Summary
The transcription factor Sox10 mediates the differentiation of neural crest–derived cells, and Sox10 labeling by immunohistochemistry (IHC) is used clinically primarily to support the diagnosis of melanoma. Sox10 expression by IHC has been previously documented in benign breast myoepithelial cells but not in breast carcinomas. Here, we report the first systematic study of Sox10 expression in invasive ductal carcinomas subclassified by IHC-defined molecular subtype (100 cases), as well as in 24 cases of ductal carcinoma in situ and 44 mammary fibroepithelial neoplasms. Tissue microarrays containing 168 primary breast tumors were subjected to IHC for Sox10. The extent of nuclear Sox10 labeling was scored by percentage labeling as follows: 0 (0%), 1+ (1%–25%), 2+ (25%–50%), 3+ (50%–75%), and 4+ (>75%). Overall, 40 (40%) of 100 invasive breast carcinomas demonstrated Sox10 immunoreactivity, which was seen primarily in the basal-like, unclassified triple-negative, and metaplastic carcinomas. Sox10 labeling was seen in 66% (38/58) of the basal-like, unclassified triple-negative, and metaplastic carcinomas as compared with 5% (2/42) of the luminal A, luminal B, and Her-2 carcinomas (P < .00001). Sox10 labeling was seen in 1 (4%) of 24 cases of ductal carcinoma in situ, which was negative for estrogen receptor/progesterone receptor. No labeling was seen in the stromal component of phyllodes tumors or fibroadenomas. These findings show that breast carcinoma must be considered in the differential diagnosis of melanoma for an S100-positive, Sox10-positive metastatic malignant neoplasm. Sox10 expression in the basal-like, unclassified triple-negative, and metaplastic carcinomas types supports the concept that these neoplasms show myoepithelial differentiation.
Keywords: Sox10, Breast carcinoma, Metaplastic carcinoma, Basal-like carcinoma, Myoepithelial
1. Introduction
The transcription factor Sox10 mediates the survival and differentiation of neural crest cells into glial cells and melanocytes [1,2]. Immunohistochemical labeling for Sox10 is used in surgical pathology primarily to support the diagnosis of melanoma [3–5] but also of nerve sheath tumors [3,4], both of which are thought to be neural crest derived. Sox10 expression by immunohistochemistry (IHC) has previously been documented in myoepithelial cells of the breast, salivary glands, and bronchial glands [3,4].
In the breast, Sox10 plays a role in Notch4 and PBP (peroxisome-proliferative–activated receptor-binding protein)–mediated mammary epithelial cell growth in vitro [6]. To our knowledge, the expression of Sox10 in breast carcinomas has only been examined in one study [3], in which no epithelial Sox10 labeling was seen in 4 cases of invasive ductal carcinoma or 4 cases of ductal carcinoma in situ (DCIS). The molecular subtype of the limited number of breast carcinomas examined was not reported. Here, we report the first systematic study of the expression of Sox10 in invasive ductal carcinomas subdivided by IHC-defined molecular subtype, including metaplastic (sarcomatoid) carcinoma, triple-negative and basal-like carcinoma, Her-2–positive carcinoma, and luminal-positive carcinoma, as well as in 24 cases of DCIS and in 44 fibroepithelial neoplasms of the breast.
2. Materials and methods
2.1. Tissue microarray construction and case selection
This study was approved by the institutional review board of the Johns Hopkins Medical Institutions. Three tissue microarrays (TMAs) were constructed from archived paraffin tissue blocks of 13 primary breast metaplastic carcinomas, 34 primary breast phyllodes tumors, and 10 fibroadenomas from different patients. The pathologic features of the metaplastic carcinomas are listed in Table 1. The metaplastic carcinomas in the series were identified from the pathology archives of our institution from 2005 to 2012. The metaplastic carcinomas in this series had at least 50% heterologous elements present in the resection specimen (lumpectomy or mastectomy), including spindled, squamous, chondroid, or osseous differentiation. All metaplastic carcinomas were grade III, except for case 5, which was grade II. The metaplastic carcinomas included were all negative for estrogen receptor (ER), progesterone receptor (PR), and Her-2.
Table 1.
Pathologic features of metaplastic (sarcomatoid) carcinomas
| Case no. |
Tumor size (cm) |
Metaplastic component(s) |
Sox10 score (0–4+) |
|---|---|---|---|
| Case 1 | 3.5 | Spindled and squamous | 0 |
| Case 2 | 4 | Chondroida | 3 |
| Case 3 | 2.7 | Spindleda and squamous | 4 |
| Case 4 | 13 | Spindled | 0 |
| Case 5 | 4.2 | Spindled | 0 |
| Case 6 | 2.3 | Chondroida | 4 |
| Case 7 | 5.5 | Spindled | 0 |
| Case 8 | 2.8 | Chondroida | 4 |
| Case 9 | 3 | Chondroida and osseousa | 4 |
| Case 10 | 10.1 | Squamous | 0 |
| Case 11 | 1.6 | Chondroida and squamous | 3 |
| Case 12 | 5.5 | Chondroid | 0 |
| Case 13 | 3 | Spindled and squamous | 0 |
NOTE. Nuclear Sox10 labeling is defined as 0, 1+ (1%–25%), 2+ (25%–50%), 3+ (50%–75%), 4+ (>75%).
Sox10 labeling was only present in the components.
Phyllodes tumors of the breast are in the diagnostic differential of metaplastic carcinomas with spindle cell differentiation, particularly in the limited material of a core biopsy, and they are thus included in this study to serve as a comparison with the metaplastic carcinomas. The series of phyllodes tumors consisted of 14 malignant, 10 borderline, and 10 benign tumors, as previously described in an abstract form [7]. The tumors were subdivided into malignant, borderline, and benign on the basis on tumor circumscription, stromal overgrowth, stromal cellularity, stromal pleomorphism, and stromal mitosis, as previously defined [8,9]. These cases were selected to be classic examples of these subtypes; cases that were ambiguous or difficult to subclassify were purposely excluded. The fibroadenomas were included in the study for completeness to serve as a comparison with the phyllodes tumors. Each TMA consisted of 99 cores, each measuring 1.4 mm in diameter, including 9 cores of control tissues. Five cores were taken per metaplastic carcinoma and phyllodes tumor and 2 cores per fibroadenoma, to minimize sampling error. One core per case contained benign breast lobules as an internal control.
In addition, we evaluated a series of previously described TMAs [10] constructed from archived paraffin tissue blocks of 87 invasive ductal carcinomas, with 5 cores per case including 1 core of benign lobules. The IHC standard surrogate panel used to define the molecular subtypes included ER, PR, Her-2, cytokeratin (CK) 5/6, and epidermal growth factor receptor (EGFR), as previously described [10–12]. Briefly, in this series, the luminal A carcinomas were defined as having nuclear ER labeling in greater than 1% of cells but negative Her-2 (IHC 0 or 1+); the luminal B carcinomas were defined as having nuclear ER or PR labeling in greater than 1% of cells as well as evidence of Her-2 amplification by IHC (3+) or fluorescence in situ hybridization (amplification ratio >2.2); the Her-2 carcinomas were defined as being completely negative for ER and PR (0% staining), but with evidence of Her-2 amplification (IHC 3+ or fluorescence in situ hybridization >2.2); the basal-like carcinomas were defined as being completely negative for ER and PR (0% staining) and Her-2 (IHC 0 or 1+) and demonstrating immunoreactivity for CK5/6 and/or EGFR; and the unclassified triple-negative carcinomas were defined as being negative for ER and PR (0% staining), Her-2 (IHC 0 or 1+), and CK5/6 and EGFR. In this series of cases, all luminal cases selected for the TMAs showed more than 70% labeling for ER, and all cases with Her2 amplification selected for the TMAs had an amplification ratio greater than 4.0, as previously described [10]. Our intention was to choose cases unequivocally positive for the IHC markers so that the cases would be more likely to reflect the categories defined by gene expression. Cases were selected as classic examples of the subtypes; cases that were ambiguous or difficult to subclassify were purposefully excluded. All of our cases that were classified as ER and PR negative showed 0% nuclear labeling for these markers.
Using this panel, the carcinoma cases were subdivided into the 5 categories of luminal A (21 cases), luminal B (7 cases), Her-2 (14 cases), basal-like (32 cases), and unclassified triple-negative carcinomas (13 cases) based on the accepted and validated IHC surrogate profiles [11,12] for molecular subtypes identified by genomic analysis [13,14].
In addition, we evaluated previously constructed TMAs constructed from archived paraffin blocks containing 24 cases of DCIS from different patients, with 4 to 5 cores per tumor. Two cases were nuclear grade 1, 11 cases were nuclear grade 2, and 11 cases were nuclear grade 3. Ten of the nuclear grade 3 cases had comedo pattern necrosis. The average tumor size was 2.52 cm, with a range from 0.4 to 5 cm and a median of 1.5 cm. Twenty cases were positive for ER/PR, and 4 cases were negative for ER and PR. Four DCIS cases were associated with invasive ductal carcinoma with known ER, PR, and Her-2 status; of these cases, 1 was luminal A, 1 was luminal B, and 2 were triple negative.
2.2. IHC and expression scoring
The TMAs were labeled by IHC for Sox10 by using the Sox10 (N-20) goat polyclonal antibody from Santa Cruz Biotechnology (Santa Cruz, CA; SC-17342) at 1:100 dilution. Briefly, unstained 5-µm sections were cut from paraffin TMA blocks; slides were deparaffinized by routine techniques, steamed for 25 minutes in sodium citrate buffer, cooled for 5 minutes, blocked with peroxidase blocking solution for 10 minutes and with 1% normal horse serum for nonspecific protein blocking for 25 minutes, and incubated with the primary antibody for 30 minutes at room temperature. The reaction was developed with detection from Biocare Goat-on-Rodent horseradish peroxidase polymer kit (reference no. GHP516H; Biocare Medical, LLC, Concord, CA), with incubation times of 15 minutes with the goat probe, 15 minutes with the goat horseradish peroxidase, and 10 minutes with diaminobenzedine for visualization. Slides were counterstained with hematoxylin and dehydrated before permanent coverslip.
Nuclear Sox10 labeling of the invasive carcinoma cells or stromal tumor cells was scored as previously described [3], with the extent of nuclear labeling graded as follows: 0 (0%), 1+ (1%–25%), 2+ (25%–50%), 3+ (50%–75%), and 4+ (>75%). Intensity of staining was recorded separately as weak, moderate, or strong. Sox10 labeling was scored blindly without knowledge of the molecular subtype of the tumor. Control tissues on the TMAs included benign brain, kidney, liver, lung, lymph node, placenta, skin, small intestine, stomach, and tonsil. Based on prior studies [3–5], melanocytes in the epidermis, neuroendocrine and mast cells in the gastrointestinal cells, and myoepithelial cells in the breast were considered positive internal controls for Sox10 staining.
3. Results
Overall, 40 (40%) of 100 invasive breast carcinomas demonstrated nuclear Sox10 immunoreactivity, and the reactivity was seen primarily in the basal-like, unclassified triple-negative and metaplastic carcinomas (Table 2). Sox10 labeling was seen in 66% (38/58) of the basal-like, unclassified triple-negative, and metaplastic carcinomas as compared with 5% (2/42) of the luminal A, luminal B, and Her-2 carcinomas (P < .00001, Fisher exact test). In the metaplastic carcinomas, Sox10 labeling was seen in spindled, chondroid, and osseous components, but not in any squamous components (Table 1).
Table 2.
Immunohistochemical labeling of Sox10 in breast neoplasms
| Tumor type | n | Sox10 a | Total positive |
||||
|---|---|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | 4+ | |||
| Luminal A–type carcinoma | 21 | 21 | 0 | 0 | 0 | 0 | 0 (0%) |
| Luminal B–type carcinoma | 7 | 6 | 0 | 0 | 0 | 1 | 1 (14%) |
| Her-2–type carcinoma | 14 | 13 | 0 | 0 | 0 | 1 | 1 (7%) |
| Basal-like carcinoma | 32 | 10 | 0 | 3 | 4 | 15 | 22 (69%) |
| Unclassified triple-negative carcinoma | 13 | 3 | 0 | 2 | 2 | 6 | 10 (77%) |
| Metaplastic carcinoma | 13 | 7 | 0 | 0 | 2 | 4 | 6 (46%) |
| DCIS | 24 | 23 | 0 | 0 | 0 | 1 | 1 (4%) |
| Phyllodes tumor | 34 | 34 | 0 | 0 | 0 | 0 | 0 (0%) |
| Fibroadenoma | 10 | 10 | 0 | 0 | 0 | 0 | 0 (0 %) |
Nuclear Sox10 labeling is defined as 0, 1+ (1%–25%), 2+ (25%–50%), 3+ (50%–75%), 4+ (>75%).
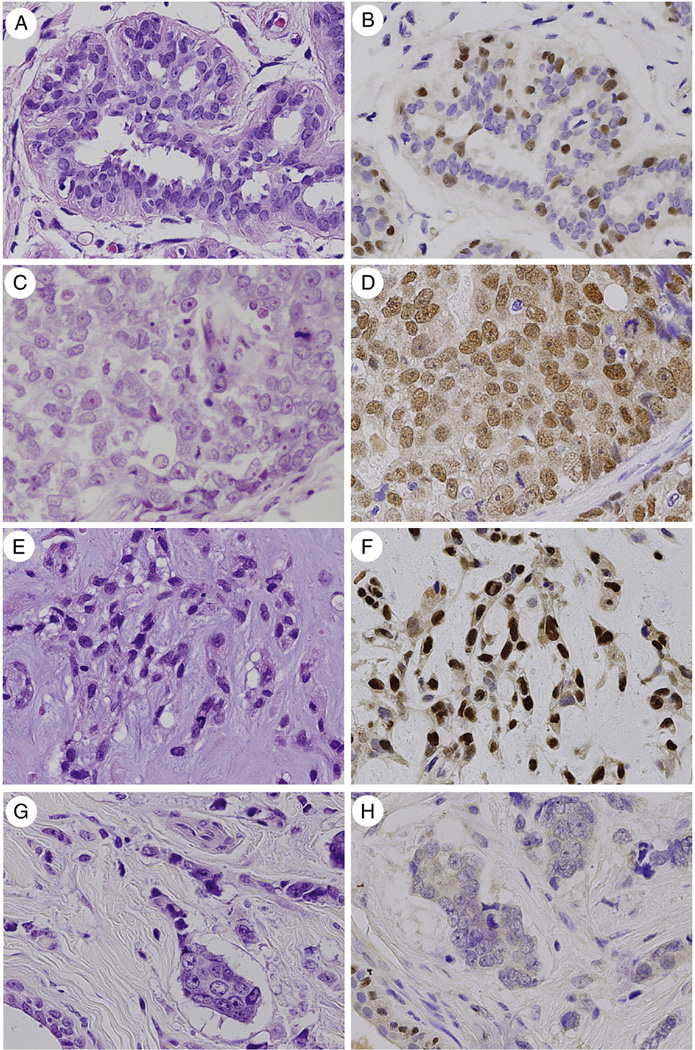
As an internal control, strong nuclear Sox10 staining was present in breast myoepithelial cells of benign lobules (Fig. A and B), consistent with previously reported findings [3,4], as well as in the myoepithelial cells of phyllodes tumors and fibroadenomas. Sox10 labeling was seen in 69% (22/32) of basal-like carcinomas (Fig. C and D), 77% (10/13) of unclassified triple-negative carcinomas, and 46% (6/13) metaplastic carcinomas (Fig. E and F). Sox10 labeling was seen in 1 case each of luminal B carcinoma (14%; 1/7) and Her-2 carcinoma (7%; 1/14). No labeling was seen in the luminal A carcinoma cells (0%; 0/21) (Fig. G and H), the phyllodes tumors’ stromal or luminal epithelial cells (0%; 0/34), or the fibroadenoma stromal or luminal epithelial cells (0%; 0/10). The internal positive controls labeled appropriately (Fig. G and H). Sox10 labeling was seen in 25% (1/4) cases of ER/PR-negative DCIS. This case of DCIS was nuclear grade 3, with comedo, solid, cribriform, and micropapillary architectural patterns and associated with a triple-negative invasive ductal carcinoma. All 20 ER-positive DCIS cases were negative for Sox10; thus, overall, Sox10 labeling was seen in 4% (1/24) of all DCIS cases.
Fig.
Nuclear Sox10 labeling is seen in breast myoepithelial cells and a subset of invasive breast carcinomas. Breast myoepithelial cells in benign lobules (A) display strong nuclear Sox10 labeling (B), but the ductal luminal cells are negative. Nuclear Sox10 labeling is seen in the invasive carcinoma cells in 66% of unclassified triple-negative carcinomas, basal-like carcinomas (C, D), and metaplastic carcinomas (E, F). No Sox10 labeling is seen in luminal A carcinomas (G, H) or mammary fibroepithelial neoplasms; positive internal control staining of myoepithelial cells in benign lobules is seen in the left lower field (G, H). Hematoxylin and eosin and Sox10 immunostain, ×400.
Of the 40 cases of invasive carcinoma positive for Sox10, most (88%; 35/40) showed diffuse labeling, with scores of 3+ to 4+. Only 13% of positive cases (5/40) showed 2+ labeling, and no cases showed 1+ or focal labeling. Of the 40 cases positive for Sox10, most (85%; 34/40) had moderate to strong nuclear labeling. Only 15% of positive cases (6/40) had weak nuclear labeling. The intensity and percentage of cells labeling with Sox10 were uniform within a given case, with very limited intratumoral heterogeneity demonstrated on the TMA sections.
4. Discussion
The Sox (Sry-related HMG box) genes are a family of transcription factors with HMG DNA-binding domains that play a role in numerous developmental processes, including the development of the nervous system, skeletal system, and immune system [15]. The Sox10 gene was first discovered in mouse embryos in 1993 [16] and subsequently described in the human genome in 1998 [17].Of all the Sox family proteins, the function of the Sox10 protein has been one of the most studied, largely because of the association of mutated Sox10 with clinical neurocristopathies such as Waardenburg-Shah syndrome [2]. Sox10 appears to have a role in the survival of neural crest cells and their maturation and differentiation into neural-crest–derived melanocytes and glia [1,2].
In the breast, the Notch signaling pathway is central to controlling stem cell maintenance and cell differentiation [18], and the Notch gene is activated in the ductal luminal progenitor cells of the mammary lobules [19]. Sox10 is required for Notch4-PBP–mediated cell growth in in vitro studies of murine mammary epithelial cells [6]. Otherwise, the exact role of Sox10 in effecting mammary cell differentiation is not well understood. Sox10 labeling by IHC has been previously demonstrated in myoepithelial cells of the breast [3,4], but not in benign ductal epithelial cells or a limited number of in situ carcinomas or invasive carcinomas of the breast [3].
Here, we demonstrate for the first time that Sox10 nuclear labeling by IHC can be seen in invasive breast carcinoma, most notably in 66% of basal-like, unclassified triple-negative, and metaplastic carcinomas of the breast. No Sox10 labeling was seen in luminal A carcinomas, phyllodes tumors, or fibroadenomas. Limited labeling was seen in luminal B and Her-2 carcinomas. Limited Sox10 labeling was seen in DCIS, specifically in a single case of ER/PR-negative DCIS associated with a triple-negative invasive ductal carcinoma. We suspect that the limited Sox10 labeling of DCIS in our study reflects the fact that most of our DCIS cases were pure DCIS without associated invasive carcinoma, which were predominantly ER positive. Pure triple-negative DCIS cases are less common on a percentage basis than triple-negative invasive carcinomas, perhaps reflecting their increased tendency to invade. Because most markers expressed in invasive breast cancers are also expressed in their corresponding subtype of DCIS, we suspect that the incidence of Sox10 expression will be highest in triple-negative DCIS, although further data are needed to support this assertion.
Triple-negative carcinomas of the breast are a heterogeneous group of invasive carcinomas that lack expression of ER, PR, and Her-2 [20,21]. There is currently no specific targeted therapy for these of tumors, and as a group, they are associated with poor overall survival. Basal-like tumors comprise a subset of triple-negative carcinomas because they lack ER, PR, and Her-2 expression, in that they express markers seen in the basal myoepithelial cells of the breast such as CK5/6 and/or EGFR [22,23]. However, basal-like carcinomas themselves are heterogeneous [23]. Notably, metaplastic carcinomas have also been shown to have a basallike phenotype by IHC [24–28] and genomic profiling [29].
Our finding that Sox10 expression is seen in a subset of invasive breast carcinoma illustrates several points. First, the presence Sox10 expression in normal breast myoepithelial cells as well as the basal-like carcinomas and metaplastic carcinomas supports the myoepithelial differentiation in these tumor types. It suggests that the unclassified triple-negative carcinomas that demonstrate Sox10 labeling also show basal-like or myoepithelial differentiation that is not detected by IHC for EGFR or CK5/6. Second, immunoreactivity for Sox10 may be useful in supporting breast origin in a metastasis from a triple-negative, basal-like, or metaplastic carcinoma, although one caveat is that the full spectrum of Sox10 immunoreactivity in human neoplasms has yet to be completely studied. Third, the lack of Sox10 labeling in phyllodes tumors suggests that this immunostain may be useful in distinguishing between malignant phyllodes tumors and metaplastic/sarcomatoid carcinomas, a distinction that can be especially difficult on core needle biopsy. However, we recognize that our case series is relatively small, and the study is limited by the TMA methodology used. Further studies are clearly needed.
To date, immunoreactivity of Sox10 has been used primarily to support the diagnosis of melanoma in primary [3–5] and metastatic [30] sites. A potential pitfall may arise when one encounters an S100-positive, Sox10-positive, CK-negative spindled, and epithelioid malignant neoplasm; in this setting, based on the published literature, one could potentially make an outright diagnosis of melanoma. A common situation in which this can occur is in the woman who presents with an axillary mass presumed to be a lymph node metastasis but no known primary, where a metastasis from occult melanoma or breast carcinoma usually tops the differential diagnosis. Similar to melanomas, metaplastic carcinomas of the breast may be negative for broad-spectrum CKs by IHC [31]. Furthermore, both primary [32,33] and metastatic [33] breast carcinomas can be immunoreactive for the S-100 protein, a finding frequently used to support the diagnosis of melanoma [3] (particularly the desmoplastic or spindle cell type). As our study shows, Sox10 labeling does not prove that such a lesion is melanoma, and breast carcinoma remains in the differential diagnosis. In this setting, use of more specific markers (such as high-molecular-weight CKs or p63 for metaplastic carcinoma, and HMB45 and Melan-A for melanoma) can often make this distinction. When they cannot, especially in the setting of a limited core biopsy, additional tissue sampling with procurement of tissue for possible electron microscopy may be helpful.
Further studies are needed to fully understand the role of Sox10 in breast myoepithelial cells and what role, if any, it has in maintaining the differentiation and survival in the associated Sox10-positive carcinomas. The expression of Sox10 in other breast neoplasms with myoepithelial differentiation, such as myoepithelial carcinoma and adenoid cystic carcinoma, is currently being investigated.
In summary, strong nuclear Sox10 labeling is seen in a subset of invasive breast carcinomas, most notably the basal-like, unclassified triple-negative, and metaplastic carcinomas. Sox10 expression in these tumor types supports myoepithelial differentiation. Sox10 reactivity cannot alone be used to support a diagnosis of metastatic melanoma, and metastatic breast carcinoma must be considered in the differential diagnosis of an S100 and Sox10-positive malignant neoplasm.
Footnotes
Disclosures: The authors have no conflicts of interest to disclose.
Funding source: Johns Hopkins Hospital Breast Cancer Research Fund (A.C.-M.), Baltimore, MD.
References
- 1.Mollaaghababa R, Pavan WJ. The importance of having your SOX on: role of SOX10 in the development of neural crest–derived melanocytes and glia. Oncogene. 2003;22:3024–3034. doi: 10.1038/sj.onc.1206442. [DOI] [PubMed] [Google Scholar]
- 2.Kelsh RN. Sorting out Sox10 functions in neural crest development. Bioessays. 2006;28:788–798. doi: 10.1002/bies.20445. [DOI] [PubMed] [Google Scholar]
- 3.Nonaka D, Chiriboga L, Rubin BP. Sox10: a pan-schwannian and melanocytic marker. Am J Surg Pathol. 2008;32:1291–1298. doi: 10.1097/PAS.0b013e3181658c14. [DOI] [PubMed] [Google Scholar]
- 4.Karamchandani JR, Nielsen TO, van de Rijn M, West RB. Sox10 and S100 in the diagnosis of soft-tissue neoplasms. Appl Immunohistochem Mol Morphol. 2012;20:445–450. doi: 10.1097/PAI.0b013e318244ff4b. [DOI] [PubMed] [Google Scholar]
- 5.Shin J, Vincent JG, Cuda JD, et al. Sox10 is expressed in primary melanocytic neoplasms of various histologies but not in fibrohistiocytic proliferations and histiocytoses. J Am Acad Dermatol. 2012;67:717–726. doi: 10.1016/j.jaad.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 6.Zhu YT, Jia Y, Hu L, et al. Peroxisome-proliferator–activated receptor-binding protein (PBP) is essential for the growth of active Notch4-immortalized mammary epithelial cells by activating SOX10 expression. Biochem J. 2009;425:435–444. doi: 10.1042/BJ20091237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cimino-Mathews A, Hicks JL, Sharma R, et al. Rb reference. A subset of malignant phyllodes tumors harbors Rb/p16 pathway alterations. Modern Pathol. 2012;25(S2):30A. doi: 10.1016/j.humpath.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellow JP, Magro G. Fibroepithelial tumors. In: Tavassoli FA, Devilee P, editors. World Health Organization Classification of Tumours: Tumours of the Breast and Female Genital Organs. Lyon, France: IARC Press; 2003. pp. 99–103. [Google Scholar]
- 9.Rosen PP. Fibroepithelial neoplasms. In: Rosen PP, editor. Rosen’s breast pathology. 3rd ed. Philadelphia (PA): Lippincott Williams & Wilkins; 2009. pp. 187–229. [Google Scholar]
- 10.Subhawong AP, Subhawong T, Nassar H, et al. Most basal-like breast carcinomas demonstrate the same Rb−/p16+ immunophenotype as the HPV-related poorly differentiated squamous cell carcinomas which they resemble morphologically. Am J Surg Pathol. 2009;33:163–175. doi: 10.1097/PAS.0b013e31817f9790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 13.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 14.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wegner M. From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res. 1999;27:1409–1420. doi: 10.1093/nar/27.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright EM, Snopek B, Koopman P. Seven new members of the Sox gene family expressed during mouse development. Nucleic Acids Res. 1993;21:744. doi: 10.1093/nar/21.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pusch C, Hustert E, Pfeifer D, et al. The SOX10/Sox10 gene from human and mouse: sequence, expression, and transactivation by the encoded HMG domain transcription factor. Hum Genet. 1998;103:115–123. doi: 10.1007/s004390050793. [DOI] [PubMed] [Google Scholar]
- 18.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 19.Bouras T, Pal B, Vaillant F, et al. Notch signaling regulated mammary stem cell function and luminal cell–fate commitment. Cell Stem Cell. 2008;3:429–441. doi: 10.1016/j.stem.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Reis-Filho JS, Tutt AN. Triple negative tumours: a critical review. Histopathology. 2008;52:108–118. doi: 10.1111/j.1365-2559.2007.02889.x. [DOI] [PubMed] [Google Scholar]
- 21.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 22.Rakha EA, Reis-Filho JS, Ellis IO. Basal-like breast cancer: a critical review. J Clin Oncol. 2008;26:2568–2581. doi: 10.1200/JCO.2007.13.1748. [DOI] [PubMed] [Google Scholar]
- 23.Badve S, Dabbs DJ, Schnitt SJ, et al. Basal-like and triple-negative breast cancers: a critical review with an emphasis on the implications for pathologists and oncologists. Mod Pathol. 2011;24:157–167. doi: 10.1038/modpathol.2010.200. [DOI] [PubMed] [Google Scholar]
- 24.Rosen PP. Carcinomas with metaplasia. In: Rosen PP, editor. Rosen’s breast pathology. 3rd ed. Philadelphia (Pa): Lippincott Williams & Wilkins; 2009. pp. 470–505. [Google Scholar]
- 25.Reis-Filho JS, Milanezi F, Paredes J, et al. Novel and classic myoepithelial/stem cell markers in metaplastic carcinomas of the breast. Appl Immunohistochem Mol Morphol. 2003;11:1–8. doi: 10.1097/00129039-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Reis-Filho JS, Milanezi F, Steele D, et al. Metaplastic breast carcinomas are basal-like tumours. Histopathology. 2006;49:10–21. doi: 10.1111/j.1365-2559.2006.02467.x. [DOI] [PubMed] [Google Scholar]
- 27.Gobbi H, Olson SJ, Simpson JF, Jensen RA, Page DL. Spindle cell metaplastic tumors of the breast (SCMTB) co-express p63, a novel myoepithelial marker, and epithelial markers. Mod Pathol. 2004;17(S1):31A. [Google Scholar]
- 28.Koker MM, Kleer CG. p63 expression in breast cancer: a highly sensitive and specific marker of metaplastic carcinoma. Am J Surg Pathol. 2004;28:1506–1512. doi: 10.1097/01.pas.0000138183.97366.fd. [DOI] [PubMed] [Google Scholar]
- 29.Weigelt B, Kreike B, Reis-Filho JS. Metaplastic breast carcinomas are basal-like breast cancers: a genomic profiling analysis. Breast Cancer Res Treat. 2009;117:273–280. doi: 10.1007/s10549-008-0197-9. [DOI] [PubMed] [Google Scholar]
- 30.Jennings C, Kim J. Identification of nodal metastases in melanoma using sox-10. Am J Dermatopathol. 2011;33:474–482. doi: 10.1097/DAD.0b013e3182042893. [DOI] [PubMed] [Google Scholar]
- 31.Dunne B, Lee AH, Pinder SE, Bell JA, Ellis IO. An immunohistochemical study of metaplastic spindle cell carcinoma, phyllodes tumor and fibromatosis of the breast. Hum Pathol. 2003;34:1009–1015. doi: 10.1053/s0046-8177(03)00414-3. [DOI] [PubMed] [Google Scholar]
- 32.Dwarakanath S, Lee AK, Delellis RA, Silverman ML, Frasca L, Wolfe HJ. S-100 protein positivity in breast carcinomas: a potential pitfall in diagnostic immunohistochemistry. Hum Pathol. 1987;18:1144–1148. doi: 10.1016/s0046-8177(87)80382-9. [DOI] [PubMed] [Google Scholar]
- 33.Stroup RM, Pinkus GS. S-100 immunoreactivity in primary and metastatic carcinoma of the breast: a potential source of error in immunodiagnosis. Hum Pathol. 1988;19:949–953. doi: 10.1016/s0046-8177(88)80011-x. [DOI] [PubMed] [Google Scholar]