Abstract
Object
Treatment options for anaplastic or malignant intramedullary spinal cord tumors (IMSCTs) remain limited. Paclitaxel has potent cytotoxicity against experimental intracranial gliomas and could be beneficial in the treatment of IMSCTs, but poor CNS penetration and significant toxicity limit its use. Such limitations could be overcome with local intratumoral delivery. Paclitaxel has been previously incorporated into a biodegradable gel depot delivery system (OncoGel) and in this study the authors evaluated the safety of intramedullary injections of OncoGel in rats and its efficacy against an intramedullary rat gliosarcoma.
Methods
Safety of intramedullary OncoGel was tested in 12 Fischer-344 rats using OncoGel concentrations of 1.5 and 6.0 mg/ml (5 μl); median survival and functional motor scores (Basso-Beattie-Bresnahan [BBB] scale) were compared with those obtained with placebo (ReGel) and medium-only injections. Efficacy of OncoGel was tested in 61 Fischer-344 rats implanted with an intramedullary injection of 9L gliosarcoma containing 100,000 cells in 5 μl of medium, and randomized to receive OncoGel administered on the same day (in 32 rats) or 5 days after tumor implantation (in 29 rats) using either 1.5 mg/ml or 3.0 mg/ml doses of paclitaxel. Median survival and BBB scores were compared with those of ReGel-treated and tumor-only rats. Animals were killed after the onset of deficits for histopathological analysis.
Results
OncoGel was safe for intramedullary injection in rats in doses up to 5 μl of 3.0 mg/ml of paclitaxel; a dose of 5 μl of 6.0 mg/ml caused rapid deterioration in BBB scores. OncoGel at concentrations of 1.5 mg/ml and 3.0 mg/ml paclitaxel given on both Day 0 and Day 5 prolonged median survival and preserved BBB scores compared with controls. OncoGel 1.5 mg/ml produced 62.5% long-term survivors when delivered on Day 0. A comparison between the 1.5 mg/ml and the 3.0 mg/ml doses showed higher median survival with the 1.5 mg/ml dose on Day 0, and no differences in median survival or BBB scores after treatment on Day 5.
Conclusions
OncoGel is safe for intramedullary injection in rats in doses up to 5 μl of 3.0 mg/ml, prolongs median survival, and increases functional motor scores in rats challenged with an intramedullary gliosarcoma at the doses tested. This study suggests that locally delivered chemotherapeutic agents could be of temporary benefit in the treatment of malignant IMSCTs under experimental settings.
Keywords: intramedullary spinal cord tumor, OncoGel chemotherapy, gliosarcoma, rat, paclitaxel, oncology
Intramedullary spinal cord tumors represent a small subset of all CNS tumors, but are associated with significant morbidity secondary to neurological compromise.18,29,43,45 Surgical debulking remains the mainstay of treatment for malignant IMSCTs18,19,38 and improved microsurgical and intraoperative monitoring techniques have had a positive, but modest, impact on patient survival, particularly in patients with high-grade tumors such as anaplastic astrocytoma or glioblastoma. Postoperative adjuvant therapies such as radiation and chemotherapy have become increasingly important in the management of high-grade, persistent, nonresectable, or recurring IMSCTs.3,18,19,29,38,43
Radiation therapy has been used as an adjuvant treatment for IMSCTs. Its clinical efficacy, however, is limited by both dose-related toxicity to the normal spinal cord and surrounding tissues, and systemic toxicity26,39,48 These adverse effects are particularly deleterious in pediatric patients, in whom a large percentage of IMSCTs occur.18 As a result, chemotherapy has been proposed as an alternative treatment for these patients.3,43 Nevertheless, chemotherapeutic options have not been thoroughly evaluated, and treatment regimens are confined to systemically administered agents selected on the basis of data from intracranial brain tumors,3, 29,43 and the role of chemotherapeutic agents in the treatment of IMSCTs remains undefined.
Chemotherapy for CNS tumors is well established in both preclinical and clinical studies for intracranial tumors, and novel drug discovery technologies as well as improved drug delivery systems continue to enhance their efficacy.9,12,21,22,24,25,34,46 Several chemotherapeutic agents that have been shown to be effective against experimental animal and human gliomas have been used empirically to treat IMSCTs with limited success.20,37 The limited effect observed with these agents could be related in part to inadequate penetration into the tumor site at tolerated doses, and severe systemic toxicity at high doses. The challenges faced with systemic chemotherapy warrant the selection of more suitable agents and development of adequate drug delivery systems for spinal cord administration.
Paclitaxel, a cellular proliferation inhibitor, has been shown to be effective against gliomas in vitro, but has poor penetration into the CNS when administered systemically, and shows dose-limiting toxicities such as sensory neuropathies, gastrointestinal disturbances, and severe myelosuppression.17,32,45,46 To enhance the efficacy of paclitaxel and limit its systemic toxicity, taxol has been incorporated into local drug delivery devices including biodegradable polymers and convection-enhanced delivery systems.21,33,42,47 Although these strategies have effectively treated intracranial experimental gliomas and await further clinical testing,20,21,33 they require placement of either a polymer/drug wafer into the resection cavity or an intratumoral catheter, and the anatomical characteristics of the spinal cord limit their use in IMSCT therapy.
An ideal drug delivery system for local chemotherapy of IMSCTs should provide consistent and precise intramedullary tumor localization, deliver drugs in a sustained controlled fashion, and be amenable for intraoperative use as well as for percutaneous image-guided administration. ReGel is a thermal gel depot–based delivery system developed by Protherics Salt Lake City, Inc.51 ReGel consists of an ABA-type (A = poly[lactide-co-glycolide], B = poly[ethylene glycol]) biodegradable thermosensitive gel, which can be tailored to deliver various agents at different rates. ReGel has been formulated with paclitaxel (OncoGel; Protherics) to provide an injectable, controlled-release, biodegradable vehicle for paclitaxel administration. OncoGel has been shown to be biocompatible in rats,2,15,44 and an OncoGel formulation containing 2 mg/ml of paclitaxel releases paclitaxel in a sustained fashion for 50 days, and is biodegraded in 4–6 weeks at 37°C.51 In vivo studies demonstrate adequate biodistribution of cytotoxic levels of paclitaxel in both human breast carcinoma xenografts as well as in the brain parenchyma following OncoGel injection.51 OncoGel has been shown to be efficacious in extending survival in experimental intracranial rodent gliomas as well as in delaying the onset of paresis in rodent breast metastatic spine tumor models.2,15,44 The results of the efficacy studies with intracranially injected OncoGel were used as the basis for initiation of a Phase I/II clinical trial for the treatment of recurrent malignant glioma.
With this encouraging data, we hypothesized that local delivery of paclitaxel could be efficacious in the treatment of IMSCTs. Therefore, in the present study, we tested the safety of intramedullary injected OncoGel in rats, an animal model developed by our group, and then monitored the ability of intramedullary OncoGel to delay hindlimb paresis and prolong survival of rats challenged with a lethal dose of intramedullary 9L gliosarcoma.
Methods
Experimental Design
Toxicity Study
To determine the biocompatibility of OncoGel after intramedullary injection, 12 Fischer-344 rats were randomized into 4 experimental groups, each containing 3 animals. The first group received a 5-μl intramedullary injection of DMEM only (untreated control; Life Technologies, Inc.); the second group received an injection of 5 μl of ReGel (vehicle control); the third group received a 5-μl intramedullary injection of OncoGel loaded with 1.5 mg/ml of paclitaxel; and the fourth group received a 5-μl intramedullary injection of OncoGel loaded with 6.0 mg/ml of paclitaxel (Table 1). Animals were evaluated and weighed daily for signs of systemic toxicity; their hindlimb functional motor performance was tested periodically as described below. Full necropsies were performed in all animals at the completion of the study (Day 28; Table 1).
Table 1. Scores on the BBB scale in the toxicity study*.
| Experimental Groups† | Mean BBB Score ± SEM on Day 13 |
Comparison p Values | Median Survival (days) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Controls | ReGel | 1.5 mg/ml | 6.0 mg/ml | |||
| DMEM (control) | 21 ± 0 | NA | >0.05 | >0.05 | <0.001 | 28 |
| ReGel (vehicle only) | 21 ± 0 | >0.05 | NA | >0.05 | <0.001 | 28 |
| 1.5 mg/ml OncoGel | 21 ± 0 | >0.05 | >0.05 | NA | <0.001 | 28 |
| 6.0 mg/ml OncoGel | 4 ± 2 | <0.001 | <0.001 | <0.001 | NA | 14 |
NA = not applicable.
Three rats in each group.
Efficacy Study: Treatment Administered on Day 0
To evaluate the efficacy of intramedullary OncoGel in treating animals with a lethal intramedullary challenge of 9L gliosarcoma, 32 Fischer-344 rats received a 5-μl intramedullary injection of 100,000 9L gliosarcoma cells in medium on Day 0 and were randomized to the following experimental groups (8 animals per group): animals in the first group received no further treatment (untreated control group), animals in the second group received an intramedullary injection of 5 μl of ReGel (vehicle control) on Day 0, the third group received an intramedullary injection of 5 μl of 1.5 mg/ml OncoGel on Day 0, and the fourth group received a 5-μl intramedullary injection of 3.0 mg/ml OncoGel on Day 0 (Tables 2 and 3).
Table 2. Scores on the BBB scale in the efficacy study.
| Experimental Groups | Mean BBB Score ± SEM on Day 13 | Comparison p Values | |||
|---|---|---|---|---|---|
|
| |||||
| Controls | ReGel | 1.5 mg/ml | 3.0 mg/ml | ||
| treatment on Day 0* | |||||
| 9L tumor Day 0 (control) | 5.38 ± 3.54 | NA | 0.621 | 0.007 | 0.004 |
| 9L tumor & ReGel (vehicle only) Day 0 | 7 ± 2.46 | 0.621 | NA | 0.003 | 0.005 |
| 9L tumor & 1.5 mg/ml OncoGel Day 0 | 15.25 ± 1.19 | 0.007 | 0.003 | NA | 0.554 |
| 9L tumor & 3.0 mg/ml OncoGel Day 0 | 14.5 ± 0.33 | 0.004 | 0.005 | 0.554 | NA |
| treatment on Day 5† | |||||
| 9L tumor Day 0 (control) | 3.2 ± 1.08 | NA | 0.162 | 0.013 | 0.016 |
| 9L tumor Day 0 & ReGel (vehicle only) Day 5 | 5.87 ± 1.35 | 0.162 | NA | 0.108 | 0.29 |
| 9L tumor Day 0 & 1.5 mg/ml OncoGel Day 5 | 9.25 ± 1.42 | 0.013 | 0.108 | NA | 0.266 |
| 9L tumor Day 0 & 3.0 mg/ml OncoGel Day 5 | 7.5 ± 0.5 | 0.016 | 0.29 | 0.266 | NA |
Eight rats in each group (32 total).
Five rats in the control group, 8 rats in each of the other 3 groups (29 total).
Table 3. Median survival values in the efficacy study.
| Experimental Groups | Median Survival (days) | Comparison p Values | |||
|---|---|---|---|---|---|
|
| |||||
| Controls | ReGel | 1.5 mg/ml | 3.0 mg/ml | ||
| treatment on Day 0* | |||||
| 9L tumor Day 0 (control) | 11 | NA | 0.438 | 0.0002 | <0.0001 |
| 9L tumor & ReGel (vehicle only) Day 0 | 13 | 0.438 | NA | 0.0003 | 0.0001 |
| 9L tumor & 1.5 mg/ml OncoGel Day 0 | NR‡ | 0.0002 | 0.0003 | NA | 0.0179 |
| 9L tumor & 3.0 mg/ml OncoGel Day 0 | 38 | <0.0001 | 0.0001 | 0.0179 | NA |
| treatment on Day 5† | |||||
| 9L tumor Day 0 (control) | 13 | NA | 0.3288 | 0.0018 | 0.0001 |
| 9L tumor Day 0 & ReGel (vehicle only) Day 5 | 13 | 0.3288 | NA | 0.0635 | 0.0439 |
| 9L tumor Day 0 & 1.5 mg/ml OncoGel Day 5 | 22 | 0.0018 | 0.0635 | NA | 0.5527 |
| 9L tumor Day 0 & 3.0 mg/ml OncoGel Day 5 | 27 | 0.0001 | 0.0439 | 0.5527 | NA |
Eight rats in each group (32 total).
Five rats in the control group, 8 rats in each of the other 3 groups (29 total).
Not reached (long-term survivors were killed on Day 97).
Efficacy Study: Treatment Administered on Day 5 (Established Tumor)
To determine the efficacy of intramedullary OncoGel against an established intramedullary 9L gliosarcoma, 29 Fischer-344 rats received a 5-μl intramedullary injection of 100,000 9L gliosarcoma cells in medium on Day 0 and were randomized into 4 experimental groups. The first group (5 rats) received no further intervention (untreated control group). On Day 5, the 8 animals in the second group were injected, at the site of the previous injection, with 5 μl of intramedullary ReGel (vehicle control), the 8 rats in the third group received an intramedullary injection of 5 μl of OncoGel with 1.5 mg/ml of paclitaxel, and the 8 rats in the fourth group received an intramedullary injection of 5 μl of OncoGel loaded with 3.0 mg/ml of paclitaxel (Tables 2 and 3).
ReGel/OncoGel Formulations and In Vitro Release of Paclitaxel
The synthesis and characterization of the ReGel (vehicle control) and OncoGel formulations have been de-scribed previously.51 Briefly, ReGel is a biodegradable thermosensitive polymer that remains water soluble at temperatures below the gel transition temperature and becomes a water-insoluble gel after injection. ReGel easily solubilizes and stabilizes sensitive drugs, including proteins. The ReGel/paclitaxel preparation (OncoGel) has been shown to provide a sustained local release of paclitaxel for approximately 50 days in vitro, with a slow clearance and minimal distribution into any organ after intratumoral injection.
Tumor Cell Lines
Rat 9L gliosarcoma cells were obtained from the University of California, San Francisco Brain Tumor Research Center, and maintained in our laboratory in DMEM with 4.5 g/L of glucose, supplemented with 10% fetal bovine serum, and penicillin/streptomycin. A tumor suspension was prepared by suspending 100,000 cells in 5 μl of DMEM.
Animal Care
Female Fischer-344 rats weighing 150–200 grams each were obtained from Charles River Laboratories. Animals were housed in standard facilities and were given free access to water and rodent chow. All animals were treated in accordance with the policies and procedures set forth by the Animal Care and Use Committee of the Johns Hopkins University School of Medicine.
Surgical Technique
We have previously described the technique for intramedullary implantation of 9L gliosarcoma in rats.8 Briefly, animals were anesthetized with an intraperitoneal injection (0.4–0.6 ml) of a stock solution consisting of ketamine hydrochloride (25 mg/ml; Hospira, Inc.), xylazine (2.5 mg/ml; Phoenix Pharmaceutical, Inc.), and 14.25% ethanol in normal saline. Animals were placed on a sterile field, and the back of each animal was shaved and prepared with a betadine solution. The prominent spinous process of T-5 was identified, a 2-cm longitudinal incision was made over the dorsal midthoracic region, the underlying fascia and paravertebral muscles were retracted, and the spinous process of T-5 was removed using rongeurs. The ligamentum flavum was removed, exposing the intervertebral space, and the cell suspension was injected through the dorsal intervertebral space using a 26-gauge Hamilton syringe (Hamilton Company). After the intramedullary injection, hemostasis was verified, the wounds were closed with surgical staples, and analgesia was provided by an intraperitoneal injection of 0.2 ml of buprenorphine (0.02 mg/ml, Abbott Laboratories) in saline.
Functional Testing
Functional testing of hindlimb strength was assessed by using the BBB scale.4,5 Animals were placed in an open field testing area, allowed to adapt, and observed for 4 minutes. Their locomotion was rated using the BBB locomotor scale, which is a 22-point scale ranging from 21 (consistent plantar stepping and coordinated gait, consistent toe clearance, predominant paw position parallel throughout stance, consistent trunk stability, tail consistently up) to 0 (no observable hindlimb movement). All animals were tested preoperatively to ensure a baseline locomotor rating of 21. Postoperatively, animals were tested at least once every other day by 3 different blinded observers; interobserver variability, however, was not determined.
Histopathological Analysis
Once the functional BBB score of an animal was less than 5 (slight movement of 2 joints and extensive movement of the third), the animals were killed by CO2 overexposure. After the animals were killed, the spinal column of each animal was exposed, and a segment of the spinal cord encompassing all visible tumor including the surgical site, along with 2 contiguous vertebral segments caudal and 2 rostral with no visible tumor, were harvested and placed in 4.0% formalin in phosphate-buffered saline. At the completion of the study, all spines were decalcified. Three thoracic spine sections (2-mm each) were sliced (2 sections through visible tumor, 1 section through normal tissue) and embedded in paraffin. Five 10-μm slices were taken from each section for H & E staining.
Statistical Analysis
In this experiment, a BBB functional score of less than 5 was the primary end point, and thus the thresh-old for subsequent animal sacrifice. Survival times were compared among groups by the log-rank (Mantel-Cox) test in Kaplan-Meier nonparametric analysis of survival; median survival is reported as the median ± SEM. Results of the BBB score on Day 13 after tumor implantation are expressed as the mean ± SEM, and treated animals were compared with controls by a 2-tailed t-test for independent samples. The statistical software program SPSS version 12.0 for Windows (SPSS, Inc.) was used for the statistical analyses.
Results
Toxicity of ReGel and OncoGel
Animals in this study were randomized to receive an intramedullary injection of DMEM only (untreated control), ReGel (vehicle control), OncoGel with 1.5 mg/ml of paclitaxel, or OncoGel with 6.0 mg/ml of paclitaxel. Injection of OncoGel with 6.0 mg/ml of paclitaxel caused rapid deterioration in hindlimb motor function. Animals in this group had a median survival of 14 days and a mean BBB score on Day 12 of 4 ± 2 (Table 1). Animals receiving either ReGel or 1.5 mg/ml OncoGel showed no functional deficits and exhibited 100% survival rates throughout the study, which was concluded on Day 28 (Fig. 1 lower). On Day 12, animals in the ReGel and the 1.5 mg/ml OncoGel groups both had mean BBB scores of 21 (Fig. 1 upper).
Fig. 1.
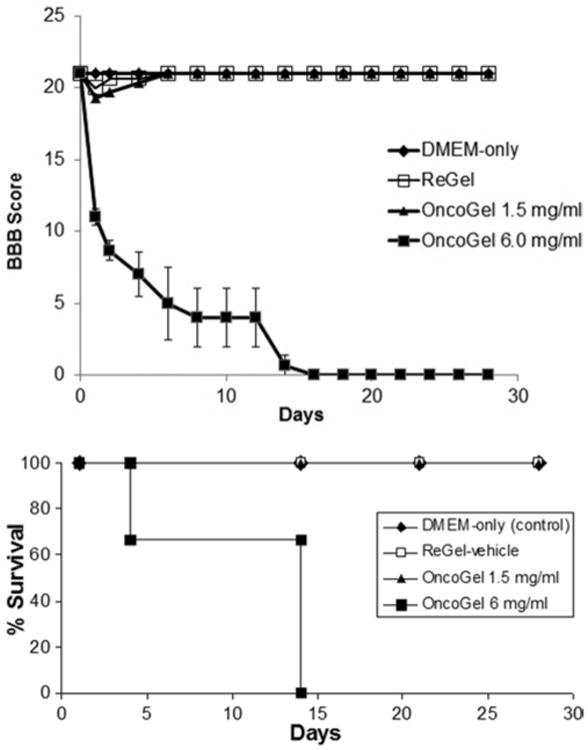
Graphs of the biocompatibility study showing BBB score progression over time (upper) and Kaplan-Meier survival curves (lower) in each group.
Efficacy of OncoGel Injected on Day 0
Animals in this study received an injection of either 9L cells alone, or 9L followed by OncoGel on the same day. On postoperative Day 13, animals receiving 9L only (untreated control) had a mean BBB score of 5.38 ± 3.54 and a median survival of 11 days, and those receiving 9L and ReGel (vehicle control) had a BBB score of 7 ± 2.46 and a median survival of 13 days (Tables 2 and 3). Animals that received 9L and treatment with 5 μl of OncoGel 1.5 mg/ml had a significantly higher mean BBB score (15.25 ± 1.19 on Day 13) when compared with untreated controls (p = 0.007) and ReGel animals (p = 0.003), and had significantly higher median survival rates. Median survival was not reached by Day 97 in this group and 62.5% of the animals were long-term survivors (p = 0.0002 vs untreated controls, and p = 0.0003 vs ReGel). Similarly, animals treated with 5 μl of OncoGel 3.0 mg/ml had a higher mean BBB score (14.5 ± 0.33 on Day 13) when compared with untreated controls (p = 0.004) and ReGel (p = 0.005), and higher median survival rates (38 days; p < 0.0001 vs untreated controls, and p = 0.0001 vs ReGel; Fig. 2).
Fig. 2.
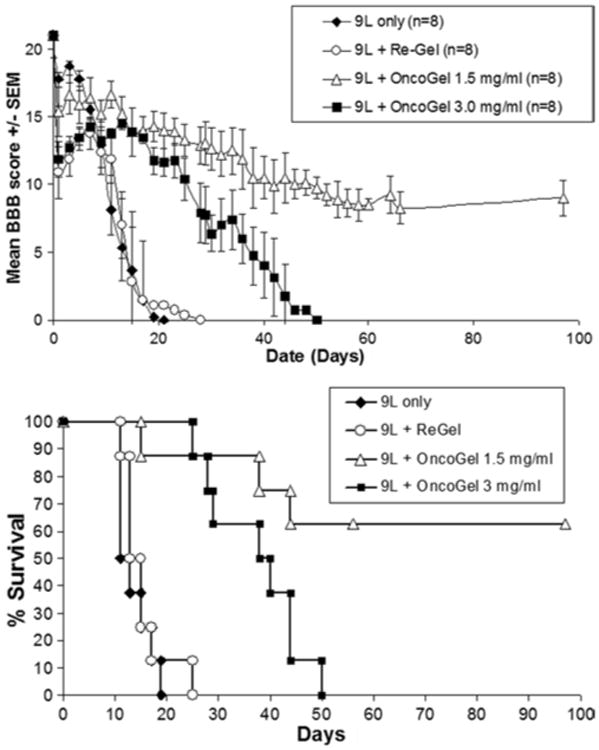
Graphs of the Day 0 treatment efficacy study showing progression of BBB scores over time (upper) and Kaplan-Meier survival curves (lower) in each group.
Animals treated with 1.5 mg/ml of OncoGel had significantly higher median survival (p = 0.0179) than animals treated with 3.0 mg/ml of OncoGel (Fig. 2 lower), although the BBB scores on Day 13 were not significantly different between the 1.5 mg/ml and 3.0 mg/ml groups (p = 0.554; Fig. 2 upper). No differences were found in BBB scores or median survival between untreated animals and animals treated with ReGel (p = 0.621 and p = 0.438, respectively).
Efficacy of OncoGel Treatment Injected on Day 5 Against an Established 9L Gliosarcoma
Animals in this study were implanted with intramedullary 9L gliosarcoma on Day 0 and were randomized to receive either no further treatment (untreated control), or treatment on Day 5 Animals that received 9L only had a mean BBB score of 3.2 ± 1.08 on postoperative Day 13, and a median survival of 13 days; those that received Re-Gel on Day 5 had a mean BBB score of 5.87 ± 1.35 on Day 13 and a median survival of 13 days. Of the groups that received OncoGel on Day 5, the group that was given 5 μl of OncoGel 1.5 mg/ml had a mean BBB score of 9.25 ± 1.42 on Day 13 (p = 0.013 vs control, p = 0.108 vs ReGel) at the end of the study median of 22 days (12.5% were long-term survivors; p = 0.0018 vs controls, p = 0.0635 vs ReGel). The group receiving 3.0 mg/ml of OncoGel had a mean BBB score of 7.5 ± 0.5 (p = 0.016 vs controls, p = 0.29 vs ReGel) and never reached median survival, with 62.5% of the animals living the length of the experiment (100 days; p = 0.0001 vs controls, p = 0.0439 vs ReGel; Fig. 3 upper).
Fig. 3.
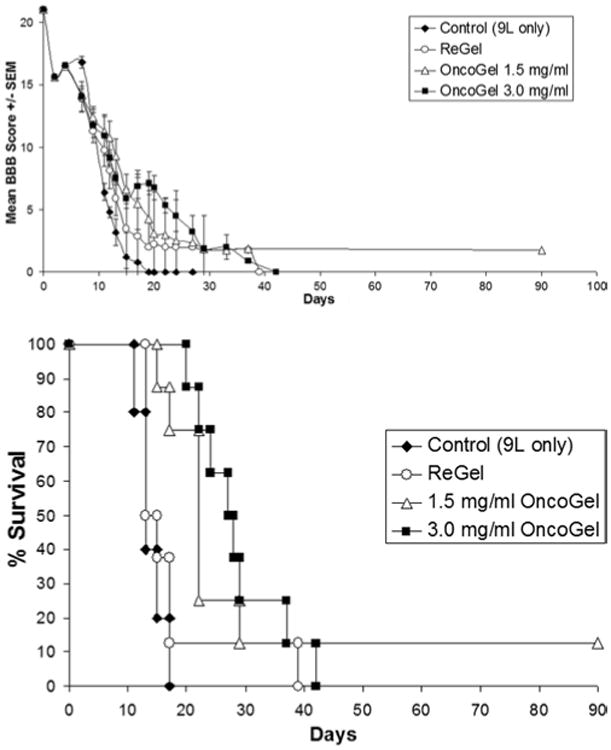
Graphs of the delayed treatment (Day 5) against an established tumor study showing BBB score progression over time (upper) and Kaplan-Meier survival curves (lower) in each group.
No significant differences in mean BBB scores (p = 0.266) or median survival (p = 0.5527) were observed between the groups treated with 5 μl of either 1.5 mg/ml or 3.0 mg/ml of OncoGel, or between the untreated control group and the ReGel group (mean BBB scores p = 0.162 and median survival p = 0.3288, respectively; Fig. 3 lower).
Histopathological Analysis
Histopathological analysis of cross-sections of the spinal cord of animals injected with 9L gliosarcoma alone, or with the 9L gliosarcoma and ReGel, showed well-circumscribed lesions infiltrating most of the gray and white matter, with compression of the remaining structures. Analysis of cross-sections of the spinal cord of animals treated with 1.5 mg/ml OncoGel showed the presence of intramedullary tumors occupying extensive areas (Fig. 4), with moderate signs of necrosis and disseminated scarring. Analysis of specimens from the group treated with 3.0 mg/ml of paclitaxel showed marked necrosis and scarring, more preservation of normal spinal cord architecture, and smaller, more circumscribed tumors. Peritumoral edema was observed in all animals in the study, but appeared to be subjectively greater in the group with 3.0 mg/ml of paclitaxel when compared with the 1.5 mg/ml and control groups. No formal analysis of peritumoral edema was conducted.
Fig. 4.
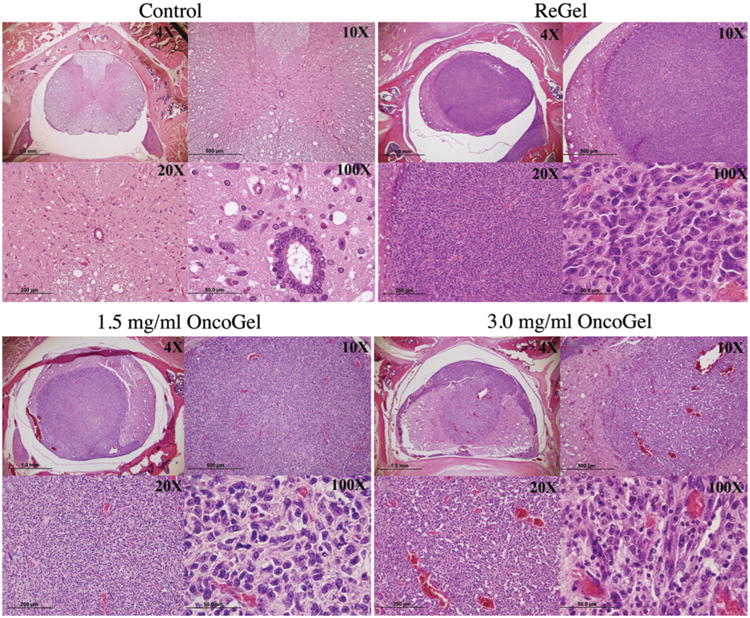
Photomicrographs of cross-sections of the spinal cord stained with H & E in a normal rat (upper left), in a rat injected with 9L and ReGel (vehicle) on Day 0 (upper right), in a rat injected with 9L and treated with intramedullary OncoGel at a dose of 1.5 mg/ml of paclitaxel on Day 0 (lower left), and in a rat injected with 9L and treated with intramedullary OncoGel at a dose of 3.0 mg/ml of paclitaxel on Day 0 (lower right).
Discussion
In the present study we evaluated the biocompatibility and efficacy of OncoGel, a paclitaxel-loaded biodegradable controlled-release injectable gel depot, against an experimental intramedullary rat gliosarcoma. We found that OncoGel can be administered safely in the spinal cord of rats at doses up to 5 μl of 3.0 mg/ml of paclitaxel, whereas a dose of 5 μl of 6.0 mg/ml caused rapid deterioration of hindlimb motor function.
Treatment with OncoGel loaded with 1.5 mg/ml of paclitaxel, on either the same day or 5 days after tumor implantation, significantly increased the functional motor scores and prolonged the median survival of animals challenged with intramedullary 9L glisarcoma, and when treatment was initiated on Day 0, 62.5% of animals were long-term survivors. Similarly, treatment with OncoGel loaded with 3.0 mg/ml of paclitaxel on either the same day or 5 days after tumor implantation significantly increased functional motor scores and prolonged median survival compared with untreated controls (9L tumor injection only).
Comparison of the 2 OncoGel formulations (1.5 and 3.0 mg/ml) used in the study showed that the 1.5 mg/ml formulation resulted in higher median survival if administered on Day 0, and that no significant differences exist between the 2 formulations in functional motor scores or median survival when given 5 days after tumor implantation. We speculate that the decreased median survival observed in the 3.0 mg/ml OncoGel group on Day 0 could be related to the progressive peritumoral edema that accompanies higher doses of chemotherapy, which was observed by histopathological examination with greater frequency in the 3.0 mg/ml OncoGel group compared with the 1.5 mg/ml OncoGel group and untreated controls. Clinical application of locally delivered paclitaxel would require coadministration of systemic corticosteroids. This combination of intramedullary OncoGel and systemic corticosteroids could be tested in the laboratory setting to confirm a decrease in edema and subsequent increase in efficacy.
To test new treatment options for IMSCTs, we had developed the rat IMSCT model described above.8 In this model, 9L tumor cells are injected directly in the intramedullary space of the fifth thoracic vertebra. Functional analysis of hindlimb motor strength resulted in a mean BBB score of 8.4 ± 2.67 on Day 11, and a median survival of 12 ± 2.9 days for the groups receiving 9L tumor alone (untreated control). Control animals injected only with DMEM showed intact functional motor scores throughout the study and no deaths. Histopathological analysis of spinal cord specimens confirmed intramedullary tumors in all of the animals. This established model will enable further characterization of the biology of IMSCTs and can be applied to the evaluation of novel treatment options for these tumors. The findings derived from studies using the intracranial 9L tumor model to study chemotherapy implants have shown that the animal model data parallels the results of subsequent Phase II randomized clinical trials.1, 6, 27, 35,41
The present study is, to our knowledge, the first to report on preclinical testing of locally delivered chemotherapy for the treatment of IMSCTs. The availability of an injectable, biodegradable, gel depot system (ReGel) was essential for adequate administration to the spinal cord in our study. ReGel constitutes an ideal system for IMSCT therapy because it is water soluble at temperatures below the gel transition temperature, and the gel form is water insoluble after injection, thereby increasing the viscosity by 4 orders of magnitude.51 ReGel forms a controlled release drug depot with delivery times that range from 1 to 6 weeks and provides solubilization and stabilization of poorly soluble or sensitive drugs. Paclitaxel is the first chemotherapeutic agent with antiglioma activity incorporated into the ReGel delivery system. The gel provides controlled release of paclitaxel for approximately 50 days, and direct intratumoral injection of OncoGel results in slow clearance of paclitaxel from the injection site, minimal systemic distribution, and in vivo efficacy at doses 10-fold lower than the equivalent maximum tolerated systemic doses.2,15, 44, 51
Paclitaxel binds primarily to microtubules and inhibits their depolymerization into tubulin, which prevents the breakdown of the mitotic spindle during mitosis.36 Paclitaxel has proven to be clinically efficacious for the treatment of ovarian and breast cancers when administered systemically,7,9,11,13,14,16,17,20,33 but despite its potent antiglioma activity in vitro, systemic administration has not shown significant benefit in patients with malignant brain tumors.9,10,17,31 Local delivery of paclitaxel via bio-degradable polymers and microspheres and convection-enhanced delivery has been shown to treat malignant brain tumors effectively in experimental settings.19, 23, 33 The efficacious findings from the intracranial OncoGel studies were the basis for a Phase I/II clinical trial, but those results have not been reported as yet.44
An additional benefit of intratumoral OncoGel is its synergistic activity when combined with radiation therapy. This increased antitumor activity has been shown using human prostate xenografts, a spinal cord metastatic tumor model, and an intracranial glioma model.15, 21,44 This increase in efficacy appears to be related to the radiosensitizing properties of paclitaxel,39,40 which blocks tumor cells in the most radiosensitive phases of the cell cycle (G2-M phase).20 The benefit of radiation therapy alone was shown in the IMSCT model; therefore, adding OncoGel to that treatment regimen should prove beneficial.30
The toxicity of systemic administration of paclitaxel has been previously determined in animal28 and human studies,14,24,49,50 and includes severe myelosuppression, sensory neuropathies, and gastrointestinal disturbances. When paclitaxel is delivered to the brain locally, levels of paclitaxel are maximized at the tumor site, systemic release of paclitaxel is minimal, and myelosuppression is avoided.19,29,44 Although side effects of locally delivered paclitaxel in the CNS such as poor wound healing, chemical and bacterial meningitis, and subdural empyema have been reported in animal33 and human studies,23 they were not observed after intramedullary injection of OncoGel in the present study.
Limitations of this study include the use of nonhuman cell lines, which can result in markedly different responses to paclitaxel, and the induction of intramedullary tumors via tumor injection, which does not take into consideration the conditions required in the tumor microenvironment to result in viable infiltrative tumor cells such as proangiogenic genotypes and immunomodulatory properties to evade immune tumor surveillance, among others. Ideal models would include injection of human-derived tumor cells into immunocompromised animal hosts or spontaneous tumor generation in transgenic animals pre-disposed to glial tumor formation through induced mutations in the platelet-derived growth factor receptor, epidermal growth factor receptor, or neurofbromatosis Type 1 pathways, or other known targets, although in published models of intracranial gliomas generated in this manner, formation of spontaneous spinal cord tumors has yet to be reported.
Conclusions
OncoGel can be safely administered into the spinal cord of rats at doses up to 5 μl of 3.0 mg/ml and it improves the median survival and functional motor scores of rats challenged with a lethal dose of intramedullary 9L gliosarcoma. These findings support the use of locally delivered chemotherapy in the treatment of experimental IMSCTs, and further studies should be performed to assess its efficacy in combination with corticosteroids, radiation, and other antitumor agents.
Acknowledgments
Disclosure: This work was partially funded by a grant from the National Cancer Institute (No. U01 CA 52857), and through a generous gift from the Malia's CORD Foundation. Protherics Salt Lake City, Inc., a BTG group company, manufactures and provided OncoGel and ReGel for this study. Dr. Fowers is a full-time employee of Protherics, Inc.
Abbreviations used in this paper
- BBB
Basso-Beattie-Bresnahan
- DMEM
Dulbecco modified Eagle medium
- IMSCT
intramedullary spinal cord tumor
Footnotes
Author contributions to the study and manuscript preparation include the following. Conception and design: Pradilla, Legnani, Jallo. Acquisition of data: Tyler, Hdeib, Caplan. Analysis and interpretation of data: Pradilla, Tyler, Caplan. Drafting the article: Pradilla, Hdeib. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Pradilla. Statistical analysis: Hdeib, Caplan. Administrative/technical/material support: Tyler, Fowers, Brem, Jallo. Study supervision: Brem.
References
- 1.Attenello FJ, Mukherjee D, Datoo G, McGirt MJ, Bohan E, Weingart JD, et al. Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: a 10-year institutional experience. Ann Surg Oncol. 2008;15:2887–2893. doi: 10.1245/s10434-008-0048-2. [DOI] [PubMed] [Google Scholar]
- 2.Bagley CA, Bookland MJ, Pindrik JA, Ozmen T, Gokaslan ZL, Witham TF. Local delivery of oncogel delays paresis in rat metastatic spinal tumor model. J Neurosurg Spine. 2007;7:194–198. doi: 10.3171/SPI-07/08/194. [DOI] [PubMed] [Google Scholar]
- 3.Balmaceda C. Chemotherapy for intramedullary spinal cord tumors. J Neurooncol. 2000;47:293–307. doi: 10.1023/a:1006499313482. [DOI] [PubMed] [Google Scholar]
- 4.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 5.Basso DM, Beattie MS, Bresnahan JC, Anderson DK, Faden AI, Gruner JA, et al. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J Neurotrauma. 1996;13:343–359. doi: 10.1089/neu.1996.13.343. [DOI] [PubMed] [Google Scholar]
- 6.Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. Lancet. 1995;345:1008–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 7.Buda A, Floriani I, Rossi R, Colombo N, Torri V, Conte PF, et al. Randomised controlled trial comparing single agent paclitaxel vs epidoxorubicin plus paclitaxel in patients with advanced ovarian cancer in early progression after platinum-based chemotherapy: an Italian Collaborative Study from the Mario Negri Institute, Milan, G.O.N.O. (Gruppo Oncologico Nord Ovest) group and I.O.R (Istituto Oncologico Romagnolo) group. Br J Cancer. 2004;90:2112–2117. doi: 10.1038/sj.bjc.6601787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caplan J, Pradilla G, Hdeib A, Tyler BM, Legnani FG, Bagley CA, et al. A novel model of intramedullary spinal cord tumors in rats: functional progression and histopathological characterization. Neurosurgery. 2006;59:193–200. doi: 10.1227/01.NEU.0000219276.44563.DA. [DOI] [PubMed] [Google Scholar]
- 9.Carpentier AF. Neuro-oncology: the growing role of chemotherapy in glioma. Lancet Neurol. 2005;4:4–5. doi: 10.1016/S1474-4422(04)00944-5. [DOI] [PubMed] [Google Scholar]
- 10.Chang SM, Kuhn JG, Robins HI, Schold SC, Jr, Spence AM, Berger MS, et al. A Phase II study of paclitaxel in patients with recurrent malignant glioma using different doses depending upon the concomitant use of anticonvulsants: a North American Brain Tumor Consortium report. Cancer. 2001;91:417–422. doi: 10.1002/1097-0142(20010115)91:2<417::aid-cncr1016>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Delfno C, Caccia G, Gonzáles LR, Mickiewicz E, Rodger J, Balbiani L, et al. Gemcitabine plus paclitaxel as first-line chemotherapy for patients with advanced breast cancer. Oncology. 2004;66:18–23. doi: 10.1159/000076330. [DOI] [PubMed] [Google Scholar]
- 12.Gallia GL, Brem S, Brem H. Local treatment of malignant brain tumors using implantable chemotherapeutic polymers. J Natl Compr Canc Netw. 2005;3:721–728. doi: 10.6004/jnccn.2005.0042. [DOI] [PubMed] [Google Scholar]
- 13.Garcia AA, O'Meara A, Bahador A, Facio G, Jeffers S, Kim DY, et al. Phase II study of gemcitabine and weekly paclitaxel in recurrent platinum-resistant ovarian cancer. Gynecol Oncol. 2004;93:493–498. doi: 10.1016/j.ygyno.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Glantz MJ, Choy H, Kearns CM, Mills PC, Wahlberg LU, Zuhowski EG, et al. Paclitaxel disposition in plasma and central nervous systems of humans and rats with brain tumors. J Natl Cancer Inst. 1995;87:1077–1081. doi: 10.1093/jnci/87.14.1077. [DOI] [PubMed] [Google Scholar]
- 15.Gok B, McGirt MJ, Sciubba DM, Garces-Ambrossi G, Nelson C, Noggle J, et al. Adjuvant treatment with locally delivered OncoGel delays the onset of paresis after surgical resection of experimental spinal column metastasis. Neurosurgery. 2009;65:193–200. doi: 10.1227/01.NEU.0000345948.54008.82. [DOI] [PubMed] [Google Scholar]
- 16.Guppy AE, Nelstrop AE, Foster T, Agarwal R, Seckl MJ, Rustin GJ. A phase II study of sequential carboplatin, paclitaxel and topotecan in patients with previously untreated advanced ovarian cancer. Br J Cancer. 2004;90:810–814. doi: 10.1038/sj.bjc.6601618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagiwara H, Sunada Y. Mechanism of taxane neurotoxicity. Breast Cancer. 2004;11:82–85. doi: 10.1007/BF02968008. [DOI] [PubMed] [Google Scholar]
- 18.Jallo GI, Freed D, Epstein F. Intramedullary spinal cord tumors in children. Childs Nerv Syst. 2003;19:641–649. doi: 10.1007/s00381-003-0820-3. [DOI] [PubMed] [Google Scholar]
- 19.Jallo GI, Kothbauer KF, Epstein FJ. Intrinsic spinal cord tumor resection. Neurosurgery. 2001;49:1124–1128. doi: 10.1097/00006123-200111000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Lapidus RG, Dang W, Rosen DM, Gady AM, Zabelinka Y, O'Meally R, et al. Anti-tumor effect of combination therapy with intratumoral controlled-release paclitaxel (PACLIMER microspheres) and radiation. Prostate. 2004;58:291–298. doi: 10.1002/pros.10331. [DOI] [PubMed] [Google Scholar]
- 21.Lesniak MS, Brem H. Targeted therapy for brain tumours. Nat Rev Drug Discov. 2004;3:499–508. doi: 10.1038/nrd1414. [DOI] [PubMed] [Google Scholar]
- 22.Li KW, Dang W, Tyler BM, Troiano G, Tihan T, Brem H, et al. Polilactofate microspheres for Paclitaxel delivery to central nervous system malignancies. Clin Cancer Res. 2003;9:3441–3447. [PubMed] [Google Scholar]
- 23.Lidar Z, Mardor Y, Jonas T, Pfeffer R, Faibel M, Nass D, et al. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: a phase I/II clinical study. J Neurosurg. 2004;100:472–479. doi: 10.3171/jns.2004.100.3.0472. [DOI] [PubMed] [Google Scholar]
- 24.Lonardi S, Tosoni A, Brandes AA. Adjuvant chemotherapy in the treatment of high grade gliomas. Cancer Treat Rev. 2005;31:79–89. doi: 10.1016/j.ctrv.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Mardor Y, Rahav O, Zauberman Y, Lidar Z, Ocherashvilli A, Daniels D, et al. Convection-enhanced drug delivery: increased efficacy and magnetic resonance image monitoring. Cancer Res. 2005;65:6858–6863. doi: 10.1158/0008-5472.CAN-05-0161. [DOI] [PubMed] [Google Scholar]
- 26.Marucci L, Niemierko A, Liebsch NJ, Aboubaker F, Liu MC, Munzenrider JE. Spinal cord tolerance to high-dose fractionated 3D conformal proton-photon irradiation as evaluated by equivalent uniform dose and dose volume histogram analysis. Int J Radiat Oncol Biol Phys. 2004;59:551–555. doi: 10.1016/j.ijrobp.2003.10.058. [DOI] [PubMed] [Google Scholar]
- 27.McGirt MJ, Than KD, Weingart JD, Chaichana KL, Attenello FJ, Olivi A, et al. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. Clinical article. J Neurosurg. 2009;110:583–588. doi: 10.3171/2008.5.17557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nieto Y, Vaughan WP. Pharmacokinetics of high-dose chemotherapy. Bone Marrow Transplant. 2004;33:259–269. doi: 10.1038/sj.bmt.1704353. [DOI] [PubMed] [Google Scholar]
- 29.Parsa AT, Lee J, Parney IF, Weinstein P, McCormick PC, Ames C. Spinal cord and intradural-extraparenchymal spinal tumors: current best care practices and strategies. J Neurooncol. 2004;69:291–318. doi: 10.1023/b:neon.0000041889.71136.62. [DOI] [PubMed] [Google Scholar]
- 30.Pennant WA, Sciubba DM, Noggle JC, Tyler BM, Tamargo RJ, Jallo GI. Microsurgical removal of intramedullary spinal cord gliomas in a rat spinal cord decreases onset to paresis, an animal model for intramedullary tumor treatment. Childs Nerv Syst. 2008;24:901–907. doi: 10.1007/s00381-008-0587-7. [DOI] [PubMed] [Google Scholar]
- 31.Pipas JM, Meyer LP, Rhodes CH, Cromwell LD, McDonnell CE, Kingman LS, et al. A Phase II trial of paclitaxel and topotecan with flgrastim in patients with recurrent or refractory glioblastoma multiforme or anaplastic astrocytoma. J Neurooncol. 2005;71:301–305. doi: 10.1007/s11060-004-2026-2. [DOI] [PubMed] [Google Scholar]
- 32.Poirier VJ, Hershey AE, Burgess KE, Phillips B, Turek MM, Forrest LJ, et al. Efficacy and toxicity of paclitaxel (Taxol) for the treatment of canine malignant tumors. J Vet Intern Med. 2004;18:219–222. doi: 10.1892/0891-6640(2004)18<219:eatopt>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 33.Pradilla G, Wang PP, Gabikian P, Li K, Magee CA, Walter KA, et al. Local intracerebral administration of Paclitaxel with the paclimer delivery system: toxicity study in a canine model. J Neurooncol. 2006;76:131–138. doi: 10.1007/s11060-005-5531-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raza SM, Pradilla G, Legnani FG, Thai QA, Olivi A, Weingart JD, et al. Local delivery of antineoplastic agents by controlled-release polymers for the treatment of malignant brain tumours. Expert Opin Biol Ther. 2005;5:477–494. doi: 10.1517/14712598.5.4.477. [DOI] [PubMed] [Google Scholar]
- 35.Recinos VR, Tyler BM, Bekelis K, Sunshine SB, Vellimana A, Li KW, et al. Combination of intracranial temozolomide with intracranial carmustine improves survival when compared with either treatment alone in a rodent glioma model. Neurosurgery. 2010;66:530–537. doi: 10.1227/01.NEU.0000365263.14725.39. [DOI] [PubMed] [Google Scholar]
- 36.Rose WC. Taxol: a review of its preclinical in vivo antitumor activity. Anticancer Drugs. 1992;3:311–321. [PubMed] [Google Scholar]
- 37.Rowinsky EK, Donehower RC. Paclitaxel (taxol) N Engl J Med. 1995;332:1004–1014. doi: 10.1056/NEJM199504133321507. [DOI] [PubMed] [Google Scholar]
- 38.Sandalcioglu IE, Gasser T, Asgari S, Lazorisak A, Engelhorn T, Egelhof T, et al. Functional outcome after surgical treatment of intramedullary spinal cord tumors: experience with 78 patients. Spinal Cord. 2005;43:34–41. doi: 10.1038/sj.sc.3101668. [DOI] [PubMed] [Google Scholar]
- 39.Shrivastava RK, Epstein FJ, Perin NI, Post KD, Jallo GI. Intramedullary spinal cord tumors in patients older than 50 years of age: management and outcome analysis. J Neurosurg Spine. 2005;2:249–255. doi: 10.3171/spi.2005.2.3.0249. [DOI] [PubMed] [Google Scholar]
- 40.Sipos EP, Brem H. Local anti-angiogenic brain tumor therapies. J Neurooncol. 2000;50:181–188. doi: 10.1023/a:1006482120049. [DOI] [PubMed] [Google Scholar]
- 41.Sipos EP, Tyler B, Piantadosi S, Burger PC, Brem H. Optimizing interstitial delivery of BCNU from controlled release polymers for the treatment of brain tumors. Cancer Chemother Pharmacol. 1997;39:383–389. doi: 10.1007/s002800050588. [DOI] [PubMed] [Google Scholar]
- 42.Tishler RB, Schiff PB, Geard CR, Hall EJ. Taxol: a novel radiation sensitizer. Int J Radiat Oncol Biol Phys. 1992;22:613–617. doi: 10.1016/0360-3016(92)90888-o. [DOI] [PubMed] [Google Scholar]
- 43.Townsend N, Handler M, Fleitz J, Foreman N. Intramedullary spinal cord astrocytomas in children. Pediatr Blood Cancer. 2004;43:629–632. doi: 10.1002/pbc.20082. [DOI] [PubMed] [Google Scholar]
- 44.Tyler B, Fowers KD, Li KW, Recinos VR, Caplan JM, Hdeib A, et al. A thermal gel depot for local delivery of paclitaxel to treat experimental brain tumors in rats. Laboratory investigation. J Neurosurg. 2010;113:210–217. doi: 10.3171/2009.11.JNS08162. [DOI] [PubMed] [Google Scholar]
- 45.Van Goethem JW, van den Hauwe L, Ozsarlak O, De Schepper AM, Parizel PM. Spinal tumors. Eur J Radiol. 2004;50:159–176. doi: 10.1016/j.ejrad.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 46.Vogelbaum MA. Convection enhanced delivery for the treatment of malignant gliomas: symposium review. J Neurooncol. 2005;73:57–69. doi: 10.1007/s11060-004-2243-8. [DOI] [PubMed] [Google Scholar]
- 47.Walter KA, Cahan MA, Gur A, Tyler B, Hilton J, Colvin OM, et al. Interstitial taxol delivered from a biodegradable polymer implant against experimental malignant glioma. Cancer Res. 1994;54:2207–2212. [PubMed] [Google Scholar]
- 48.Werner-Wasik M, Yu X, Marks LB. Normal-tissue toxicities of thoracic radiation therapy: esophagus, lung, and spinal cord as organs at risk. Hematol Oncol Clin North Am. 2004;18:131–160. x–xi. doi: 10.1016/s0889-8588(03)00150-3. [DOI] [PubMed] [Google Scholar]
- 49.Winer EP, Berry DA, Woolf S, Duggan D, Kornblith A, Harris LN, et al. Failure of higher-dose paclitaxel to improve outcome in patients with metastatic breast cancer: cancer and leukemia group B trial 9342. J Clin Oncol. 2004;22:2061–2068. doi: 10.1200/JCO.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 50.Wist EA, Sommer HH, Ostenstad B, Risberg T, Fjaestad K. Weekly one-hour paclitaxel as first-line chemotherapy for metastatic breast cancer. Acta Oncol. 2004;43:11–14. doi: 10.1080/02841860310017748. [DOI] [PubMed] [Google Scholar]
- 51.Zentner GM, Rathi R, Shih C, McRea JC, Seo MH, Oh H, et al. Biodegradable block copolymers for delivery of proteins and water-insoluble drugs. J Control Release. 2001;72:203–215. doi: 10.1016/s0168-3659(01)00276-0. [DOI] [PubMed] [Google Scholar]


