Abstract
Background
The adverse effects of smoking on various health conditions such as cancer, diabetes, and cardiovascular disease have been well documented. Many orthopaedic conditions, such as fracture healing, wound repair, and bone mineral density, have been reported to be adversely affected by smoking. However, no known systematic reviews have investigated the effects of smoking on ligament and cartilage knee surgery.
Purpose
We hypothesized that smoking would have a negative influence from both a basic science and clinical outcome perspective on these types of knee surgeries.
Study Design
Systematic review.
Methods
A systematic review of multiple medical databases was performed evaluating clinical and basic science studies to determine the effects of smoking on ligament and cartilage knee surgery.
Results
Fourteen studies were found for inclusion and analysis. Eight of these studies addressed the relationship between smoking and knee ligaments, and 6 investigated the relationship between smoking and articular cartilage. With the exception of 1, all of the basic science and clinical studies exploring the relationship between smoking and knee ligaments found a negative association of smoking, either molecularly, biomechanically, or clinically. One basic science and 3 clinical studies found a negative influence of smoking on articular cartilage of the knee. No studies were found that investigated the relationship of smoking and menisci.
Conclusion
The current literature reveals a negative influence of smoking on the results of knee ligament surgery, both from a basic science and clinical perspective, implying that smoking cessation would benefit patients undergoing these procedures. The association between smoking and knee articular cartilage was less clear, although the literature still suggests an overall negative influence and highlights the need for further investigation.
Keywords: Smoking, nicotine, meniscus, articular cartilage, anterior cruciate ligament, knee
Introduction
Currently, it is estimated that 46 million people in the United States, or 20.6% of all adults, smoke cigarettes.7 Smoking and other tobacco use is the leading preventable cause of morbidity and mortality in the United States, and it accounts for approximately 443,000 deaths, or 1 of every 5 deaths, in the United States each year.5 The adverse effects of smoking on conditions such as cancer, diabetes, and cardiovascular disease have been well documented.4
Nicotine, the addictive component in cigarette smoke, has been implicated in the pathogenesis of a variety of diseases by increasing platelet aggregation, reducing microvascular prostacyclin levels, and perhaps more importantly in surgery, inhibiting the function of fibroblasts, red blood cells, and macrophages.14,28 In addition, vasoconstriction, hypoperfusion, and ischemia contribute to nicotine’s adverse affect on vascularized tissues involved in surgery.
It has been reported that many orthopaedic conditions have been complicated and adversely affected by tobacco, including fracture healing, spinal fusion, wound repair, bone mineral density, lumbar disk disease, and rate of hip fracture.20 Theoretically, nicotine could also adversely affect knee surgery involving articular cartilage, menisci, and ligaments. Although orthopaedic surgeons have known about these potential complications of smoking on knee surgery and there have been individual reports addressing this relationship, most have mentioned it in a cursory manner and there are no known comprehensive reviews on the topic. The purpose of this systematic review is to provide an analytical summary of the current literature regarding the relationship and effects of smoking specifically after ligamentous, meniscal, or articular cartilage surgery of the knee. We hypothesized that smoking would have a negative influence from both a basic science and clinical outcome perspective on these types of knee surgeries.
Methods
We conducted a systematic review of the available English language literature according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-analyses) standards and a PRISMA checklist (Figure 1). Initial search citation strategy terminology was based on “knee AND [smoke OR smoking OR nicotine OR tobacco] LIMIT English language.” The search was performed independently by 3 authors (P.K., J.D.H., D.C.F.). The search was performed on November 4, 2011. The following databases were used: PubMed (1950-present), CINAHL (Cumulative Index to Nursing and Allied Health Literature; 1994-present), SPORTDiscus (1975-present), and Cochrane Central Register of Controlled Trials (1994-present). Levels I, II, III, and IV evidence (for diagnostic studies) were included (according to the Oxford Centre for Evidence Based Medicine used by the American version of the Journal of Bone and Joint Surgery 18). Both print journals and e-published journals were eligible for inclusion. Meeting and/or conference abstracts were not eligible for inclusion. If there was any disagreement among authors regarding inclusion of an article, the senior author (D.C.F.) made the final decision on article inclusion. All references within included studies were checked to assess for potentially inclusive articles if missed by the initial search criteria. after meniscal surgery.
Figure 1.
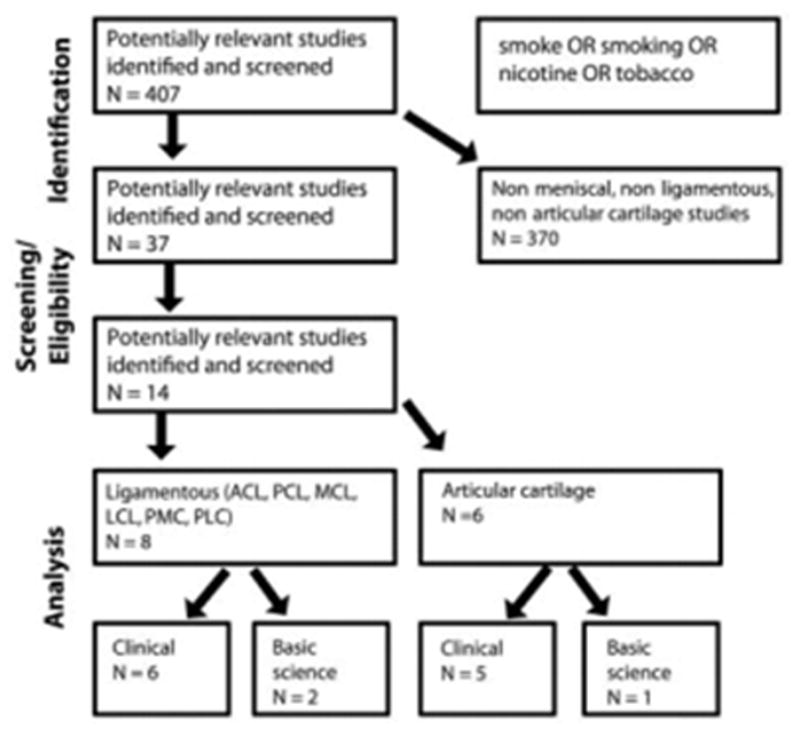
Search strategy according to PRISMA guidelines. Fourteen studies were identified for inclusion. No studies identified an analysis of smoking on outcomes after meniscal surgery.
Inclusion criteria included any English language clinical outcomes studies following ligamentous, meniscal, or cartilage surgery of the knee with evidence levels I through IV. Ligamentous surgery was defined as anterior and posterior cruciate ligament reconstruction, medial and lateral (and/or posteromedial and/or posterolateral corner) collateral ligament repair and/or reconstruction, and multi-ligamentous repair and/or reconstruction. Meniscal surgery was defined as meniscal repair, allograft transplantation, and partial meniscectomy/debridement. Cartilage surgery was defined as cartilage palliation techniques (articular cartilage defect debridement, joint lavage, and loose body removal), cartilage repair (marrow-stimulation techniques like microfracture, subchondral bone drilling, and abrasion arthroplasty), and cartilage restoration (autologous chondrocyte implantation, osteochondral autograft transfer, osteochondral allograft, cartilage autograft implantation system, juvenile allograft articular cartilage implantation). Also included were basic science studies analyzing the anatomy, histology, pathology, biomechanics, and imaging characteristics after ligamentous, meniscal, or cartilage surgery of the knee. Duplicate patient populations (within different studies) were included only if reporting separate outcome parameters; however, if duplicate patient populations reported only separate lengths of follow-up, then the more recent study was included.
Exclusion criteria were any clinical outcomes and basic science studies analyzing outcomes after surgery in any joint other than the knee. In addition, clinical outcomes and basic science studies analyzing outcomes after fracture reduction and internal fixation or external fixation around the knee, total and unicompartmental knee arthroplasty, oncologic mass excision around the knee, and osteotomy around the knee (including valgus-inducing, closing- or opening-wedge; proximal tibial osteotomy and varus-inducing opening-wedge distal femoral osteotomy; and tibial tubercle realignment osteotomy) were excluded. Length of follow-up in clinical outcomes studies was not an exclusion criterion. Non–English language studies and studies of level V evidence were excluded.
Descriptive statistics were calculated. Continuous variable data were reported as means ± standard deviations from the mean. Categorical variable data were reported as frequency with percentages. Data range was reported as minimum to maximum absolute values. For all statistical analysis within all studies analyzed, P < .05 was deemed significant.
Results
Fourteen studies were identified for inclusion and analysis. Eight of these studies addressed the relationship of smoking and knee ligaments (2 basic science and 6 clinical studies; Table 1, A and B). Six of these studies investigated the effects of smoking on knee cartilage (1 basic science and 5 clinical studies; Table 2, A and B). No studies were found that specifically explored the effects of smoking on menisci.
TABLE 1.
Studies on Smoking and Knee Ligamentsa
| A. Basic Science Studies
| ||||
|---|---|---|---|---|
| Author (Year) | Journal | Type of Study | Injury | Outcomes |
| Gill et al11 (2006) | J Orthop Res | Animal model (mouse) | MCL | Smoking decreased cellular density and type I collagen expression in injured MCL of mice exposed to cigarette smoke versus control mice not exposed to cigarette smoke |
| Wright et al27 (2010) | J Knee Surg | Animal model (mouse) | MCL | MCL of mice exposed to cigarette smoke were biomechanically weaker and less stiff than the ligaments of control mice |
| B. Clinical Studies
| |||||
|---|---|---|---|---|---|
| Author (Year) | Journal | No. of Patients (mean age, y) | Injury | Surgical Intervention | Pertinent Outcomes |
| Dunn et al8 (2003) | J Bone Joint Surg Am | 2192 (28) | ACL | Diagnostic arthroscopy or ACLR | The outcome of interest was development of disability following the initial hospitalization related to an ACL injury Cigarette smoking was significantly correlated (P =.01) to a significantly higher rate of disability discharge from active duty following an ACL injury (10% of all ACL injuries) |
| Karim et al15 (2006) | J Bone Joint Surg Br | 304 (32.8) | ACL | ACLR | Smokers had worse outcomes for the mean subjective IKDC score (P < .001), the frequency (P = .005), and intensity (P = .005) of pain Smokers were also less likely to return to their original level of pre-injury sport (P = .003) and had an overall worse IKDC grade score (P = .007), a calculation based on effusion, deficit in passive movement, ligament examination, compartment findings, pathology of the harvest site, radiological findings, and functional knee tests Smokers had more objective laxity as measured by Westminster cruciometry recordings (P = .001) |
| Kowalchuk et al16 (2009) | Arthroscopy | 402 (27.3) | ACL | ACLR | Subjective IKDC scores were used to measure patient-reported outcomes following ACL reconstruction Lower patient-reported outcomes were associated with smoking, as smokers had 0.36 times the odds of having a successful outcome as subjects who did not smoke (P = .02) |
| Dunn et al9 (2010) | Am J Sports Med | 390 (27) | ACL | ACLR | Smoking within 6 mo prior to an ACL reconstruction was associated with lower postoperative activity levels at a follow-up of 2 y as measured by the Marx activity level (OR, 0.55; 95% CI, 0.33–0.92; P = .02) |
| Spindler et al24 (2011) | Am J Sports Med | 378 (27) | ACL | ACLR | Six years after ACL reconstruction, smoking was correlated with worse outcomes across several measures, including a longer return to sports function and knee-quality of life (as measured by IKDC and KOOS) |
| Li et al17 (2011) | Am J Sports Med | 249 (26.4) | ACL | ACLR | Attempted to determine the overall prevalence of and risk factors for the development of radiographic knee OA using the Kellgren-Lawrence scale after single-bundle ACL reconstruction Reported that smoking was not a statistically significant risk factor (P = .18) for the development of knee OA |
ACL, anterior cruciate ligament; ACLR, anterior cruciate ligament reconstruction; CI, confidence interval; IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; MCL, medial collateral ligament; OA, osteoarthritis; OR, odds ratio.
TABLE 2.
Studies on Smoking and Knee Articular Cartilagea
| A. Basic Science Studies
| ||||
|---|---|---|---|---|
| Author (Year) | Journal | Type of Study | Injury | Pertinent Outcomes |
| Schmal et al21 (2011) | Am J Sports Med | Descriptive laboratory study | Circumscribed cartilage lesions | Synovial fluid of microfracture and ACI subjects: reduced expression of IGF-1 and bFGF in smokers vs nonsmokers |
| B. Clinical Studies
| |||||
|---|---|---|---|---|---|
| Author (Year) | Journal | No. of Patients (mean age, y) | Injury | Surgical Intervention | Pertinent Outcomes |
| Spahn et al23 (2006) | Arthroscopy | 156 (51.6) | Isolated Kellgren-Lawrence grade 2 medial-compartment knee OA | Arthroscopy (debridement or microfracturing of chondral defects) | KOOS determined in all patients; outcome rated poor if score was less than 114 points or if further surgery required Smoking associated with worse outcomes (follow-up, 47–54 mo) as measured by the KOOS (OR, 3.8; 95% CI, 1.6–9.1) Rate of poor results 61.5% in nonsmokers and 83.6% in smokers (P < .001) Complication rate 2.6%; 3 patients had deep vein thromboses; 1 patient had recurrent effusion and underwent revision arthroscopy at 3 wk |
| Spahn et al22 (2008) | Knee Surg Sports Traumatol Arthrosc | 60 (43.3) | Medial meniscus tear and idiopathic ICRS grade II defect of the medial femoral condyle | Partial meniscectomy and either bipolar radiofrequency- based chondroplasty or mechanical shaver debridement | Smokers had significantly worse KOOS outcomes than nonsmokers: debridement, nonsmokers (59.5) vs smokers (46.6; P = .02); radiofrequency chondroplasty: nonsmokers (83.9) vs smokers (73.8; P < .001) |
| Jaiswal et al13 (2009) | J Bone Joint Surg Br | 129 (33.9) | Full-thickness condral defects | ACI with a synthetic type I/III collagen scaffold or matrix-carried procedure | Mean Modified Cincinnati Knee score was significantly lower in smokers (n = 48) than in nonsmokers (n = 66) both before and after surgery (P < .05) Smokers experienced significantly less improvement in the knee score 2 y after surgery (P < .05) Graft failures seen only in smokers (P = .02) Strong negative correlation between the number of cigarettes smoked and the outcome following surgery (Pearson correlation coefficient, 0.65, P = .004) Arthroscopically, higher proportion of excellent and good results according to ICRS scale in nonsmokers versus smokers (P = .01) Smokers were less likely to form hyaline or hyaline-like cartilage than nonsmokers, although not statistically significant owing to the low numbers of biopsies performed (P = .46) |
| Balain et al3 (2009) | Osteoarthritis Cartilage | 53 (42 [median age]) | Full-thickness chondral defects | Knee microfracture | Smoking status did not significantly affect the response shift, a change in the internal standards of a patient as determined by patient-reported function and pain scores, following microfracture |
| Ollat et al19 (2011) | Orthop Traumatol Surg Res | 142 (31) | Osteochondral defects | Autologous osteochondral mosaicplasty | Evaluated prognostic factors associated with cartilage defects of the knee in patients treated by mosaicplasty (follow-up, 53–158 mo; mean, 96 mo) Smoking did not have significant effect on the functional results as measured by IKDC score, ICRS score, or Hughston score at final follow-up Complication rate, 13% (n = 19) including hemarthrosis (6 cases), sepsis (1 case), complex regional pain syndrome (1 case) |
ACI, autologous chondrocyte implantation; bFGF, basic fibroblast growth factor; CI, confidence interval; ICRS, International Cartilage Repair Society; IGF-1, insulin-like growth factor-1; IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; OA, osteoarthritis; OR, odds ratio.
Ligaments
Two basic science studies used animal models to investigate the effects of smoking on medial collateral ligament (MCL) injuries (surgically induced rupture) in mice.11,27 Negative effects of smoking on the injured ligament were seen, both at the molecular level in terms of cellular density and type I collagen expression and biomechanically in terms of strength and stiffness. In comparison with sham surgery on mice MCL (skin incision only), the surgically ruptured MCL demonstrated (via immunohistochemical staining and mRNA in situ hybridization) significant negative effects of cigarette smoke on the injured ligament. Exposure to smoke in surgically ruptured MCL mice resulted in significant decreases (versus sham) in cellular density (P = .0111), type I collagen gene expression (alpha 1 chain11), strength (P = .0227), and stiffness (P = .0127).
Five of the 6 clinical studies investigated the effects of smoking on anterior cruciate ligament (ACL) injuries and postoperatively after ACL reconstruction.8,9,15–17,24 The clinical studies on ligamentous knee injury reflect clinical outcomes in approximately 4000 young adults (mean age, 28 years) after ACL reconstruction. Smoking status was a significant factor related to the following subjective and objective outcomes after surgery: (1) permanent disability discharge from United States Army (P = .018); (2) subjective International Knee Documentation Committee (IKDC; P < .00111; P = .0216; P = .0224); (3) mean overall IKDC function score (P = .00715); (4) rate of return to sport (P = .00315); (5) objective cruciometry laxity (P = .00115); (6) activity levels based on validated activity scales (P = .029); and (7) quality of life as measured by the Knee Injury and Osteoarthritis Outcome Score (KOOS; P = .0324). Rate of development of radiographic knee osteoarthritis (via Kellgren-Lawrence scale) in 249 patients over 8-year follow-up was not significantly (P = .1817) affected by smoking status.
Articular Cartilage
The only basic science study reporting the effect of smoking on knee articular cartilage was a descriptive laboratory study by Schmal et al.21 In that study, knee lavage fluids of patients with circumscribed cartilage lesions treated by either microfracture or autologous chondrocyte implantation were analyzed. The knee lavage fluids were collected intraoperatively, whereby fluid was first instilled into the knee before the procedure and then samples were aspirated after repeated passive-flexion maneuvers and manipulations of the supra- and infrapatellar regions. Synovial expression of total protein content, insulin-like growth factor-I (IGF-I), and basic fibroblast growth factor (bFGF) was significantly diminished in smokers compared with nonsmokers.
Three of the clinical studies found a negative effect of smoking after surgical treatment of cartilage defects in the knee as measured by postoperative clinical outcome measures, such as the KOOS and the mean Modified Cincinnati Knee score.13,22,23 Two studies by Spahn et al found that smokers had significantly worse outcomes (debridement/microfracture P < .00123; debridement P = .0222; radiofrequency chondroplasty P < .00122) than nonsmokers as measured by the KOOS. Jaiswal et al found that in patients undergoing autologous chondrocyte implantation for full-thickness chondral defects, smokers had significantly worse clinical outcomes than nonsmokers both subjectively (as measured by the mean Modified Cincinnati Knee score [P < .05]) and objectively (as measured by the number of graft failures [P = .016] and the arthroscopic International Cartilage Repair Society (ICRS) score with visual assessment [P =.0137] and biopsy [P = .4613]). Additionally, the only graft failures were seen in smokers.13 However, 2 additional clinical studies3,19 were found that reported that smoking was not a significant risk factor in predicting poor outcomes after knee surgery targeted at addressing cartilage lesions. At a mean follow-up of 8 years, Ollat et al19 showed no significant difference (P > .05) in outcomes (subjective IKDC and Hughston scores, ICRS score, and IKDC radiological scores) after mosaicplasty between smokers and nonsmokers. Balain et al3 demonstrated no significant difference (P > .05) between smokers and nonsmokers undergoing microfracture in response shift comparisons of Lysholm and modified IKDC scores, visual analog scales, and patient satisfaction.
Discussion
Smoking has been implicated in causing complications in many orthopaedic conditions, such as fracture healing, spinal fusion, wound repair, bone mineral density, lumbar disk disease, and rate of hip fracture.20 Given the latter, the potential certainly exists for smoking to adversely affect outcomes of knee surgery, but there have been no known comprehensive reviews on this topic. This review shows that the current literature suggests smoking has a negative effect on knee ligament and articular cartilage surgery, while no studies in the literature examine the effect on meniscal surgery.
From a basic science perspective, the current literature clearly indicates that smoking negatively affects both knee ligaments and articular cartilage.27 The 2 ligamentous studies describe negative effects of smoking on the medial collateral ligament in mice, at the molecular level (both cellular density and extracellular matrix) and in terms of biomechanical integrity (strength and stiffness). Smoking also seems to negatively affect articular cartilage of the knee from a basic science perspective.21 Significant decreases in knee synovial expression of total protein content, IGF-I, and bFGF were found in smokers versus nonsmokers. These specific cytokines are thought to be directly involved in cartilage repair mechanisms, with the implication that smoking negatively influences synovial cytokine expression related to cartilage metabolism.
Clinically, there have been several studies addressing the relationship between smoking and both knee ligaments and articular cartilage. In regard to effects on ligaments, the current literature (6 level II and III evidence studies) suggests a negative effect of smoking on both subjective and objective clinical outcomes.8–9,15–16,24 Despite the negative clinical outcomes associated with smoking in these studies, the accountable biochemical and pathophysiological mechanisms were not reported and therefore, remain yet unknown and based on theory and basic science research. No randomized controlled trials of knee ligament surgery exist (or likely will ever exist) comparing smokers and nonsmokers (with smoking as the variable of analysis), thus level II evidence is likely to be the highest level of evidence achievable.
The clinical literature regarding the effects of smoking on articular cartilage of the knee is less clear, yet still demonstrates a negative effect of smoking after articular cartilage repair or restoration.13,22–23 Three studies found a significantly (P < .05) negative influence of smoking on outcomes (KOOS, subjective IKDC, modified Cincinnati, and ICRS visual cartilage assessment scores) of debridement, microfracture, radiofrequency chondroplasty, and autologous chondrocyte implantation. Nevertheless, 2 more recent clinical studies were found in which smoking was not reported to be an independent risk factor for negative outcomes of procedures targeted at treating articular knee cartilage.3,19 These studies were not without limitations, though. Balain et al used a method called the “then-test” to evaluate the response shift, or the change in internal standards of the patients, after surgery.3 This test relies on the patients’ ability to recall their preoperative health status, and an inaccurate recall may lead to both increased random error and systematic error (recall bias).
The findings of Jaiswal et al essentially link the basic science and clinical mechanisms of cartilage restoration.13 The visual arthroscopic findings (P < .05), combined with the hyaline and hyaline-like biopsies (P > .05), demonstrate that even at 1-year follow-up, the repair tissue in smokers was inferior. This smoking-based difference was also seen clinically in the modified Cincinnati score. This finding suggests that smoking negatively influences both functional outcomes after articular cartilage restoration, but it also leads to inferior repair tissue macroscopically, based on appearance with arthroscopy, and microscopically, based on the amount of hyaline cartilage formed after the procedure.13 Taking the findings from Schmal et al into consideration,21 these results are to be expected. If at the molecular level, cartilage metabolism is adversely affected by smoking, it would make sense that the repair tissue in smokers would appear worse at the macroscopic and microscopic level. It would follow that with inferior tissue, smokers would also have poorer clinical outcomes, as was the case in the results reported by Jaiswal et al.13
The current literature seems to suggest a negative influence of smoking on knee ligaments, both from a basic science and clinical outcomes perspective. Orthopaedic surgeons have been advocating smoking cessation for patients undergoing ligamentous knee surgery for years. Our findings suggest that this practice should be continued and may lead to better clinical outcomes, especially in the setting of ACL reconstruction. In terms of the relationship of smoking and articular cartilage, the literature is slightly less conclusive. From a basic science perspective, articular cartilage metabolism seems to be adversely affected by smoking, and clinical studies tend to find worse outcomes among smokers or no significant relationships. Given the nutrition and metabolism of articular cartilage via synovial fluid interaction (plasma interstitial fluid diffusion), it can be speculated that the negative vascular and cytokine effects of smoking would similarly impair perioperative articular cartilage health. Future studies should be more targeted at addressing a specific relationship between smoking and articular cartilage. For example, the effect of smoking on cartilage repair using restoration techniques that rely upon direct vascular supply (eg, microfracture, osteochondral autograft transfer) and indirect supply (eg, autologous chondrocyte implantation, cartilage autograft implantation systems) for successful healing are potential areas of future study. In addition, future investigations should determine whether there is a time-dependent relationship of smoking cessation or dose-dependent relationship of number of cigarettes on clinical outcomes. These studies would allow the orthopaedic community to make specific recommendations on the benefits of smoking cessation in patients undergoing knee surgery for ligament or articular cartilage lesions. In addition, as we found no studies addressing the relationship of smoking and menisci, future research on this topic would be a beneficial addition to our current fund of knowledge.
The exact component involved in cigarette smoking that contributes to negative health effects, especially when it comes to orthopaedic surgery, is not completely clear. Cigarette smoking consists of nearly 500 different gases, including nitrogen, carbon monoxide and hydrogen cyanide, and roughly 3500 different chemicals, of which one is nicotine.12 Nicotine has been shown to increase platelet aggregation, reduce microvascular prostacyclin levels, and perhaps more important in surgery, inhibit the function of fibroblasts, red blood cells, and macrophages.14,28 Also, the associated vasoconstriction, hypoperfusion, and ischemia contribute to nicotine’s adverse affect on vascularized tissues involved in surgery. Theoretically, it is these mechanisms that contribute to cigarette smoke’s negative effects on knee surgery, which often involves vascularized structures and tissues. However, to our knowledge, no basic science studies have investigated the specific component in cigarette smoke that seems to adversely affect knee surgery outcomes.
This systematic review has a few limitations to consider. A source of selection bias is inherent in its study inclusion criteria relating to the use of only English language studies. The prevalence of cigarette smoking is unique to global geographic regions (categorized dichotomously by the World Health Organization and United States Centers for Disease Control and Prevention as “developing” [low- or middle-income countries] and “developed” [middle- or high-income] countries).6,26 As a result of smoking cessation programs, smoking rates have either declined or remained stable since the mid-1990s in both the English- and non-English-speaking countries of North America and western Europe and in other “developed” countries. In these countries, smokers generally include those with mental health problems, alcohol and drug problems, criminals, and the homeless, all populations in which smoking cessation programs do not reach anyway or are simply ignored. Globally, of the 1.3 billion smokers, 1 billion (84%) live in “developing” or “transitional” economies.1,10 Further, the smoking rate is increasing in the latter group by 3.4% per year.2 In this systematic review, all studies were from “developed” countries (United States, United Kingdom, Germany, and France). Obtaining data from “developing” countries would be very useful, given the disparity in smoking rates versus “developed” countries. In addition, this study examined only smoking tobacco, and not smokeless tobacco or nicotine-enriched gum. One recent study (not analyzed for inclusion in this review) has compared outcomes between cigarette smoking versus smokeless “snuff” tobacco and nonsmokers.25 It was shown that oral snuff smokeless tobacco use did not delay bone healing or increase the risk of perioperative complications after high tibial osteotomy, whereas smoking cigarettes did delay healing and increase complications. These differences warrant further study and could potentially elucidate the causative component that influences knee and other orthopaedic surgical outcomes.
Other sources of potential selection, performance, and detection bias include the analysis of only the knee joint (versus other musculoskeletal regions and tissue types, which likely are equally affected by the systemic effects of smoking), different ligaments (ACL and MCL; intra-articular vs extra-articular anatomic differences), different surgical techniques, multiple different outcome measurements (IKDC, KOOS, Modified Cincinnati Knee score, etc), and different types and doses of cigarettes. As a result of these differences, it becomes a challenge to generalize and draw conclusions between the various studies with several different variables. In addition, as mentioned above, it has not been identified which component of cigarette smoke (nicotine vs other additives) contributes to the reported negative effects. All 14 studies in this review had relatively few subjects. This fact further reinforces the need for future studies in this area.
Conclusion
This systematic review reveals a negative effect of smoking on knee ligament surgery, both from a basic science and clinical perspective. These findings suggest that smoking cessation would benefit patients undergoing these procedures. The relationship of smoking and outcomes from articular cartilage surgery was less clear, although an overall negative influence has been suggested. There are no studies looking at the effect of smoking on outcomes from meniscal surgery, implying the need for more specific studies addressing these topics.
Acknowledgments
The investigation was performed by the Cartilage Outcomes and MEniscal Treatment (COMET) study group.
Footnotes
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution.
An online CME course associated with this article is available for 1 AMA PRA Category 1 Credit™ at http://ajsm-cme.sagepub.com. In accordance with the standards of the Accreditation Council for Continuing Medical Education (ACCME), it is the policy of The American Orthopaedic Society for Sports Medicine that authors, editors, and planners disclose to the learners all financial relationships during the past 12 months with any commercial interest (A ‘commercial interest’ is any entity producing, marketing, re-selling, or distributing health care goods or services consumed by, or used on, patients). Any and all disclosures are provided in the online journal CME area which is provided to all participants before they actually take the CME activity. In accordance with AOSSM policy, authors, editors, and planners’ participation in this educational activity will be predicated upon timely submission and review of AOSSM disclosure. Noncompliance will result in an author/editor or planner to be stricken from participating in this CME activity.
References
- 1.ASH. [Accessed May 21, 2012];Tobacco: Global trends. Available at: http://www.ash.org.uk/files/documents/ASH_562.pdf.
- 2.ASH. Tobaco and the developing world. [Accessed May 21, 2012]; Available at: http://ash.org.uk/files/documents/ASH_126.pdf.
- 3.Balain B, Ennis O, Kanes G, et al. Response shift in self-reported functional scores after knee microfracture for full thickness cartilage lesions. Osteoarthritis Cartilage. 2009;17(8):1009–1013. doi: 10.1016/j.joca.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Bartecchi CE, MacKenzie TD, Schrier RW. The human costs of tobacco use (1) N Engl J Med. 1994;330(13):907–912. doi: 10.1056/NEJM199403313301307. [DOI] [PubMed] [Google Scholar]
- 5.CDC. Cigarette smoking among adults and trends in smoking cessation - United States, 2008. MMWR Morb Mortal Wkly Rep. 2009;58(44):1227–1232. [PubMed] [Google Scholar]
- 6.CDC. Annual smoking-attributable mortality, years of potential life lost, and economic costs - United States, 1995–1999. Morbidity and Mortality Weekly Report. 2002;51(14):300–303. [PubMed] [Google Scholar]
- 7.CDC. Vital signs: current cigarette smoking among adults aged >/= 18 years - United States, 2009. Morbidity and Mortality Weekly Report. 2010;59(35):1135–1140. [PubMed] [Google Scholar]
- 8.Dunn WR, Lincoln AE, Hinton RY, Smith GS, Amoroso PJ. Occupational disability after hospitalization for the treatment of an injury of the anterior cruciate ligament. J Bone Joint Surg Am. 2003;85-A(9):1656–1666. doi: 10.2106/00004623-200309000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Dunn WR, Spindler KP. Predictors of activity level 2 years after anterior cruciate ligament reconstruction (ACLR): a Multicenter Orthopaedic Outcomes Network (MOON) ACLR cohort study. Am J Sports Med. 2010;38(10):2040–2050. doi: 10.1177/0363546510370280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esson KM. The Millennium development goals and tobacco control: an opportunity for global partnership. WHO; [Accessed May 21, 2012]. [Google Scholar]
- 11.Gill CS, Sandell LJ, El-Zawawy HB, Wright RW. Effects of cigarette smoking on early medial collateral ligament healing in a mouse model. J Orthop Res. 2006;24(12):2141–2149. doi: 10.1002/jor.20234. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann D, Hoffmann I. The changing cigarette, 1950–1995. J Toxicol Environ Health. 1997;50(4):307–364. doi: 10.1080/009841097160393. [DOI] [PubMed] [Google Scholar]
- 13.Jaiswal PK, Macmull S, Bentley G, Carrington RW, Skinner JA, Briggs TW. Does smoking influence outcome after autologous chondrocyte implantation?: A case-controlled study. J Bone Joint Surg Br. 2009;91(12):1575–1578. doi: 10.1302/0301-620X.91B12.22879. [DOI] [PubMed] [Google Scholar]
- 14.Jorgensen LN, Kallehave F, Christensen E, Siana JE, Gottrup F. Less collagen production in smokers. Surgery. 1998;123(4):450–455. [PubMed] [Google Scholar]
- 15.Karim A, Pandit H, Murray J, Wandless F, Thomas NP. Smoking and reconstruction of the anterior cruciate ligament. J Bone Joint Surg Br. 2006;88(8):1027–1031. doi: 10.1302/0301-620X.88B8.17189. [DOI] [PubMed] [Google Scholar]
- 16.Kowalchuk DA, Harner CD, Fu FH, Irrgang JJ. Prediction of patient-reported outcome after single-bundle anterior cruciate ligament reconstruction. Arthroscopy. 2009;25(5):457–463. doi: 10.1016/j.arthro.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li RT, Lorenz S, Xu Y, Harner CD, Fu FH, Irrgang JJ. Predictors of radiographic knee osteoarthritis after anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39(12):2595–2603. doi: 10.1177/0363546511424720. [DOI] [PubMed] [Google Scholar]
- 18.Obremskey W, Pappas N, Attallah-Wasif E, Tornetta P, Bhandari M. Levels of Evidence in Orthopaedic Journals. Journal of Bone and Joint Surgery, American. 2005;87(12):2632–2638. doi: 10.2106/JBJS.E.00370. [DOI] [PubMed] [Google Scholar]
- 19.Ollat D, Lebel B, Thaunat M, et al. Mosaic osteochondral transplantations in the knee joint, midterm results of the SFA multicenter study. Orthop Traumatol Surg Res. 2011;97(8 Suppl):S160–166. doi: 10.1016/j.otsr.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Porter SE, Hanley EN., Jr The musculoskeletal effects of smoking. J Am Acad Orthop Surg. 2001;9(1):9–17. doi: 10.5435/00124635-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Schmal H, Niemeyer P, Sudkamp NP, Gerlach U, Dovi-Akue D, Mehlhorn AT. Pain perception in knees with circumscribed cartilage lesions is associated with intra-articular IGF-1 expression. Am J Sports Med. 2011;39(9):1989–1996. doi: 10.1177/0363546511406851. [DOI] [PubMed] [Google Scholar]
- 22.Spahn G, Kahl E, Muckley T, Hofmann GO, Klinger HM. Arthroscopic knee chondroplasty using a bipolar radiofrequency-based device compared to mechanical shaver: results of a prospective, randomized, controlled study. Knee Surg Sports Traumatol Arthrosc. 2008;16(6):565–573. doi: 10.1007/s00167-008-0506-1. [DOI] [PubMed] [Google Scholar]
- 23.Spahn G, Muckley T, Kahl E, Hofmann GO. Factors affecting the outcome of arthroscopy in medial-compartment osteoarthritis of the knee. Arthroscopy. 2006;22(11):1233–1240. doi: 10.1016/j.arthro.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Spindler KP, Huston LJ, Wright RW, et al. The prognosis and predictors of sports function and activity at minimum 6 years after anterior cruciate ligament reconstruction: a population cohort study. Am J Sports Med. 2011;39(2):348–359. doi: 10.1177/0363546510383481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.W-Dahl A, Toksvig-Larsen S. No delayed bone healing in Swedish male oral snuffers operated on by the hemicallotasis technique: a cohort study of 175 patients. Acta Orthop. 2007;78(6):791–794. doi: 10.1080/17453670710014563. [DOI] [PubMed] [Google Scholar]
- 26.WHO. [Accessed May 21, 2012];Tobacco Free Initiative (TFI): Surveillance and monitoring. Available at: http://www.who.int/tobacco/surveillance/en/
- 27.Wright R, Mackey RB, Silva M, Steger-May K. Smoking and mouse MCL healing. J Knee Surg. 2010;23(4):193–199. doi: 10.1055/s-0030-1268695. [DOI] [PubMed] [Google Scholar]
- 28.Zevin S, Gourlay SG, Benowitz NL. Clinical pharmacology of nicotine. Clin Dermatol. 1998;16(5):557–564. doi: 10.1016/s0738-081x(98)00038-8. [DOI] [PubMed] [Google Scholar]


