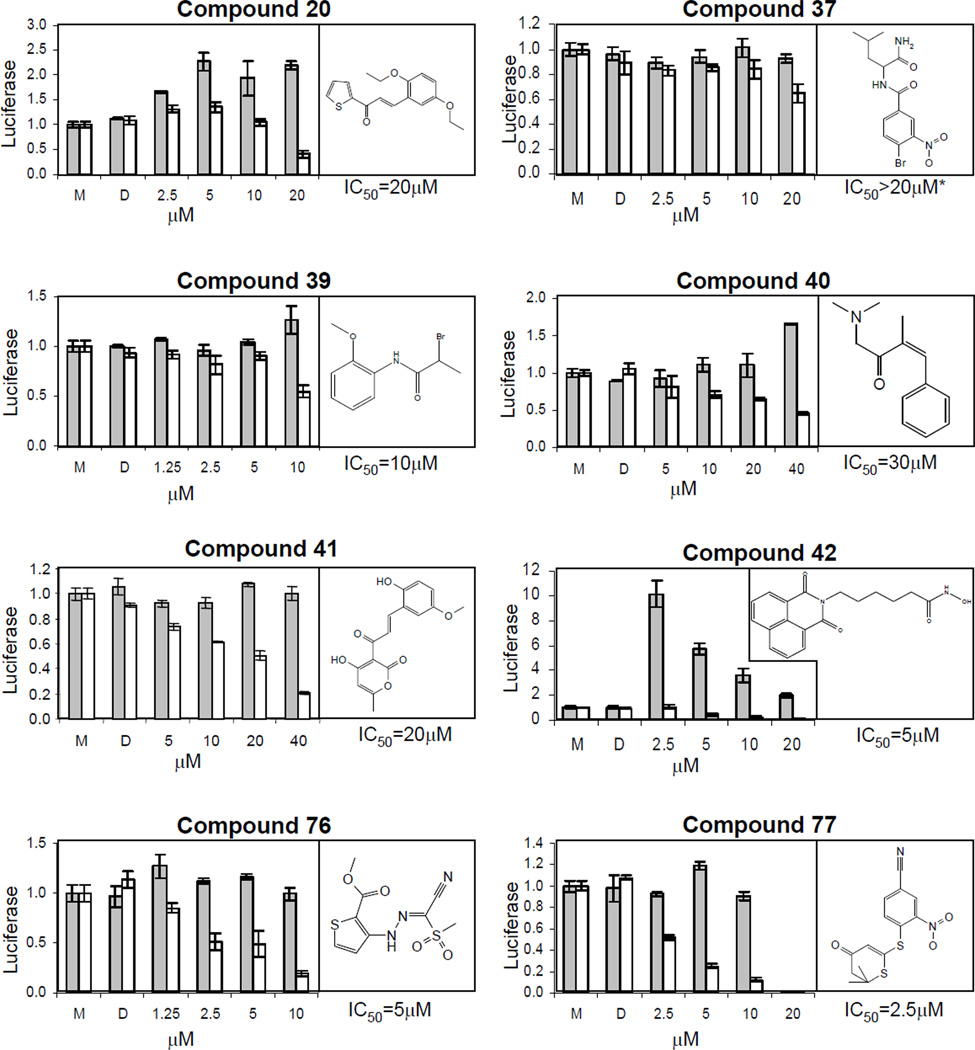
786-O cells were untreated (M for medium only), mock-treated (D for DMSO) or treated with compounds for two days before assaying for normalized luciferase activity. The resulting dose response curves are shown along with the compound structures (Figure 1). The full chemical name of the compounds is listed in Table S1, online. Compounds exhibited apparent IC50 values ranging from 2.5–40 µM. Based on the potency of the compounds and comparative lack of cellular toxicity at IC50 concentration, we chose to focus our mechanistic analysis on compounds 40, 41, 76 and 77.
Figure 1. Structure and dose response of HIF inhibitor compounds.
Compounds were added at the indicated concentrations for two days prior to measuring normalized luciferase activity. Gray bars, 7SV (SV40-luciferase) cells; white bars, 7H4 (HRE-luciferase) cells. All experiments were performed in triplicate. Error bars represent standard error of the mean (SEM).
Gene expression profiling suggests compounds preferentially affect HIF target gene expression
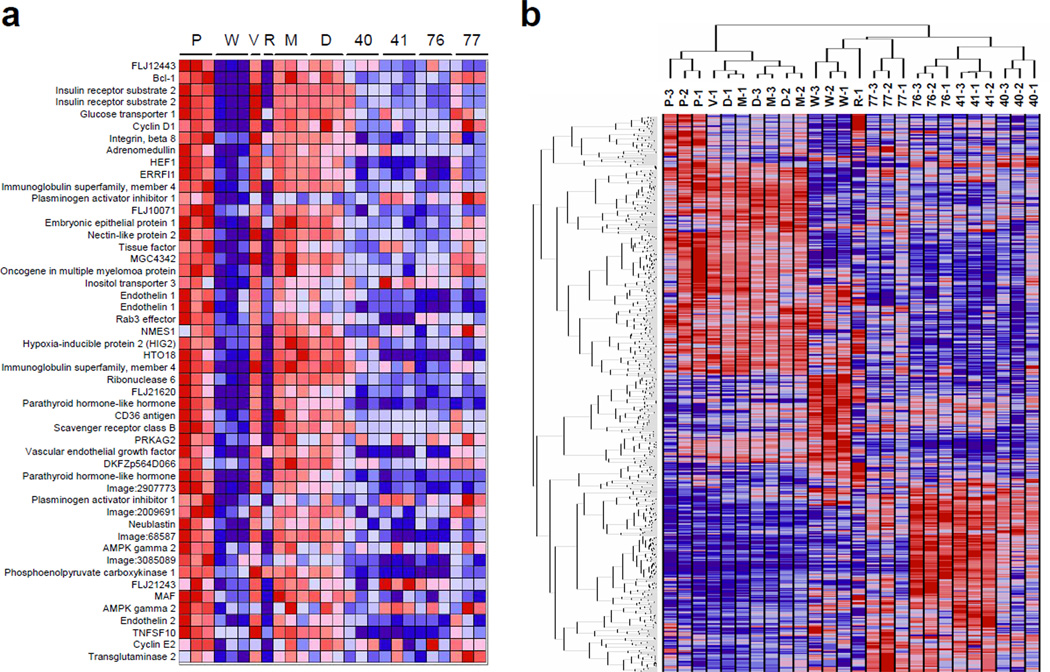
Total RNA was extracted from stable 786-O derived VHL-reconstituted (WT8), HIF-2a knock down (pTR) and their respective vector only control (PRC3 and pTV) cells and labeled cRNA was hybridized to oligonucleotide arrays. Comparison of WT8 to PRC3 shows genes that were downregulated by pVHL while comparison of pTR to pTV identifies direct HIF-2a target genes since 786-O cells express only the HIF-2a isoform. Compound-treated parental 786-O cells were subjected to the same analysis. Overall, genes downregulated by either VHL-reconstitution or HIF-2a-targeting shRNA expression were also coordinately downregulated by exposure to compounds 40, 41, 76 and 77 in parental 786-O cells (Figure 2a). The Gene Set Enrichment Analysis (GSEA) algorithm was used to investigate whether the compounds affected the expression of genes identified as VHL and HIF targets in other cell types (Mootha et al., 2003). We found that genes that were upregulated in VHL or HIF gene sets were downregulated by compounds 40, 41, 76 and 77; and genes that were downregulated in the VHL gene set were upregulated by compounds 41, 76 and 77 (Figure 2a and Table S2).
Figure 2. Gene expression profile of compound treated 786-O cells.
For all panels, the cell lines used were: P, PRC3; W, WT8; V, pTV; R, pTR; or parental 786-O cells treated with M, medium only; D, DMSO; or compounds 40, 41, 76 and 77 as indicated, at the experimentally determined IC50 concentrations (a) Identified compounds coordinately downregulate HIF target genes. The genes illustrated are the top 50 markers of 786-O derived vector only (P for PRC3) cells relative to matched isogenic VHL-reconstituted (W for WT8) cells, which are also downregulated by the HIF-2a-targeting shRNA (R for pTR) cells relative to an empty vector control (V for pTV) cells. (b) Hierarchical clustering of samples and genes reveals similarities between the activities of compounds. Samples were clustered in the space of 800 genes comprised of the 100 genes most powerfully upregulated and the 100 genes most powerfully downregulated in cells treated with compounds, and in PRC3 versus WT8 cells.
Hierarchical clustering reveals one tight cluster formed by samples in which HIF is constitutively active, including PRC3 and pTV cells, and 786-O cells that were untreated or mock treated with DMSO. A separate cluster included samples with inactive HIF, including WT8 and pTR cells, and 786-O cells treated with the identified compounds. Independent, replicate experiments clustered together, demonstrating the distinctive and highly reproducible gene expression profiles associated with each compound. Compounds 41 and 76 have highly similar molecular profiles (Figure 2b).
Conditioned medium from compound treated cells suppress HUVEC growth in vitro
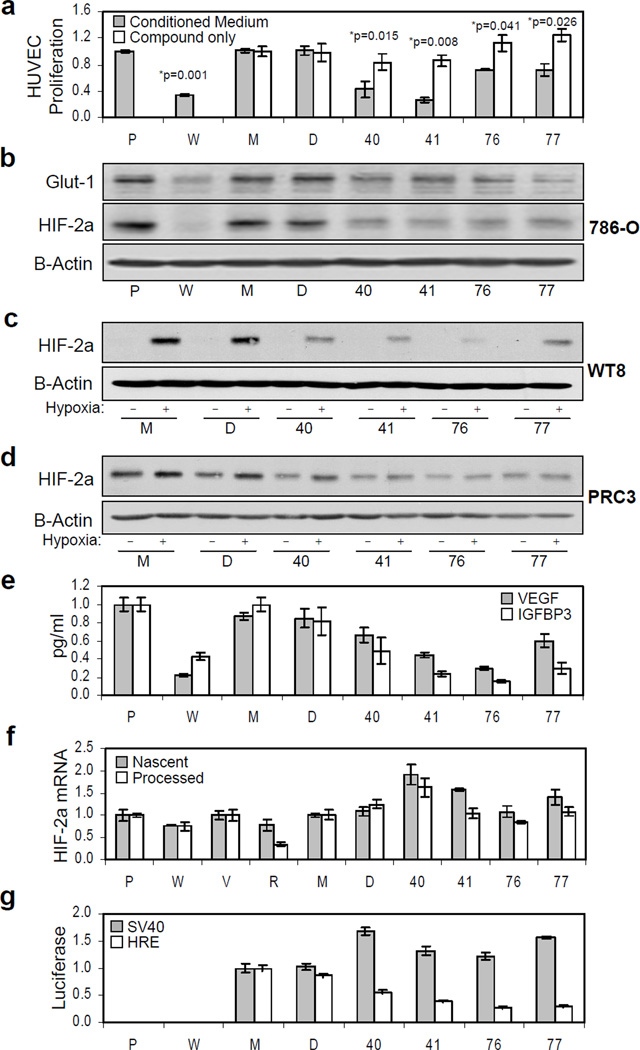
Conditioned tissue culture medium from compound-treated 786-O cells was tested for its ability to induce human umbilical vein endothelial cell (HUVEC) proliferation. Compound application decreased the ability of conditioned tissue culture medium to sustain HUVEC proliferation while the compounds themselves exhibited no significant affect on HUVEC proliferation when applied directly at the same concentrations (Figure 3a).
Figure 3. Effect of compounds on the angiogenic activity of renal carcinoma cell supernatant, the expression of endogenous HIF-2a protein, its cognate mRNA and HIF-downstream target genes.
For all panels: P, PRC3; W, WT8; V, pTV; R, pTR, or parental 786-O cells treated with M, medium only; D, DMSO; or compounds, as indicated. HUVEC proliferation, ELISA, qRT-PCR and luciferase measurements were performed in triplicate. Error bars represent standard error of the mean (SEM). (a) HIF inhibitors decrease the angiogenic activity of renal carcinoma cell supernatant. HUVEC proliferation after incubation with conditioned tissue culture supernatant from control or compound-treated 786-O cells. Conditioned supernatant from identically treated VHL-reconstituted WT8 (W) and vector-only PRC3 (P) cells were used as positive controls. P-values were determined using a Student's paired t-Test, with a two-tailed distribution. (b) Effect of compounds on endogenous HIF-2a and Glut-1 protein expression. HIF-2a, Glut-1 and B-Actin expression was analyzed by Western blot. (c) Effect of compounds on VHL-reconstituted WT8 cells. Western blot for HIF-2a and B-Actin in which WT8 cells were cultured in the presence of compound, plus or minus 24 hours hypoxia. (d) Effect of compounds on HIF-2a in matched isogenic PRC3 cells. Western blot on PRC3 cells were performed as described above. (e) Effect of compounds on secreted VEGF and IGFBP3 expression. The concentration of VEGF and IGFBP3, secreted HIF target genes, were determined in tissue culture supernatant from the above cells, as measured by ELISA. (f) Compounds do not decrease HIF-2a mRNA expression. Quantitative RT-PCR was performed on matched 786-O derived vector-only (pTV) or HIF-2a-targeting shRNA expressing (pTR), PRC3 or WT8 clones, as well as medium only, DMSO and compound treated 7H4 cells. Data shows relative expression of both the nascent (unspliced, gray bars) and processed (spliced, white bars) HIF-2a message normalized to B-2-microglobulin (B2M) for control. (g) Effect of compounds of HRE-luciferase reporter activity. In parallel with the qRT-PCR analysis, luciferase activity was measured from identically treated 7SV (gray bars) and 7H4 (white bars) cell lysates.
Compounds decrease HIF-2a protein and HIF-2a target gene expression in normoxia and hypoxia
All compounds decreased endogenous HIF-2a protein expression (Figure 3b). Hypoxia stabilized HIF-2a in VHL-reconstituted WT8 cells, but treatment with compounds diminished hypoxia-induced HIF-2a expression (Figure 3c). Normoxic HIF-2a expression was similarly decreased in VHL-deficient PRC3 cells (Figure 3d). The expression of known HIF-2a target genes, Glut-1, VEGF and IGFBP3, was also concomitantly decreased in compound treated 786-O cells (Figure 3b, e).
Compounds decrease neither HIF-2a mRNA expression nor HIF-2a protein stability
Quantitative RT-PCR was performed on total RNA harvested from 7H4 cells treated with medium only, DMSO or compound. In no case was the expression of nascent (unspliced) or processed (spliced) HIF-2a mRNA decreased, while a luciferase assay performed in parallel shows that the compounds were active (Figure 3f, g). To test whether the compounds alter HIF-2a stability, we assessed protein half-life following the addition of cycloheximide in compound versus DMSO-only treated cells (Figure S3). These experiments provided no evidence that any of the compounds affected HIF-2a protein stability.
Compounds decrease HIF-2a mRNA translation in an mTOR independent manner
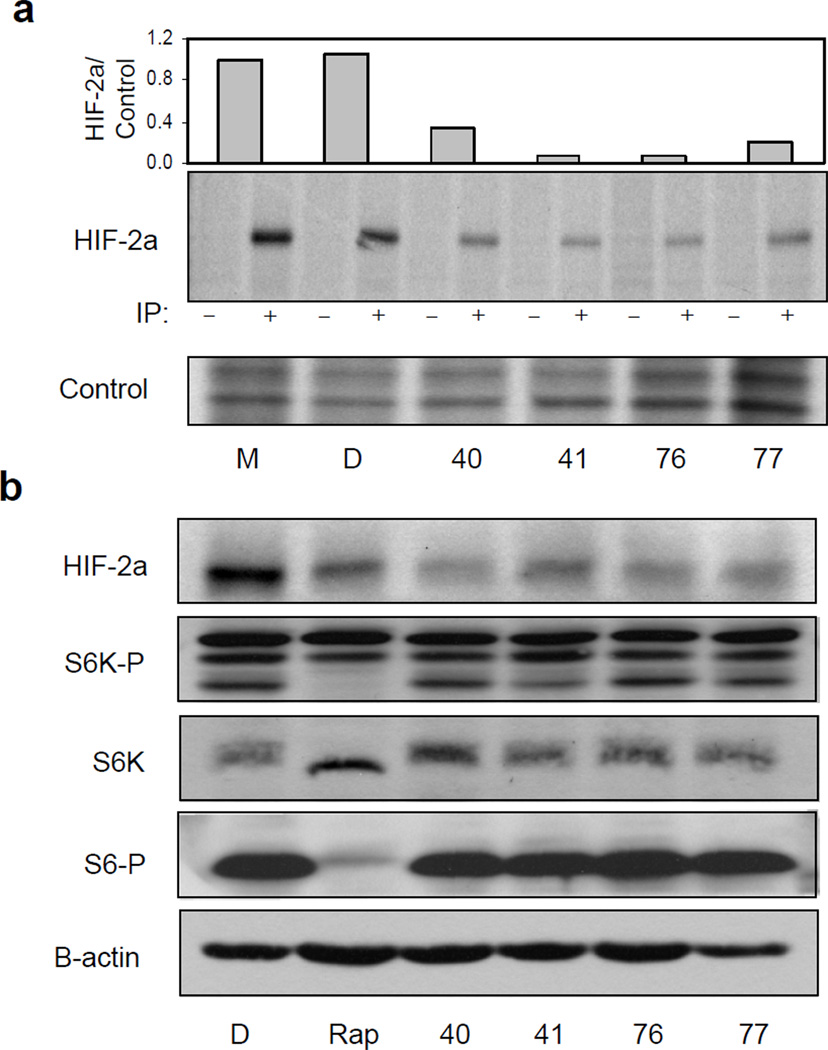
The effect of HIF inhibitors on protein translation was determined by 35S-methionine pulse labeling of DMSO or compound treated cells. All compounds significantly decreased the amount of newly synthesized HIF-2a protein (Figure 4a and S4). Pulse chase experiments confirm that this decrease is not attributable to any effect of the compounds on HIF-2a protein half-life (Figure S5).
Figure 4. Compounds decrease HIF-2a mRNA translation in an mTOR independent manner.
(a) Compound decrease HIF-2a mRNA translation. Compound treated 786-O cells were subjected to 35S-methionine pulse label followed by immunoprecipitation (IP) as described in Experimental Procedures. (−) indicates anti-HA control IP, (+) indicates IP with anti-HIF-2a antibody. Loading control is a 1:1000 dilution of the lysate. Quantification of 35S incorporation into the radiolabeled HIF-2a protein was determined by densitometry relative to control lanes and the resulting graph is shown (top). (b) Effect of compounds is mTOR independent. 786-O cells were treated with D, DMSO; Rap, rapamycin, or the indicated compounds. HIF-2a, total p70S6K, phospho-T389 p70S6K and phospho-S235/6 S6 expression and B-Actin was analyzed by Western blot.
Any effect on translation suggests that the compounds might inhibit mTOR activity (Brugarolas et al., 2004; Hudson et al., 2002; Majumder et al., 2004). We therefore compared the effects of compound versus rapamycin treated 786-O cells on HIF-2a, phospho-S6, p70S6K and phospho-p70S6K expression, as measured by Western blot, using total or phospho-specific antibodies. As is the case for HIF-1a, HIF-2a protein expression was decreased by rapamycin. This was also the case for HRE-luciferase reporter activity (data not shown). However, only rapamycin appeared to have any effect on p70S6K and S6 phosphorylation (Figure 4b).
Sensitivity to the compounds is 5’-UTR dependent and heterologously transferable
We generated stable 786-O derived clones expressing luciferase reporter constructs driven by the endogenous HIF-2a promoter alone or the promoter with the 5’-UTR. The compounds had little effect on luciferase activity when driven by the HIF-2a promoter alone. However, the same luciferase reporter containing the 5’-UTR recapitulated the effect of the compounds on HRE-driven luciferase reporter activity (Figure 5a).
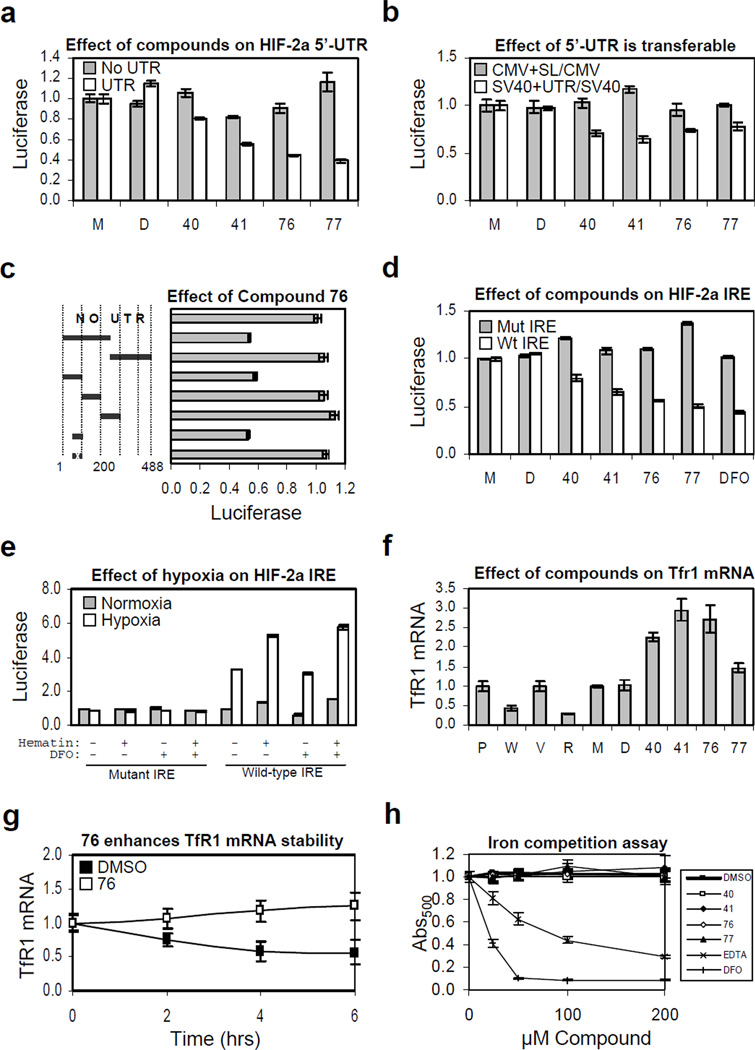
Figure 5. The HIF-2a IRE is necessary and sufficient for mediating the effect of HIF inhibitors and it is de-repressed by hypoxia.
For all panels: P, PRC3; W, WT8; V, pTV; R, pTR, or parental 786-O cells treated with M, medium only; D, DMSO; or compounds, as indicated. Experiments were done in triplicate and error bars represent standard error of the mean (SEM). (a) Effect of compound is dependent upon presence of 5’-UTR. (b) Effect of 5’-UTR is heterologously transferable. Gray bars, ratio of cells expressing CMV-luciferase with a synthetic RNA helicase reporter 5’-UTR stem loop over those expressing luciferase from the CMV promoter alone; white bars, ratio of cells expressing SV40-luciferase with the HIF-2a 5’-UTR over those expressing luciferase from the SV40 promoter alone. Shown are p-values determined using the Student's unpaired homoscedastic t-Test with a two-tailed distribution, comparing the ratios of SV40 driven luciferase with the HIF-2a 5’-UTR over promoter alone versus CMV-driven luciferase with a synthetic stem loop over promoter alone. (c) Effect of compound 76 localizes to IRE element within the 5’-UTR of the HIF-2a message. A series of 5’-UTR deletion mutants were engineered into the HIF promoter driving luciferase. Shown is the normalized ratio of cells treated with 10µM 76 over DMSO only control for each reporter relative to the construct without any 5’-UTR element. The 50 nucleotide sequence with an “x” denotes mutated IRE. (d) All compounds work via the Iron-Responsive Element (IRE). The effect of all compounds was tested on luciferase reporter lines containing wild-type or mutant 5’-UTR IRE element. (e) Hypoxia mediates translational de-repression of the HIF-2a message via the 5’-UTR IRE. Mutant or wild-type HIF-2a IRE luciferase reporter lines were plated in duplicate and treated with medium only, 10µM DFO, 100µM hematin, or both, as indicated and subjected to 24 hours normoxia (gray bars) or hypoxia (white bars). Luciferase measurements were normalized separately for wild-type or mutant IRE to corresponding normoxic reporter line treated with medium only. Raw mutant IRE luciferase activity was roughly 10-fold higher than that of wild-type. (f) Compounds increase expression of Transferrin Receptor 1 mRNA. Quantitative RT-PCR was performed on WT8, PCR3, pTR, and pTV lines as well as compound treated 786-O cells. (g) Compound 76 increases TfR1 mRNA stability. Quantitative RT-PCR showing normalized TfR1 mRNA expression in DMSO versus compound 76 treated 786-O cells following the addition of Actinomycin D. (h) Compounds are not iron chelators. Displacement of iron from ethyl-3,4-dihydroxybenzoate (EDHB) complex was measured as a decrease in absorbance at 500 nm in the presence compounds as well as EDTA and DFO.
We next stably transfected 786-O cells with luciferase reporter constructs driven by the HIF independent SV40 promoter with or without the presence of the HIF-2a 5’-UTR (Figure 5b). To control for general RNA helicase activity, we also stably transfected 786-O cells with luciferase reporters, with or without a synthetic 5’-UTR stem loop that serves as a reporter for RNA helicase activity, driven by the CMV promoter (Yang et al., 2004). We found that the 5’-UTR is sufficient to confer compound sensitivity and that this effect was independent of RNA helicase activity.
The Iron-Responsive Element (IRE) within the 5’-UTR of HIF-2a is necessary and sufficient for compound sensitivity
A series of 5’-UTR deletions were engineered into the HIF promoter-luciferase construct. We found that nucleotides 50–100 of the HIF-2a 5-UTR were necessary and sufficient for mediating the effect of our HIF inhibitors (Figure 5c). This region contains a near consensus Iron-Responsive Element (IRE) that was recently shown to interact with IRP1 (Sanchez et al., 2007). Point mutations within the consensus loop, in which the 5’-CAGUGU-3’ loop sequence is changed to 5’-CAAAGU-3’, ablated the effect of all compounds and DFO (Figure 5d).
Hypoxia upregulates HIF-2a translation via its 5’-UTR IRE
The luciferase reporter containing the wild-type, but not mutant, HIF-2a IRE was significantly activated under hypoxic conditions. Raw luciferase activity was approximately 10-fold higher in cells harboring the mutant reporter (data not shown). Addition of exogenous iron, as a positive control for IRE-reporter activity, promoted translation and synergized with the effect of hypoxia. Low concentration (10µM) of the iron chelator DFO repressed reporter activity and diminished the effect of hypoxia (Figure 5e).
Compounds promote stability of Transferrin Receptor 1 (TfR1) mRNA
To test whether the compounds promote IRP activity in general we examined their effect on Transferrin Receptor 1 (TfR1) mRNA stability. IRP1 binds to TfR1 IREs located in the 3’-UTR and promotes TfR1 stability. We found that expression of TfR1 mRNA is increased in compound treated cells relative to medium or DMSO treated control cells (Figure 5f). TfR1 has been reported to be a HIF-1a target gene (Tacchini et al., 2008) and our data indicate that it appears to be also a HIF-2a target, since TfR1 message is decreased in VHL-reconstituted or HIF-2a shRNA targeting 786-O cells (Figure 5f). The fact that net mRNA is increased rather than decreased by these HIF inhibitors is therefore very likely due to an affect on mRNA stability. To directly support this, we show that the stability of TfR1 mRNA is increased in compound 76 relative to DMSO treated 786-O cells following the addition of Actinomycin D. (Figure 5g).
Compounds do not act by iron chelation
We tested whether the compounds bind iron using a competition assay in which the displacement of iron from ethyl-3,4-dihydroxybenzoate (EDHB), a weakly chelating ester, was measured as a decrease in absorbance at 500 nm. We found that, even at concentrations as high as 200 µM, there was no evidence of any compound binding to iron, whereas the iron chelators EDTA and DFO readily displaced iron from EDHB (Figure 5h). In addition, we used the Connectivity Map (Lamb et al., 2006) to compare the gene-expression effects of our compounds and those of low- and high- dose DFO. We found no significant similarity between any of our compounds and DFO at a similar (low) concentration (Figure S6). Moreover, there was a trend towards a modest negative correlation between the effects of our compounds and those of DFO when administered at a (high) concentration (Figure S6) sufficient to chelate iron from EGLNs and FIH, and thereby stabilize and activate HIF.
IRP1 is solely responsible for hypoxic de-repression of HIF-2a translation
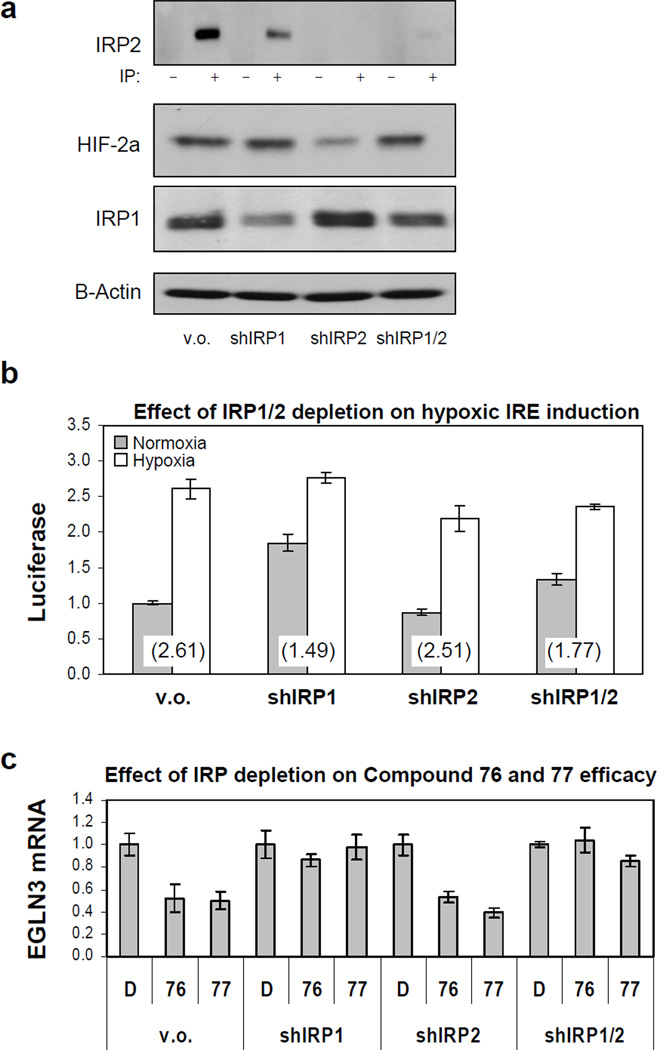
We generated wild-type HIF-2a IRE luciferase reporter lines in which the expression of each isoform individually or both isoforms together was acutely knocked down by RNA interference. Depletion of IRP1 resulted in slight enhancement of IRP2 expression and vice versa (Figure 6a). IRP1 depletion slightly increased HIF-2a expression, whereas IRP2 depletion decreased it (Figure 6a). These lines were subjected to a 24 hours normoxia or hypoxia treatment and HIF-2a IRE activity was measured by luciferase. We found that inhibition of IRP1 attenuated the hypoxic de-repression of HIF-2a translation, while inhibition of IRP2 had little effect (Figure 6b). This attenuation of hypoxic response by IRP1 shRNA was the result of increased basal translation in the IRP1 knock down line in normoxia, as the relative luciferase activities between the vector only and IRP1 knock down line in hypoxia were similar. It is likely that the residual increase in hypoxia-induced translation in the IRP1 knock down line is due to residual IRP1 expression in this line, as the effect of IRP1 and IRP2 double knock down is similar to that of knocking down IRP1 alone. Basal luciferase activity in the IRP2 knock down line is decreased relative to that of vector only control, which is compatible with the hypothesis that depletion of IRP2 results in a compensatory increase in IRP1 expression and activity, as shown by western blot for IRP1 and HIF-2a in these lines (Figure 6a).
Figure 6. Hypoxic de-repression of HIF-2a translation and compound induced repression are mediated solely by IRP1.
(a) Effect of IRP1 and IRP2 reduction on HIF-2a. 786-O cells expressing wild-type HIF-2a IRE luciferase reporter, were infected with lentiviral shRNAs targeting IRP1, IRP2 or both. (b) Inhibition of IRP1 is sufficient to prevent hypoxic de-repression of HIF-2a translation. Luciferase counts were normalized to vector only (v.o.) in normoxia. The number in parentheses is the fold induction by hypoxia for each cell line. (c) Inhibition of IRP1 is sufficient to block effect of compounds on HIF-2a activity. qRT-PCR was performed on the HIF-2a target gene EGLN3 in the above IRP knock down lines treated with D, DMSO, 76 or 77. All measurements were done in triplicate and error bars represent standard error of the mean (SEM).
Inhibition IRP1 is sufficient to inhibit the effect of compounds on HIF-2a
Quantitative RT-PCR for the HIF-2a target gene EGLN3 shows that inhibition of IRP1 blocked the effect of the compounds on HIF-2a activity, whereas knocking down the expression of IRP2 had no measurable effect. Knocking down the expression of both IRP1 and IRP2 together likewise rendered the compounds ineffective (Figure 6c).
Compounds increase and hypoxia decreases IRP1 binding to HIF-2a IRE
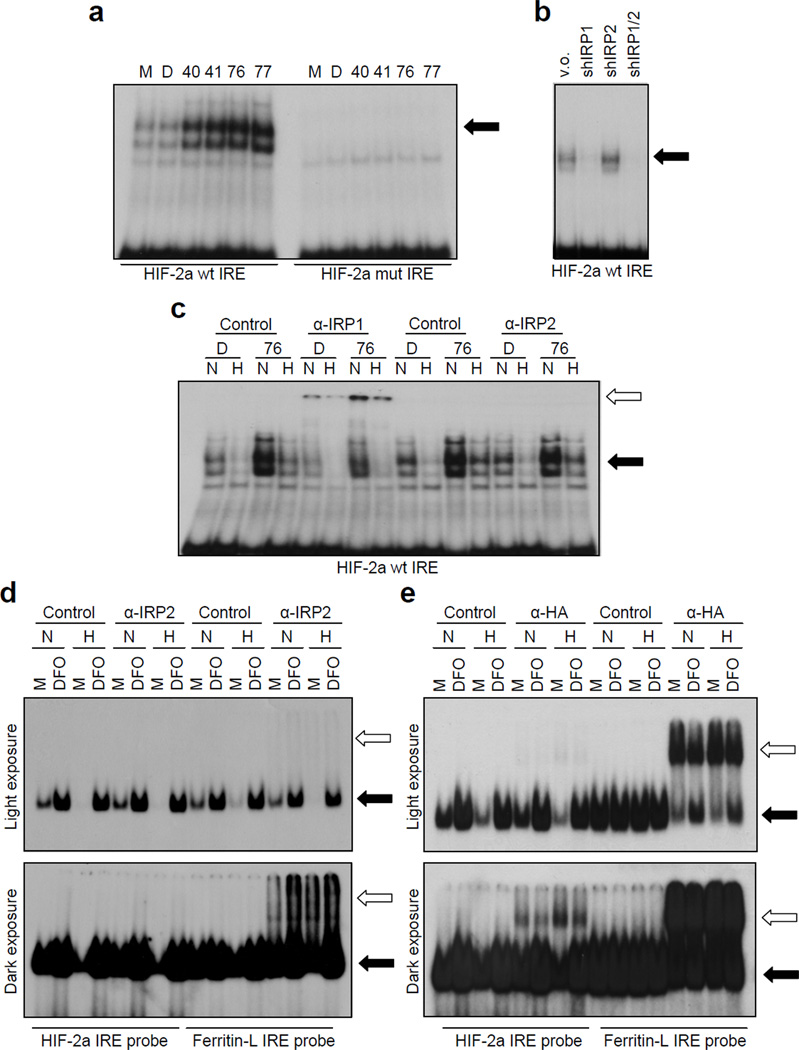
Electrophoretic mobility shift assays (EMSAs) show that compound application increased the binding of IRP1 to radiolabeled wild type but not mutant HIF-2a IRE probe (Figure 7a). EMSA using lysates from the IRP knock down lines suggest that IRP1 is the predominant species involved in HIF-2a IRE binding and corroborates the observation that depletion of IRP2 leads to enhanced IRP1 activity (Figure 7b). Consistent with our luciferase reporter assays, hypoxia dramatically decreased the intensity of the shifted IRP bands (Figure 7c). This is due to decreased mRNA binding, as western blots confirm that neither compound application nor hypoxia affects IRP1 protein expression (data not shown). The involvement of IRP1 was further confirmed by supershifting with IRP1 antibody (Figure 7c and S7). No supershifted bands were observed using IRP2 antibody. In order to determine if our compounds increased binding of IRP1 to the HIF-2a IRE, even in conditions of hypoxia, 786-O cells were treated with compound 76 followed by 24 hour exposure to normoxia or hypoxia. We found that compound 76 increased the residual amount of IRP1 bound to the HIF-2a IRE in hypoxia, although not to the same extent as that observed in normoxic cells.
Figure 7. Compounds enhance binding of IRP1 to the HIF-2a IRE.
(a) Compounds enhance wild-type but not mutant HIF-2a IRE probe gel shift. (b) IRP1 is the major species binding to HIF-2a IRE. EMSA was performed on the 786-O derived IRP knock down lines using the wild-type HIF-2a IRE probe, as indicated. (c) Binding of IRP1 to HIF-2a IRE decreases in hypoxia and it is enhanced by compound 76 under both normoxia and hypoxia. 786-O cells were treated with DMSO (D) or compound 76 and subjected to 24 hours normoxia (N) versus hypoxia (H). EMSA was performed on the resulting lysates following incubation with control, IRP1 or IRP2 antibodies. Pre-immunization polyclonal rabbit serum and purified B-Actin monoclonal were used as controls for IRP1 and IRP2, correspondingly. (d) No detectable binding of endogenous IRP2 to HIF-2a IRE. EMSA was performed on 786-O lysates either left untreated (medium only, M) or treated with 150 µM DFO in normoxia or hypoxia using either the Ferritin-L or HIF-2a IRE radiolabeled probe and supershifted with either control or anti-IRP2 (2 µL UT29) antibody in the presence of 1 mM DTT. (e) Exogenous HA-IRP2 can bind to HIF-2a IRE. EMSA was performed on lysates form U2OS cells transiently transfected with a vector expressing HA-IRP2 and either left untreated (medium only, M) or treated with 150 µM DFO in normoxia or hypoxia using either the Ferritin-L or HIF-2a IRE radiolabeled probe and supershifted with either control or anti-IRP2 (2 µL UT29) antibody in the presence of 1 mM DTT. Black arrows: position of the shifted band, white arrows: position of the supershifted band.
The absence of detectable contribution of endogenous IRP2 to HIF-2a IRE activity in these cells led us to further investigate the involvement of IRP2 in HIF-2a IRE. To this end we compared, side by side, IRP2 binding to HIF-2a and Ferritin-L IRE in 786-O cells, in conditions of normoxia or hypoxia and after treatment with compound 76 or DFO (Figure 7c). While IRP2 clearly bound the Ferritin-L IRE, we were not able to detect IRP2 binding to the HIF-2a IRE even after prolonged exposure. In these experiments we used 1mM DTT in the cell lysis buffer since this addition has been reported to enhance IRP2 binding (Wang et al., 2004). Even under these conditions we found no evidence for endogenous IRP2 binding to the HIF-2a IRE, while IRP2 was clearly detected on Ferritin-L IRE (Figure 7d). In keeping with a previous report (Hanson et al., 1999), hypoxia slightly increased IRP2 binding to Ferritin-L IRE and compound 76 also increased this binding (Figures 7d and S8). To test whether IRP2 has the ability the HIF-2a IRE when overexpressed, we transiently transfected U2OS cells with HA-IRP2; under these conditions we can detect weak binding of IRP2 to the HIF-2a IRE, compared to the much stronger binding of IRP2 to Ferritin-L IRE (Figure 7e). Hypoxia significantly decreased the intensity of the shifted HIF-2a IRE probe in these lysates, suggesting that the majority of HIF-2a IRE, even under conditions of IRP2 overexepression, is bound by IRP1 (Figure 7e).
DISCUSSION
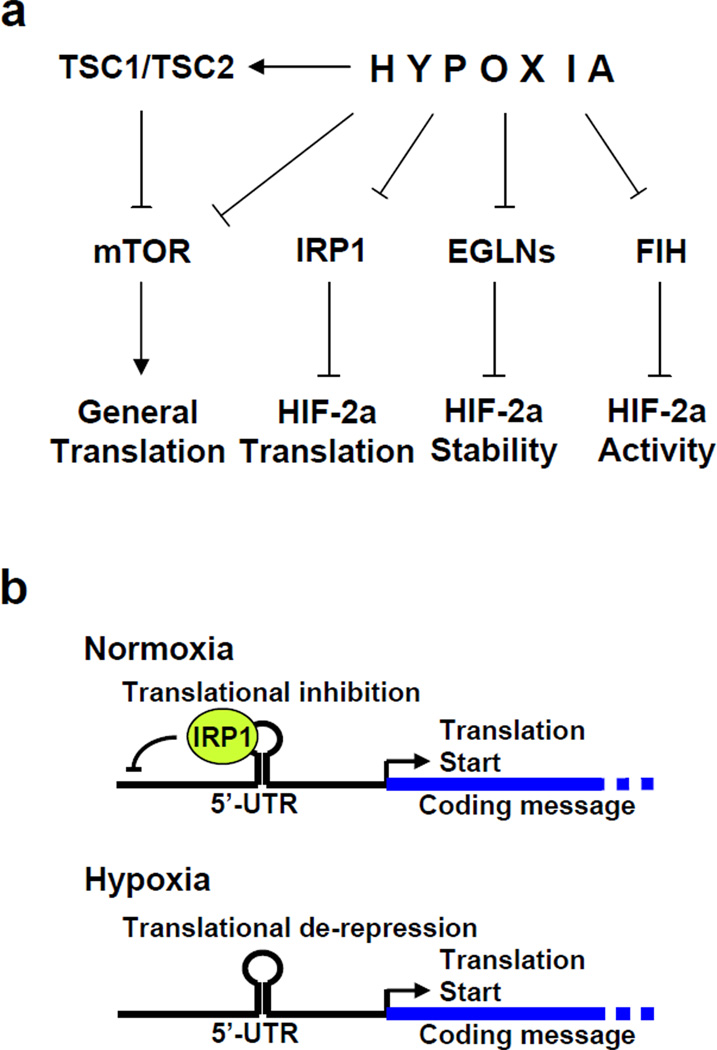
We employed a cell-based assay specific for HIF activity and identified several small molecule HIF inhibitors that decreased both constitutive and hypoxia-induced HIF-2a protein expression. Used as chemical genetics probes, these HIF inhibitors highlighted IRP1 as a hypoxia sensitive regulator of HIF translation, akin to EGLN- and FIH-mediated hypoxic regulation of HIF stability and transcriptional activity. Hypoxia therefore coordinately regulates HIF translation, stability and transcriptional activity (Figure 8a). Translation features prominently among the mechanisms of action for other compounds reported to inhibit HIF activity (Table S3). This indicates that HIF translation is clearly amenable to drug development and it is possible that several of these compounds may inhibit HIF translation in an IRP1-dependent manner.
Figure 8. Hypoxic regulation of HIF-2a.
(a) Effect of hypoxia on HIF-2a and global translation. (b) Model for IRP1-mediated hypoxic de-repression of HIF-2a translation.
Hypoxia globally decreases translational initiation through inhibition of mTOR and phosphorylation of the translation elongation factor eIF2 (Arsham et al., 2003; Blais et al., 1994; Brugarolas et al., 2004; Liu et al., 2006), which may explain why tumor cells select for increased mTOR activity through various mutations in the PI3-K/PKB signaling pathway—to maintain translation during conditions of chronic hypoxia. In order to cope with cellular hypoxia and other forms of environmental stress, cells need to efficiently translate the appropriate stress response genes during conditions in which global mRNA translation is attenuated. In fact, the translation of several transcripts, including FGF2, VEGFB, ATF4, Glut-1, Angiopoietin-like 4, Hypoxia-inducible Gene 2 and ribosomal protein S6 is increased during hypoxia (Blais et al., 1994; Thomas and Johannes, 2007). It has been proposed that this selective translational specificity is mediated largely through the 5’- and 3’-UTR (Spicher et al., 1998; Wouters et al., 2005).
HIF-2a, a critical factor for repairing hypoxic insult, conforms to the above paradigm. Our data suggest that hypoxia dramatically reduced IRP1 binding to HIF-2a and Ferritin-L IRE, while it slightly increased binding of IRP2. Our proposed model for how the IRP1 system works on the HIF-2a message is shown in Figure 8b. Under normoxic conditions, IRP1 binds to the HIF-2a IRE to repress basal translation. Hypoxia de-represses HIF-2a translation by mediating post-translational changes in IRP1 that impairs its ability to bind mRNA. This explains the observed increase in HIF-2a expression in hypoxic VHL−/− cells (Figure 3d). Previous reports include conflicting data; Christova et al. and Toth et al. describe an increase in IRP1 binding to IREs by hypoxia, while Hanson et al. and Schneider et al. suggest that hypoxia decreases IRP1 binding (Christova and Templeton, 2007; Hanson and Leibold, 1998; Schneider and Leibold, 2003; Toth et al., 1999). We employed biochemical and functional assays to address this question and our data strongly indicate that hypoxia dramatically decreases binding of IRP1 to IRE elements under hypoxic conditions and suggest that IRP1 serves as a direct or indirect sensor of hypoxia.
Hypoxia has been reported to stabilize IRP2 and enhance its binding to the Ferritin-L IRE (Hanson et al., 1999). Sanchez et al. first identified the IRE element on HIF-2a 5’-UTR and they suggested that both IRP1 and IRP2 bind to it (Sanchez et al., 2007). However, we could not detect endogenous IRP2 binding to the HIF-2a IRE. In contrast, we found that binding of IRP2 to the Ferritin-L IRE was clearly detectable under the same experimental conditions and was slightly enhanced by hypoxia, compound 76 and DFO. These data suggest that IRP1 is the primary regulator the HIF-2a IRE, at least in the context of renal cells. Indeed, IRP2 has been shown to bind more tightly to IREs containing an internal loop bulge (such as Ferritin-L), a feature that is missing in the HIF-2a IRE (Ke et al., 1998). Nonetheless, we were able to detect binding of IRP2 to HIF-2a IRE when it was overexpressed by transient transfection, consistent with the observation that recombinant IRP2 can bind the HIF-2a IRE in vitro (Sanchez et al., 2007). Our data, while not excluding that endogenous IRP2 binds to HIF-2a IRE in vivo, suggest that the main regulatory role of hypoxia on HIF translation is exerted through IRP1. In light of these findings, it is interesting to note that mouse kidney is highly enriched in IRP1 and that it is the only organ (together with brown fat) that showed a phenotype (misregulation of TfR1 and Ferritin) in IRP1−/− mice (Meyron-Holtz et al., 2004). Lastly, it is possible that the relative affinity of IRP1 and IRP2 for specific IREs changes within different cell/tissue contexts.
Our compounds also decreased the expression of HIF-1a in cell lines that express this isoform (Figure S9a). Scanning the 5’-UTR of the HIF-1a message suggests a putative, non-canonical consensus IRE loop (Figure S9b). However, we found that this near-consensus IRE did not confer sensitivity to the compounds or respond to hypoxia (Figure S9c). Likewise, no shifted band was observed when using this putative IRE as a probe in EMSAs (Figure S9d). Knocking down the expression of HIF-2a in VHL-deficient UMRC2 cells did not affect the ability of the compounds to decrease expression of HIF-1a (data not shown). We conclude that the effect of the compounds on HIF-1a is indirect and independent of HIF-2a. The lack of a functional IRE in HIF-1a represents a clear dichotomy between the two HIF isoforms that allows their translation to be controlled by a different regulatory system.
The data presented in this work therefore bring insights into the mechanism of HIF regulation by hypoxia, and beg the question of whether cryptic IREs might exist in other hypoxia inducible genes. We imagine that this might allow for the coordinate regulation of such genes at the level of translation somewhat akin to how bacteria package genes of like function into operons to provide coordinate regulation at the level of transcription. A recent examination of mRNAs enriched on hypoxic polysomes shows that only a small subset of mRNAs are thus affected (approximately 100) and that the list contains many known HIF target genes (Thomas and Johannes, 2007).
In summary, we identified and characterized four small molecule inhibitors of HIF translation that work via an Iron-Responsive Element (IRE) located within the 5’-UTR of the HIF-2a message. IRP1 binding to the HIF-2a IRE represses its translation during normoxia in several cell lines. Hypoxia impairs the IRP1/IRE interaction, allowing for efficient net translation of the message during conditions of cellular hypoxia. The relative abundance of HIF inhibitor compounds identified over the past few years that affect translation strongly suggests that it is an exquisitely sensitive and pharmacologically amenable component of HIF regulation, and the compounds identified in this work will certainly be useful chemical genetic tools by which to further study the biochemical mechanism of how IRP1 affects HIF signaling. Lastly, structure activity relationship (SAR) analysis and targeted chemical modifications may develop these lead compounds into clinically useful agents that will target renal cell carcinoma as well as prevalent solid tumors of the prostate, breast and colon.
EXPERIMENTAL PROCEDURES
Cell culture and transfections
Detailed information on cell lines and plasmids can be found in Tables S4 and S5. Cells were grown in DMEM with 10% Fetal Clone. All standard buffers and solutions were made as described (Sambrook et al., 1989) and chemicals ordered through Sigma. Transient transfections were performed with FuGENE6 (Roche). Expression of IRP1, IRP2 or both was stably knocked down in 786-O lines expressing the IRE-luciferase reporter. Due to the excess capacity of IRP1 in cells (Wang et al., 2007b), it was necessary to concomitantly use two shRNAs targeting IRP1. Luciferase assays were performed using the Promega’s Dual-luciferase Assay Reporter System (Promega, E1910).
High throughput small molecule screen for HIF-2a inhibitors
The screen was performed at the Institute for Chemical and Cellular biology, Harvard Medical School. 7SV or 7H4 cells were plated onto 384-well plates and compounds were added robotically the next day to a final concentration of approximately 10 µM. Luciferase activity was measured 24 hours later with an Analyst plate reader (LJL Biosystems). All experiments were performed in duplicate and the identity of lead compounds were confirmed by mass spectroscopy.
Quantitative real time polymerase chain reaction (qRT-PCR)
RNA was harvested using TRI-Reagent LS (Molecular Research Center, TS 120), treated with RNase-free DNaseI (Worthington) to remove contaminating genomic DNA and purified over RNeasy Mini Kit columns (Qiagen, 74104). Reverse transcription was performed using random hexamer primers and Superscript III (Invitrogen). PCR was performed using IQ SYBR Green Supermix (BioRad) and run on a MyIQ Single Color Real-Time PCR machine (BioRad) using the following intron spanning primers: 5’-TTTCATCCATCCGACATTGA-3’ and 5’-ATCTTCAAACCTCCATGATG-3’ for B-2-microglobulin (B2M); 5’-GGATCAGCGCACAGAGTTC-3’ and 5’-GTACTGGGTGGCGTAGCACT-3’ for HIF-2a; 5’-AAGCGGTTCTTGGTACCAGC-3’ and 5’-GGATAATCTGTGTCCTCGCA-3’ for TFR1; 5’-GCTACGATTGCTAACATGTG-3’ and 5’-GAGGCCTTTTGGGTCCACTA-3’ for IRP1; 5’-TGTGATTCTGGAGAACTAGG-3’ and 5’-CCCTAAACCATTCACCATCG-3’ for IRP2. Nascent HIF-2a message levels were determined using primers located within the first intron of the unprocessed message: 5’-AGACGGTGGACTCCGCCA-3’ and 5’-TTAAAGGGAGGGGTACAC-3’.
Gene expression profiling and Connectivity Map analysis
RNA from compound treated 786-O cells was isolated as described for qRT-PCR experiments. Labeled cRNA was prepared and hybridized to Affymetrix U133_Plus_2.0 microarrays according to the manufacturer’s protocols. Scans were processed using Affymetrix MAS5 software and median scaled. Gene expression values less than a minimum threshold of 20 or a maximum threshold of 16000 were set to 20 and 16000, respectively. Genes with minimal variation across the dataset were discarded (maximum/minimum < 3 or maximum – minimum < 100). Class neighbors were identified with GenePattern software (available at www.broad.mit.edu/cancer) using the signal to noise metric (Golub et al., 1999). Hierarchical clustering was performed using dCHIP software. Gene set enrichment analysis was performed as described (Mootha et al., 2003) and significance was determined by permutation of the gene labels. The GEO accession number for the complete dataset is... Gene expression signatures were analyzed using the Connectivity Map webtool (build 02), found at www.broad.mit.edu/cmap. Full details of the Connectivity Map dataset and analytics are provided elsewhere (Lamb et al., 2006).
HUVEC proliferation assay
HUVEC cells were plated in a 96 well plate in HUVEC minimal medium (Chembrex, EBM-2). 24 hours later, medium was replaced with a 1:4 mix of tissue culture medium that had been conditioned from compound or DMSO-only treated 786-O cells and HUVEC minimal medium. HUVEC proliferation was quantified by a colorimetric proliferation assay (WST-1, Roche, Germany).
Western blots
Western Blots were performed as described (Zimmer et al., 2004). Antibodies: Monoclonal anti-HIF-2a (Novus, NB100-132), polyclonal anti-HIF-2a (Novus, NB100-122), monoclonal anti-HIF-1a (BD Biosciences, 610958), polyclonal Glut-1 (Alpha Diagnostics, GT-11-A), polyclonal anti-p70S6K (Cell Signaling Technologies, 9202), polyclonal anti-phospho T389 p70S6K (Cell Signaling Technologies, 9205S), polyclonal anti-phospho S235/6 S6 (Cell Signaling Technologies, 2211S), or monoclonal anti-B-Actin (Novus, ab8226). IRP1 antibody has been described before (Wang et al., 2007a). The polyclonal IRP2 antibody (UT30) was a generous gift from Dr. B. Leibold. Secondary HRP-conjugated antibodies were purchased from Pierce (31432, 31462). Hypoxia induction: Two identical plates of cells were changed into fresh medium and incubated at 37°C, 5% CO2, and ambient O2, for 24 hours. After 24 hours, one set was kept at 37°C, 5% CO2, and ambient O2, while the other was placed into an ESPEC hypoxic incubator (1% O2, 5% CO2, 37°C) for another 24 hours.
Enzyme-Linked Immunosorbent Assay (ELISA)
VEGF or IGFBP3 ELISA was performed on the conditioned supernatant using Quantikine immunoassay kits (R&D Systems, DVE00 for VEGF, DGB300 for IGFBP3).
Cycloheximide experiments
Cells were treated with medium only, DMSO, or compound as described above. Following the second day of compound incubation, 10 µg/mL cycloheximide (CHx) was added to halt de novo protein synthesis and time points were taken. Protein quantification and Western blots were performed as described.
In vivo 35S-methionine pulse-label experiments
Compound-treated cells were pulse-labeled for 30 minutes at 37°C with 0.5 mCi 35S-Methionine (New England Nuclear) per p100 plate and lysed as described for Western blots. 500 µg lysate was immunoprecipitated with 2 µg polyclonal anti-HIF-2a (Novus, NB100-122) or control polyclonal anti-HA Y-11 (Santa Cruz, sc-805) antibodies. Chase experiments were performed with excess cold methionine (3 mg/mL methionine). Immunoprecipitated samples were resolved by SDS-PAGE. Western blot and autoradiograph images were scanned and optical density of bands determined using a UVP BioImaging System and LabWorks Image Acquisition and Analysis Software (UVP, Inc.).
Electrophoretic Mobility Shift Assays (EMSAs)
EMSAs were performed as described (Fillebeen et al., 2003). Briefly, 50 µg lysate from 786-O cells treated with medium only, DMSO, or compounds were incubated with 25,000 cpm wild-type or mutant radiolabeled HIF-2a IRE probe for 20 minutes at room temperature before loading onto non-denaturing acrylamide gels. Supershifts were performed identically, save that 0.2 µg control, IRP1 or IRP2 antibodies were also added. Total protein content was estimated by Bradford Assay (BioRad, 500-0006), and even loading for all experiments was confirmed by Western blot for B-Actin.
In vitro iron competition assay
This assay was performed as described (Wang et al., 2002). Displacement of iron from ethyl-3,4-dihydroxybenzoate (EDHB) complex was measured as a decrease in absorbance at 500 nm. Ferric iron, prepared as a 5 mM stock solution of FeCl3 in 100 mM sodium citrate, was diluted to 25 µM in the presence of 750 µM EDHB. 1 ml aliquots of this solution were then mixed with 0, 25, 50, 100 or 200 micromolar compound 40, 41, 76, or 77 as well as EDTA and DFO for controls. With the exception of compound 41, only the Fe-(EDHB)3 complex absorbed significantly at 500 nm. The self absorbance of compound 41 in a matched solution lacking Fe-(EDHB)3 was subtracted from the A500 value obtained in the presence of the Fe-(EDHB)3 complex.
Supplementary Material
AKNOWLEDGEMENTS
We thank Drs. Matthias Hentze and Betty Leibold for invaluable advice and reagents. We acknowledge the significant contribution of Tim Mitchison and Carolyn Shamu of the Harvard Institute of Chemistry and Cell Biology through continuous discussion of data, of Drs. Nick Dyson and Jeff Settleman for critically reading the manuscript and of Billy Andriopoulos for technical advice. We also thank Michele Pagano for the pCMV and pCMV-SL constructs, Betty Liebold for the Ferritin-L probe and IRP2 antibody, and Jim Rocco for the pLentiLox3.7 puromycin, hygromycin and blasticidin vectors. This work has been supported by the NIH 5R01CA104574 (to OI), the VHL Family Alliance Award, the Dana-Farber/Harvard Cancer Center Kidney Cancer Program Developmental Award (to MZ) and the National Cancer Institute's Initiative for Chemical Genetics (contract no. N01-CO-12400).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING FINANCIAL INTERESTS STATEMENT
The authors declare no competing financial interests.
REFERENCES
- Arsham AM, Howell JJ, Simon MC. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J Biol Chem. 2003;278:29655–29660. doi: 10.1074/jbc.M212770200. [DOI] [PubMed] [Google Scholar]
- Bert AG, Grepin R, Vadas MA, Goodall GJ. Assessing IRES activity in the HIF-1alpha and other cellular 5' UTRs. Rna. 2006;12:1074–1083. doi: 10.1261/rna.2320506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blais JD, Filipenko V, Bi M, Harding HP, Ron D, Koumenis C, Wouters BG, Bell JC. Activating transcription factor 4 is translationally regulated by hypoxic stress. Mol Cell Biol. 1994;24:7469–7482. doi: 10.1128/MCB.24.17.7469-7482.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, Kaelin WG. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18:2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- Christova T, Templeton DM. Effect of hypoxia on the binding and subcellular distribution of iron regulatory proteins. Mol Cell Biochem. 2007;301:21–32. doi: 10.1007/s11010-006-9393-2. [DOI] [PubMed] [Google Scholar]
- DeYoung MP, Horak P, Sofer A, Sgroi D, Ellisen LW. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev. 2008;22:239–251. doi: 10.1101/gad.1617608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, et al. Celegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Fillebeen C, Chahine D, Caltagirone A, Segal P, Pantopoulos K. A phosphomimetic mutation at Ser-138 renders iron regulatory protein 1 sensitive to iron-dependent degradation. Mol Cell Biol. 2003;23:6973–6981. doi: 10.1128/MCB.23.19.6973-6981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub TR, Slonim D, Tamayo P, Huard C, Gaasenbeek M, Mesirov J, Coller H, Loh M, Downing JR, Caligiuri MA, et al. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science. 1999;286:531–537. doi: 10.1126/science.286.5439.531. [DOI] [PubMed] [Google Scholar]
- Gordan JD, Simon MC. Hypoxia-inducible factors: central regulators of the tumor phenotype. Curr Opin Genet Dev. 2007;17:71–77. doi: 10.1016/j.gde.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson ES, Foot LM, Leibold EA. Hypoxia post-translationally activates iron-regulatory protein 2. J Biol Chem. 1999;274:5047–5052. doi: 10.1074/jbc.274.8.5047. [DOI] [PubMed] [Google Scholar]
- Hanson ES, Leibold EA. Regulation of iron regulatory protein 1 during hypoxia and hypoxia/reoxygenation. J Biol Chem. 1998;273:7588–7593. doi: 10.1074/jbc.273.13.7588. [DOI] [PubMed] [Google Scholar]
- Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG. HIF1a targeted for VHL-mediated destruction by proline hydroxylation: implications for oxygen sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- Jaakkola P, Mole D, Tian YM, Wilson MI, Gielbert J, Gaskel lSJ, Kriegsheim AA, Hebestreit HF, Mukherji M, Schofield CJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau Ubiquitylation Complex by O2-Regulated Prolyl Hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- Ke Y, Wu J, Leibold EA, Walden WE, Theil EC. Loops and bulge/loops in iron-responsive element isoforms influence iron regulatory protein binding. Fine-tuning of mRNA regulation? J Biol Chem. 1998;273:23637–23640. doi: 10.1074/jbc.273.37.23637. [DOI] [PubMed] [Google Scholar]
- Lamb J, Crawford ED, Peck D, Modell JW, Blat IC, Wrobel MJ, Lerner J, Brunet JP, Subramanian A, Ross KN, et al. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313:1929–1935. doi: 10.1126/science.1132939. [DOI] [PubMed] [Google Scholar]
- Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002a;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science. 2002b;295:858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- Lang KJ, Kappel A, Goodall GJ. Hypoxia-inducible factor-1alpha mRNA contains an internal ribosome entry site that allows efficient translation during normoxia and hypoxia. Mol Biol Cell. 2002;13:1792–1801. doi: 10.1091/mbc.02-02-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Cash TP, Jones RG, Keith B, Thompson CB, Simon MC. Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol Cell. 2006;21:521–531. doi: 10.1016/j.molcel.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Simon MC. Regulation of transcription and translation by hypoxia. Cancer Biol Ther. 2004;3:492–497. doi: 10.4161/cbt.3.6.1010. [DOI] [PubMed] [Google Scholar]
- Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- Maxwell PH, Pugh CW, Ratcliffe PJ. Activation of the HIF pathway in cancer. Current Opinion in Genetics and Development. 2001;11:293–299. doi: 10.1016/s0959-437x(00)00193-3. [DOI] [PubMed] [Google Scholar]
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- Meyron-Holtz EG, Ghosh MC, Iwai K, LaVaute T, Brazzolotto X, Berger UV, Land W, Ollivierre-Wilson H, Grinberg A, Love P, Rouault TA. Genetic ablations of iron regulatory proteins 1 and 2 reveal why iron regulatory protein 2 dominates iron homeostasis. Embo J. 2004;23:386–395. doi: 10.1038/sj.emboj.7600041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, Pavletich N, Chau V, Kaelin WG. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol. 2000;2:423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- Raval RR, Lau KW, Tran MG, Sowter HM, Mandriota SJ, Li JL, Pugh CW, Maxwell PH, Harris AL, Ratcliffe PJ. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol. 2005;25:5675–5686. doi: 10.1128/MCB.25.13.5675-5686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, Fritsch, Maniatis, editors. Molecular Cloning: a Laboratory Manual. CSHL Press; 1989. [Google Scholar]
- Sanchez M, Galy B, Muckenthaler MU, Hentze MW, editors. Iron-regulatory proteins limit hypoxia-inducible factor-2alpha expression in iron deficiency. Nat Struct Mol Biol. 2007;14:420–426. doi: 10.1038/nsmb1222. [DOI] [PubMed] [Google Scholar]
- Schepens B, Tinton SA, Bruynooghe Y, Beyaert R, Cornelis S. The polypyrimidine tract-binding protein stimulates HIF-1alpha IRES-mediated translation during hypoxia. Nucleic Acids Res. 2005;33:6884–6894. doi: 10.1093/nar/gki1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider BD, Leibold EA. Effects of iron regulatory protein regulation on iron homeostasis during hypoxia. Blood. 2003;102:3404–3411. doi: 10.1182/blood-2003-02-0433. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1747–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- Spicher A, Guicherit OM, Duret L, Aslanian A, Sanjines EM, Denko NC, Giaccia AJ, Blau HM. Highly conserved RNA sequences that are sensors of environmental stress. Mol Cell Biol. 1998;18:7371–7382. doi: 10.1128/mcb.18.12.7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacchini L, Gammella E, De Ponti C, Recalcati S, Cairo G. Role of HIF-1 and NF-kappaB transcription factors in the modulation of transferrin receptor by inflammatory and anti-inflammatory signals. J Biol Chem. 2008;283:20674–20686. doi: 10.1074/jbc.M800365200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, Johannes GJ. Identification of mRNAs that continue to associate with polysomes during hypoxia. Rna. 2007;13:1116–1131. doi: 10.1261/rna.534807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth I, Yuan L, Rogers JT, Boyce H, Bridges KR. Hypoxia alters iron-regulatory protein-1 binding capacity and modulates cellular iron homeostasis in human hepatoma and erythroleukemia cells. J Biol Chem. 1999;274:4467–4473. doi: 10.1074/jbc.274.7.4467. [DOI] [PubMed] [Google Scholar]
- Wang J, Buss JL, Chen G, Ponka P, Pantopoulos K. The prolyl 4-hydroxylase inhibitor ethyl-3,4-dihydroxybenzoate generates effective iron deficiency in cultured cells. FEBS Lett. 2002;529:309–312. doi: 10.1016/s0014-5793(02)03389-6. [DOI] [PubMed] [Google Scholar]
- Wang J, Chen G, Muckenthaler M, Galy B, Hentze MW, Pantopoulos K. Iron-mediated degradation of IRP2, an unexpected pathway involving a 2-oxoglutarate-dependent oxygenase activity. Mol Cell Biol. 2004;24:954–965. doi: 10.1128/MCB.24.3.954-965.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Fillebeen C, Chen G, Biederbick A, Lill R, Pantopoulos K. Iron-dependent degradation of apo-IRP1 by the ubiquitin-proteasome pathway. Mol Cell Biol. 2007a;27:2423–2430. doi: 10.1128/MCB.01111-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Di X, D'Agostino RB, Jr, Torti SV, Torti FM. Excess capacity of the iron regulatory protein system. J Biol Chem. 2007b;282:24650–24659. doi: 10.1074/jbc.M703167200. [DOI] [PubMed] [Google Scholar]
- Wouters BG, van den Beucken T, Magagnin MG, Koritzinsky M, Fels D, Koumenis C. Control of the hypoxic response through regulation of mRNA translation. Semin Cell Dev Biol. 2005;16:487–501. doi: 10.1016/j.semcdb.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Yang HS, Cho MH, Zakowicz H, Hegamyer G, Sonenberg N, Colburn NH. A novel function of the MA-3 domains in transformation and translation suppressor Pdcd4 is essential for its binding to eukaryotic translation initiation factor 4A. Mol Cell Biol. 2004;24:3894–3906. doi: 10.1128/MCB.24.9.3894-3906.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer M, Doucette D, Siddiqui N, Iliopoulos O. Inhibition of Hypoxia Inducible Factor is Sufficient for Growth Suppression of VHL−/− Tumors. Mol Cancer Res. 2004;2:89–95. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.