Abstract
Lymphatic vessels transport fluid, antigens, and immune cells to the lymph nodes to orchestrate adaptive immunity and maintain peripheral tolerance. Lymphangiogenesis has been associated with inflammation, cancer metastasis, autoimmunity, tolerance and transplant rejection, and thus, targeted lymphatic ablation is a potential therapeutic strategy for treating or preventing such events. Here we define conditions that lead to specific and local closure of the lymphatic vasculature using photodynamic therapy (PDT). Lymphatic-specific PDT was performed by irradiation of the photosensitizer verteporfin that effectively accumulates within collecting lymphatic vessels after local intradermal injection. We found that anti-lymphatic PDT induced necrosis of endothelial cells and pericytes, which preceded the functional occlusion of lymphatic collectors. This was specific to lymphatic vessels at low verteporfin dose, while higher doses also affected local blood vessels. In contrast, light dose (fluence) did not affect blood vessel perfusion, but did affect regeneration time of occluded lymphatic vessels. Lymphatic vessels eventually regenerated by recanalization of blocked collectors, with a characteristic hyperplasia of peri-lymphatic smooth muscle cells. The restoration of lymphatic function occurred with minimal remodeling of non-lymphatic tissue. Thus, anti-lymphatic PDT allows control of lymphatic ablation and regeneration by alteration of light fluence and photosensitizer dose.
Electronic supplementary material
The online version of this article (doi:10.1007/s10456-013-9365-6) contains supplementary material, which is available to authorized users.
Keywords: Endothelium, Lymphatic collectors, Intravital imaging, Lymphangiogenesis, Lymphangiography, Lymphatic ablation, Reactive oxygen species, Verteporfin, Visudyne, PDT
Introduction
Lymphatic vessels drain fluid, antigens, and immune cells from the interstitial space to lymph nodes (LNs) and eventually back into the systemic blood circulation [1]. Initial lymphatic capillaries consist of a monolayer of lymphatic endothelial cells (LECs) attached to a thin basement membrane, and unlike larger lymphatic vessels, they are deprived of smooth muscle cells (SMCs). They collect interstitial fluid, forming lymph, and drain to the pre-collecting and then collecting lymphatic vessels that are surrounded by SMCs and segmented with bicuspid valves. This physiological process regulates the tissue fluid homeostasis, leukocyte recirculation, and transport of antigen-presenting cells to the lymph nodes, crucial for adaptive immunity [2, 3]. Post-developmental lymphangiogenesis occurs primarily during inflammation [4, 5], both in the inflamed tissue as well as its draining lymph node, and in lymph nodes following infection and vaccination [6, 7] or after tissue transplantation [8]. Although its precise immunological functions remain unclear [4, 9], inflammatory lymphangiogenesis has been associated with both immune tolerance as well as immunogenicity. In melanoma, tumor-driven lymphangiogenesis promoted tolerance induction by suppression and deletion of tumor-reactive T cells [10], and lymphangiogenesis has been shown to help resolve inflammation after lung injury [11]. However, corneal lymphangiogenesis is a major risk factor for corneal transplant rejection [12–15], and blocking lymphangiogenesis after experimental islet transplantation helped prevent rejection [16]. VEGF-C or VEGF-D-driven lymphangiogenesis can be prevented by function-blocking antibodies against the lymphatic receptor VEGFR-3 [17–19]. However, anti-lymphangiogenic treatment has no influence on the function of pre-existing lymphatic vessels. Thus, there is great potential, for both fundamental lymphatic research as well as immunotherapeutic strategies, for methods that can locally destroy lymphatic vessels, and particularly lymphatic collectors.
Photodynamic therapy (PDT) is a clinical treatment for ablating unwanted or pathological blood vessels based on the administration of a non-toxic photosensitizer followed by sub-thermal light exposure that induces photosensitizer toxicity via generation of reactive oxygen species (ROS), resulting in thrombosis and vascular occlusion [20–23]. This strategy can be used to treat cancer, as well as non-oncological conditions, such as age-related macular degeneration (AMD) [24] or polypoidal choroidal vasculopathy [25]. Visudyne®, a liposomal formulation of verteporfin (the benzoporphyrin derivative monoacid ring A), is a clinically used photosensitizer administrated intravenously in patients for photodynamic treatment of pathologic cornea neovascularization [26, 27] AMD [28] or polypoidal choroidal vasculopathy [25]. Light-activated verteporfin induces a cascade of reactions leading to the formation of highly reactive and toxic ROS, mainly singlet oxygen [29, 30]. Although the effects of PDT on the blood vessels have been extensively studied during the past several decades [20, 23] the use of PDT for lymphatic ablation has only begun to be explored, namely for targeting peritumoral lymphatics and the in-transit tumor cells they contained to treat metastatic disease [31].
Our goal was to determine whether lymphatic vessels could be specifically targeted in skin, and to identify the optimal conditions to selectively close lymphatic collecting vessels without injuring the blood vasculature. More specifically, we focused on (1) the timing and the mechanism of PDT-induced lymphatic-specific closure using optimal light fluencies and photosensitizer doses, and (2) the kinetics of ensuing lymphatic regeneration. Such information, although presumably specific to mouse dermal lymphatics, is necessary to further develop lymphatic-specific PDT, for example to control metastatic spread or perform basic lymphatic research.
Materials and methods
PDT on the mouse ear dermis
All experiments were carried out in BALB/c mice according to a protocol approved by the Committee for Animal Experiments for the Canton Vaud, Switzerland (permits 2316 and 2464). Three days before each experiment, ears were treated with depilation cream (Veet® Hair Removal Cream, Slough, UK) for 10 s followed by thorough rinsing with water. For PDT, mice were anesthetized with 2.5 % isofluorane (Rothhacher GmbH, Bern, Switzerland) and kept under 1.5 % isofluorane for the duration of the experiment. Mouse core temperature was maintained at 37 °C throughout the experiment (DC Temperature Control System, FHC Inc., Bowdoin, MA). A liposomal formulation of verteporfin (Visudyne®, Novartis Ophthalmics, Hettlingen, Switzerland) was freshly reconstituted from powder as previously described [32] and injected (0.5 μl) into the tip of the ear dermis using a microsyringe (Hamilton, Reno, NV, USA) (Fig. 1a). The injection site was covered with an aluminum foil (Fig. 1b) and after 5 min, the entire ear was irradiated (Fig. 1c) with non-thermal laser light (Biolitec AG, Jena, Germany) at 689 ± 2 nm delivered through an optical fiber containing a frontal light distributor with lens (Medlight SA, Ecublens, Switzerland) with the laser power of 0.1–1.56 W, and light fluencies between 0.4 and 25 J/cm2. Light fluencies were adjusted with neutral density filters and measured with a calibrated Field-Master GS power meter (Coherent Inc, Santa Clara, CA, USA).
Fig. 1.
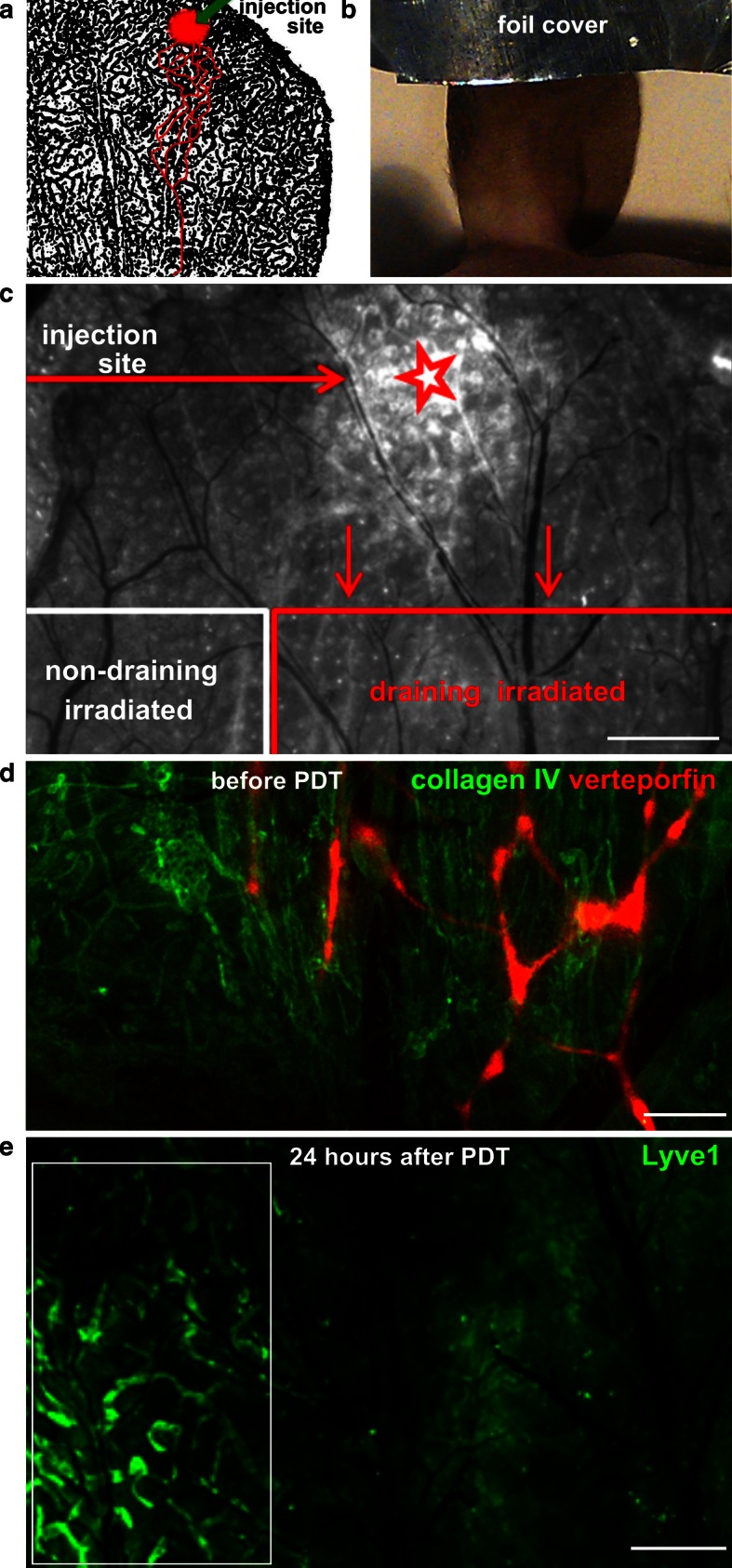
Lymphatic-specific ablation by verteporfin-PDT in the mouse ear. a Schematic representation of the injection site and draining lymphatic vessels against a LYVE-1-stained image of the mouse ear lymphatic vasculature (black). (red circle). b The site of verteporfin injection was covered with foil and the rest of the ear was irradiated. c Superimposition of verteporfin fluorescence over the bright field image of the exposed dorsal ear dermis 24 h after PDT. Image shows the site of verteporfin injection (star) and the verteporfin-draining and non-draining (control) regions in the dorsal ear dermis after removal of ventral skin and intermediate cartilage. Star depicts the site of verteporfin injection. Bar 0.5 mm Red star depicts the site of verteporfin injection (as in a). d Fluorescence image showing verteporfin (red) draining within lymphatic vessels in the exposed dorsal skin after immunostaining for collagen IV (green). e Destruction of lymphatic endothelium is seen 24 h after PDT by intravital immunofluorescence for LYVE-1. Intact lymphatic vessels are seen in the control (non-draining) region (white box). Scale bar correspond to 500 μm
Fluorescence microlymphangiography
In order to identify the effective verteporfin dose necessary to ablate lymphatic vessels selectively, 25 or 100 ng of verteporfin was injected (in 0.5 μl) into the tip of the ear 5 min prior to PDT and ears were imaged with an automated fluorescence stereomicroscope (M205 FA, Leica Microsystems GmbH, Wetzlar, Germany) equipped with a 647 nm filter and camera (DFC350 FX, Leica), controlled by a Leica AF software. Two hours after PDT, 0.5 μl TRITC-dextran (150 kDa; 10 mg/ml; Sigma-Aldrich) was injected in the tip of the ear, near the location of the verteporfin injection. Draining collecting lymphatics were imaged using fluorescence stereomicroscopy with a 594 nm filter (Leica). This was repeated 24 h later to verify the effectiveness of anti-lymphatic PDT.
Fluorescence angiography
To identify functional, blocked, or leaky blood vessels in the ear, 200 μl TRITC-dextran was injected into the tail vein 24 h after PDT, and the ears were imaged at low magnification. To observe blood perfusion at the level of individual blood vessels, the dorsal and ventral skin of the ear were separated 24 h after PDT, the tail was injected with 200 μl TRITC-dextran, and exposed dorsal circulation in the ear was imaged at high magnification.
Intravital immunofluorescence lymphangiography
This method is based on the observation that intravital immunolabeling of tissue structures is dependent on intradermal fluid currents (i.e., functional lymphatic drainage) and allows detection of dysfunctional skin drainage basins in the whole explanted tissue despite the presence of intermediate functional lymphatics [33]. Briefly, 24 h after PDT, the ventral skin with underlying cartilage was separated and removed from the dorsal skin, leaving it innervated and functionally connected to the mouse circulation. Then, the tissue was incubated with rabbit anti-Lyve-1 antibody (1 μg/ml, ReliaTech GmbH, Wolfenbüttel, Germany) 15 min, followed by washing in Ringer’s buffer (102 mM NaCl, 5 mM KCl, 2 mM CaCl2, 28 mM sodium lactate, 25 mM HEPES), 15 min incubation with Alexa 488 anti-rabbit antibody (1 μg/ml, Invitrogen, Carlsbad, CA, USA). Within this short incubation time, only functionally draining lymphatic vessels became brightly stained for Lyve-1, since convective flows carried the antibodies to draining lymphatic vessels, where they concentrated. In contrast, areas that lacked functional lymphatic drainage had weaker staining because diffusive transport was less significant over this short time period.
After bathing the skin in ascorbate-Ringer solution (140 mM sodium ascorbate, 25 mM HEPES, 4 mM KCl and 2 mM CaCl2, at a pH of 7.5) to prevent phototoxicity [33], a coverslip was placed over the ear and lymphatic capillaries were imaged using fluorescence microscopy as described above.
Intravital immunofluorescence of lymphatic vessels
Immunostaining on the live ear tissue was performed with modifications as described [33]. To detect necrotic cells within lymphatic vessels before PDT, the middle part of the ventral ear skin and cartilage were separated and removed from the dorsal skin, leaving a “dorsal dermis window”. Fcγ receptor blocking solution was placed on the tissue, followed by 15 min incubation with biotinylated rabbit anti-collagen IV (1 μg/ml; Abcam, Cambridge, MA USA), a brief rinse with Ringer’s solution and 15 min incubation with streptavidin-Pacific Blue (1 μg/ml; Invitrogen, Zug, Switzerland). PDT was then performed as described above; the dorsal dermis was mounted under a coverslip in ascorbate-Ringer solution supplemented with propidium iodide (1 μg/ml; Ebioscience, San Diego, CA USA) and imaged for 3 h with the stereomicroscopy setup described above.
To image both the initial and collecting lymphatic vessels, the entire ventral skin and cartilage of the ear was removed and the dorsal dermis was incubated in Fcγ-receptor blocking solution followed by rabbit anti-Lyve-1 and goat anti-podoplanin (R&D Systems, Minneapolis, MN) antibodies for 15 min. After washing with Ringer’s buffer, Alexafluor-labeled secondary antibodies (1 μg/ml, Invitrogen) were applied for another 15 min. Following another wash and 15 min blocking with 20 % goat serum, the ear was incubated in biotinylated goat anti-perlecan antibody (1 μg/ml; Abcam), followed by rinsing and 15 min incubation of fluorescently labeled streptavidin (1 μg/ml). The stained lymphatic structures were then imaged as above.
Time of lymphatic occlusion and regeneration
PDT was performed with an optimal dose of verteporfin and light fluence of either 3.6 or 25 J/cm2. Fluorescence microlymphangiography (above) was performed immediately after PDT as well as at various time points thereafter. After each injection, ears were washed with Betadine solution (MundiPharma) and animals were returned to cages. Lymphatic function was considered “recovered” when the lymphatic drainage was observed in at least one continuous lymphatic collecting vessel spanning the entire ear.
For time of lymphatic occlusion, PDT was performed with an optimal dose of verteporfin and two light doses 25 and 3.6 J/cm2. To test lymphatic perfusion fluorescence lymphangiography was performed with TRITC-dextran injected 3, 6, 9 and 24 h post PDT under isoflurane anesthesia.
Whole-mount staining of the ear dermis
Mice were sacrificed with CO2 and exsanguinated by intracardiac perfusion with 20 ml Ringer’s buffer (supplemented with 10,000 IU/l heparin, 0.1 % glucose, 0.1 % procaine and 25 mM HEPES (Sigma-Aldrich); pH 7.5, 330 mOsM) at a constant gravitational pressure of 120 mm Hg. Ringer’s buffer was then replaced to osmolarity-corrected zinc fixative (Zn-fix) solution (4.5 mM CaCl2, 52 mM ZnCl2, 32 mM Zn(CF3COO)2, 2 mM Tris, 38 mM glycine; pH 6.5, 340 mOsm/l) [34]. Subsequently, the ears were cut and placed in ice-cold Zn-fix with 1 % Triton® X-100 (Fluka) for at least 24 h. After that, the ventral part of the skin was removed together with cartilage and muscle, and the dorsal dermis was washed in TBS (140 mM NaCl, 25 mM Tris, pH 7.5) for 6 h, blocked with 0.5 % casein in TBS (blocking solution) for 2 h, and incubated for 24 h with the following primary antibodies (1 μg/ml): VE-cadherin, anti-Lyve1, biotinylated anti-collagen type IV, anti-α smooth muscle actin (1A4) (Abcam, Cambridge, UK), biotinylated anti-CD45 (BD Biosciences), biotinylated anti-perlecan (Life Technologies), anti-CD31 (GeneTex) or anti-podoplanin (R&D Systems). After washing in TBS with 0.1 % Tween® 20 (Sigma-Aldrich), the tissue was incubated with 1 μg/ml of each of the appropriate secondary antibodies (all from Abcam or Invitrogen) for another 24 h, subsequently washed in TBS and dehydrated with 70 and 100 % ethanol. Thereafter, the sample was cleared with 2:1 benzyl benzoate/benzyl alcohol solution (refractive index 1.56) supplemented with 25 mg/ml propyl gallate The tissue was mounted on a glass slide and imaged using HC PL APO 20x, NA 0.70 or HCX PL APO 63x, NA 1.40 lenses and a confocal microscope (Leica SP5). Images were analyzed with Imaris 7.1 (Bitplane AG, Zürich, Switzerland).
Statistical analysis
The effect of verteporfin dose on blood vessel leakage was tested using Fisher’s exact test. Survival curves were compared using the Mantel-Cox test using Prism software (Graphpad, San Diego, CA). Figures were assembled in Cytosketch (Cytokode, Auckland, New Zeland). Results were considered significant when p < 0.05.
Results
Lymphatic ablation with PDT in the mouse ear dermis
After injecting 100 ng Verteporfin (0.5 μl of 200 μg/ml solution) into the dorsal ear skin as described above (Fig. 1a, b), the naturally fluorescent verteporfin was observed in the draining lymphatic vessels within 2–4 min after injection. Since only a few lymphatic vessels drained the injection area, subsequent irradiation of the entire ear 5 min after injection, with a constant light dose of 3.6 J/cm2 produced both a treated region (irradiated, verteporfin-draining) as well as internal control region (irradiated, non-Verteporfin draining) (Fig. 1c). This treatment led to occlusion of the lymphatic collecting vessels in the verteporfin-draining area, but not in the control areas, as shown by intravital immunofluorescence lymphangiography (Fig. 1d, e), which detects the functional drainage basins in the whole tissue [33]. Lymphatic vessels in the areas that did not drain the photosensitizer (box, Fig. 1e) remained unaffected and served as internal controls in the imaging experiments.
Effects of photosensitizer dose and light fluence on lymphatic ablation
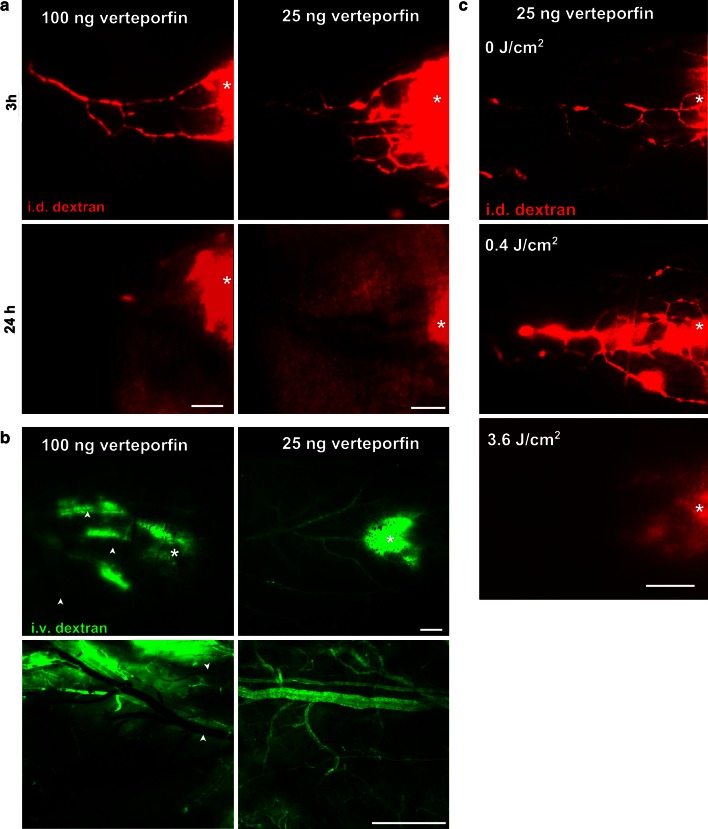
Our first aim was to determine the PDT conditions, including doses of verteporfin and light that allowed for effective and specific closure of lymphatic vasculature. With a fixed light fluence of 25 J/cm2, we found that the lowest dose of verteporfin (5 ng) did not cause any visible lymphatic damage (data not shown), while both 25 and 100 ng verteporfin led to lymphatic occlusion, as seen using fluorescence microlymphangiography 24 h post-PDT (Fig. 2a). However, intradermal administration of the high dose (100 ng) of verteporfin caused blood vessel damage as well, as seen by leakage and occlusion of blood vessels at the irradiation site (Fig. 2b, left). In contrast, blood vessels maintained patency when PDT was performed with 25 ng verteporfin, with (Fig. 2b right).
Fig. 2.
Lymphatic-specific targeting by PDT can be achieved with low doses of intradermally injected photosensitizer. a Fluorescence microlymphangiography 3 and 24 h post-PDT shows blockage of lymphatic drainage with both 100 ng (left) or 25 ng (right) of verteporfin. b Fluorescence angiography 24 h after PDT. Blood vessel integrity as revealed by intravascular injection of FITC-dextran (green) 24 h post-PDT shows vascular blockage with 100 ng verteporfin (left) in all 7 ears tested but no apparent vascular blockage with 25 ng verteporfin (right) in 6 out of 7 tested ears (p = 0.0047, Fisher exact test). In any case, dextran leaked from blood vessel at the sites of verteporfin injection (star). Top panel fluorescence angiography in dorsal ear skin imaged through the intact ear. Bottom panel high resolution fluorescence angiography in dorsal ear after surgical resection of ventral skin and cartilage. c Lymphatic drainage function 24 h after PDT (with 25 ng verteporfin) by fluorescence microlymphangiography with TRITC-dextran (red) shows that a light fluence of 3.6 J/cm2 but not 0.4 J/cm2 could occlude lymphatic drainage in the ear. Bar 500 μm
Once the lymphatic-specific dose of verteporfin was identified, we then determined the minimal light fluence required to destroy the lymphatics. As visualized 24 h after PDT with fluorescence microlymphangiography, a light fluence of 0.4 J/cm2 did not induce lymphatic ablation, but only increased the permeability of lymphatic vasculature, leading to visible dextran leakage (Fig. 2c). However, PDT with light doses of 3.6 J/cm2 and above led to a complete occlusion of the lymphatic vessels without affecting blood vessel permeability (Fig. 2b).
Lymphatic occlusion is delayed compared to lymphatic endothelial cell death
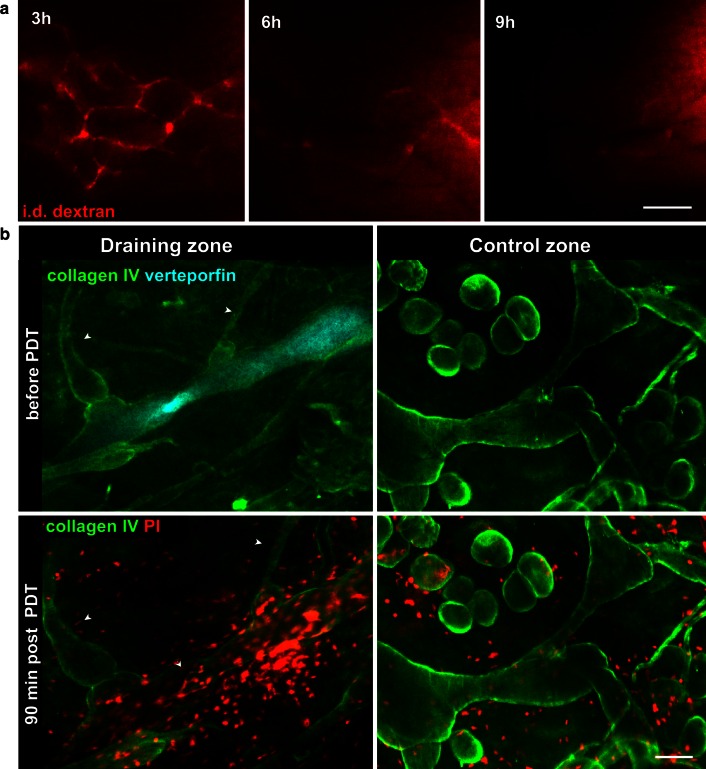
Fluorescence microlymphangiography revealed that lymphatic-specific PDT with 25 J/cm2 and 25 ng verteporfin led to lymphatic occlusion after 6–9 h in all mice tested (Fig. 3a), in contrast to blood-vessel-targeted PDT, in which irradiated, i.v.-administered verteporfin destroys local blood vessels within 3 h [23]. In both lymphatic and blood vessel-specific PDT, vessels carrying the photosensitizer are occluded when irradiated; thus, the discrepancy between the timing for blood versus lymphatic vessel occlusion may reflect different mechanisms of vessel blockade rather than administration routes of photosensitizer.
Fig. 3.
Lymphatic occlusion is delayed as compared to lymphatic endothelial cell death. a Lymphatic drainage did not become occluded until 6–9 h after PDT. b Intravital immunostaining of ear dermis with collagen IV antibody (green), verteporfin before PDT (cyan; top) and propidium iodide (PI, red; bottom) 1.5 h after PDT demonstrates large number of necrotic cells within verteporfin-draining lymphatic collectors, including nuclei located in the lymphatic valve (arrow) as well as peri-lymphatic cells (left). Few necrotic cells (PI-positive nuclei) in and around lymphatic vessels of non-draining control region (right). In addition, non-draining lymphatics (arrowheads) that were directly connected with verteporfin-draining vessels remained PI-negative. Scale bars in a and b correspond to −500 μm; in c to −100 μm
In order to determine the mechanism of PDT-dependent toxicity, the dorsal ear dermis was live-immunostained for collagen IV and propidium iodide (PI), which labels nuclei of necrotic cells (Fig. 3b). Collecting lymphatics were identified morphologically by their bulbous shapes, varying diameters, and characteristic asymmetric (pre-collectors) or bicuspid valves (collectors) located at branch points with sparse pericyte coverage as deduced from their basement membrane imprints (Supplementary Fig. 1). In addition, due to the thinness of the dorsal dermis it was possible to visualize the separate networks of nerves, lymphatics and blood vessels, which allowed us to readily identify lymphatic vessels from other structures. We further confirmed this morphological identification by co-staining with podoplanin, a lymphatic-specific marker in the skin [35].
We found that after only 1.5 h, a number of elongated nuclei within lymphatic vessels, including those near the valves, as well as peri-lymphatic cells, were necrotic (i.e., PI-positive). At the same time, cells associated with lymphatic vessels in non-draining (control) regions, including those that were directly connected to verteporfin-draining vessels, remained PI-negative (Fig. 3b). The early onset of the necrotic death of lymphatic endothelial cells or peri-lymphatic cells did not correlate with lymphatic occlusion occurring 6–9 h after PDT suggesting that at least initially intact lining of collecting lymphatic is not essential for collector function.
Regeneration of muscle coverage but not endothelial lining correlates with recovery of lymphatic drainage
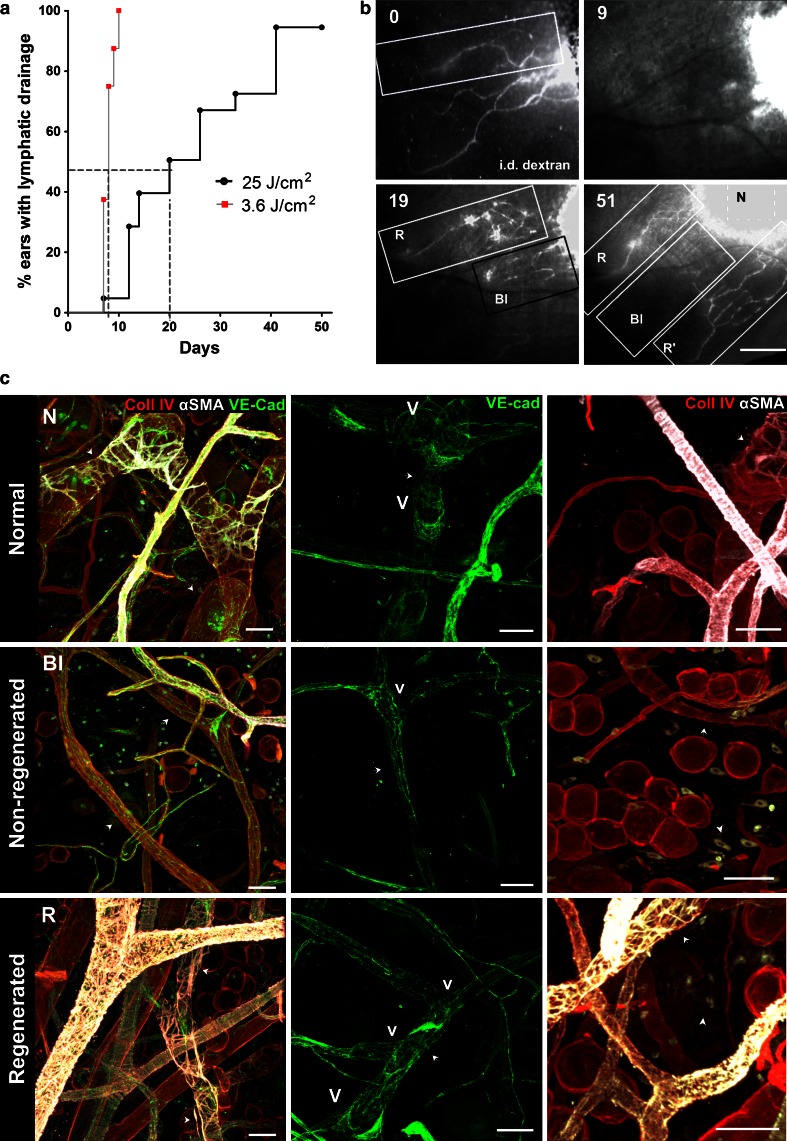
In order to investigate whether and how PDT-occluded lymphatic vessels can functionally regenerate, we followed lymphatic drainage by performing fluorescence microlymphangiography every 2–3 days over a period of 8 weeks (Fig. 4a). Lymphatic drainage was considered recovered when at least one collecting vessel could be observed draining the dextran from the site of injection to the base of the ear (Fig. 4b), even if some dysfunctional, PDT-occluded lymphatic vessels were still present in neighboring areas. Nevertheless, this definition of recovery was used for each ear because it implied that overall drainage was restored in the tissue. In 21 ears, the time to recovery of lymphatic function after PDT varied between 8 and 51 days, with median recovery times of 20 days for 25 J/cm2 and 8 days for 3.6 J/cm2 (p < 0.0001, Mantel-Cox test) (Fig. 4a).
Fig. 4.
Kinetics of functional recovery and characteristics of regenerated lymphatics after PDT. a Kaplan-Mayer plot showing percent of ears with functional lymphatic drainage versus time for 3.6 versus 25 J/cm2 dose of light, with median recovery times of 8 and 20 days, respectively (p < 0.0001). The regeneration of lymphatic drainage was concluded when at least one collector drained TRITC-dextran injected in the region that was initially injected with verteporfin. b Lymphatic drainage recovery as detected by fluorescence microlymphangiography. Fluorescence microlymphangiography with intradermally (i.d.) injected TRITC-dextran performed on the same ear immediately after PDT (0) and again on days 9, 19, and 51 days after PDT. Three distinct skin zones could be identified: zone with normal (control) (N), blocked and non-regenerated (Bl), and functionally regenerated (arrows) (R). c Representative confocal images of lymphatic vessels in each zone 51 days post-PDT (the same mouse ear as in B and Supplementary Fig. 2) after whole-mount immunostaining for collagen IV (red), α-smooth muscle actin (αSMA, white) and VE-cadherin (green). Left column αSMA-positive pericytes that are normally sparse in control lymphatic vessels (N) are absent in non-regenerated lymphatics (Bl) but overabundant around regenerated lymphatic vessels (R). Middle column Valves (v) that normally exist at the lymphatic junctions (N) were absent at the junctions of non-regenerated lymphatics (Bl) and sparsely located at the junctions of regenerated collectors (R). Right column Myofibroblasts expressing αSMA that are lacking in normal tissue (N) are present in the tissue in areas with non-regenerated (Bl) and regenerated (R) vessels (arrowheads). Arrows indicate lymphatic vessels. Bars in b correspond to: 500 μm, in c to 50 μm
Because fluorescence microlymphangiography combined with brightfield images allows specific collecting lymphatic vessels to be identified on different days by their relative position to major blood vessels (Fig. 4b and Supplementary Fig. 2a), we could compare the locations of regenerated versus original lymphatic vessels (Fig. 4b). Positions of vessels identified with fluorescence microlymphangiography could be also traced in fixed whole-mount ear preparations stained with vascular markers such as αSMA (Supplementary Fig. 2b–c). Ears were considered to have recovered lymphatic drainage function when at least one vessel could be seen draining the injected dextran. In these ears, we could also classify the major lymphatic vessels as either normal (N), which located in non-draining or control zones; blocked (Bl) or non-functional PDT-treated lymphatic vessels; or regenerated (R) which were initially blocked but functionally recovered (Fig. 4c, Supplementary Fig. 2b). Whole mount preparations were then stained for VE-cadherin (to identify endothelial cells), α-smooth muscle actin (SMA, to identify smooth muscle cells and myofibroblasts), and collagen IV (to identify basement membrane). In contrast to normal collecting vessels with sparse SMC coverage (Fig. 4c, N), regenerated lymphatic collectors were wrapped with vein-like dense SMC support (Fig. 4c, R). Surprisingly, blocked or non-regenerated lymphatic collectors were almost completely lined with lymphatic endothelium (Fig. 4c, Bl), although they were completely deprived of SMCs and their average diameter was approximately half that of control and regenerated lymphatics. Non-vascular αSMA-positive cells (myofibroblasts) were also present in the tissue stroma surrounding the occluded and regenerated lymphatics throughout the regeneration time (arrowheads in Fig. 4c). This was not paralleled with the formation of highly vascular granulation tissue or intense tissue remodeling that normally is associated with myofibroblasts-driven tissue remodeling processes [36, 37].
Discussion
Aberrant lymphangiogenesis has been associated with tumor metastasis [4, 38], transplant rejection [12–15, 39], and tumor immune tolerance [10, 40], and thus developing strategies to destroy pathological lymphatic vessels may be therapeutically useful. For example, in allogenic corneal transplant, which is the standard of care for keratopathy, blocking lymphatic drainage may reduce the probability of transplant rejection [15, 41]. In addition, therapeutic antibodies that block lymphangiogenesis can reduce tumor metastasis in mouse models [4, 38].
PDT has been used following intravenously administered photosensitizer as anti-vascular and anti-tumor therapies for many decades [42]. Photosensitizer, located in tumor cells or in the blood vessels, transfers its energy to oxygen upon irradiation, causing the formation of a toxic singlet state oxygen radical that reacts with cell membrane components, proteins, lipids and nucleic acids, leading to cell death. This, in turn, results in biphasic blood-flow stasis and vessel hyperpermeability [23]. Unlike VEGFR-3 blocking strategies, which can only prevent new lymphatic growth, PDT can also ablate pre-existing lymphatic vessels. Recently, its potential for destroying peri-tumoral lymphatics and in-transit tumor cells was demonstrated by Tammela et al. [31]. Other potential therapeutic uses of lymphatic-specific PDT might include inhibition of spread of lymphatic-trafficking parasites or pathogens as well as slowing the clearance of locally delivered drugs [43, 44]. However, the ability to selectively destroy lymphatic vessels without also destroying local blood vessels has not been established.
We demonstrated here that local and specific ablation of dermal lymphatic vessels in mouse skin could be achieved with low doses of locally (intradermally) administered verteporfin and light, and that light dose can be tuned to modulate the time of lymphatic recovery. For example, low light doses of 3.5 J/cm2 could block lymphatic drainage for approximately 1 week, while 25 J/cm2 could prolong lymphatic recovery time to 3 weeks without adversely affecting blood vessel perfusion. This indicates that the higher light fluence was more effective in killing LECs and perilymphatic SMCs without augmenting the toxic bystander effects of PDT on blood vessels. Such flexibility is not only useful for a wide range of lymphatic physiology studies, but also e.g., for exploring the roles of lymphatic transport in immunity, since leukocyte trafficking from the periphery to the lymph nodes depends on intact lymphatic drainage [3].
We also found that varying doses of intradermally administrated verteporfin had distinct effects on blood versus lymphatic vessel occlusion. The use of higher photosensitizer doses (here, 100 ng) that target both blood and lymphatic vessels might be advantageous for example before cornea keratoplasty [13], where the simultaneous destruction of both vessels are desired. The lower doses of verteporfin (25 ng and below) could specifically block lymphatic drainage while leaving the blood vasculature functional.
In addition, we observed the recruitment and persistence of myofibroblasts, in the irradiated tissue throughout the period of lymphatic remodeling. Myofibroblasts, cells characteristic for tissue healing [45] were present in the tissue even though the tissue injury was limited to the lymphatic endothelial cells of collecting lymphatics and their SMCs coverage. Interestingly, only SMC-covered regenerated lymphatic vessels were functional, and their SMC coverage was notably denser than around normal vessels. In contrast, SMCs were completely absent in non-recovered lymphatic vessels, indicating that SMC recruitment was a necessary step in functional lymphatic regeneration. The higher SMC coverage around regenerated lymphatic vessels correlated with smaller vessel diameters, potentially suggesting that the smaller diameters were due to increased contractile forces from higher SMC coverage. Dense pericyte coverage of regenerating lymphatic vessels might also help stabilize lymphatic vessels similar to their effects during sprouting angiogenesis [46].
In conclusion, PDT can be used to selectively ablate lymphatic vessels and in turn block lymphatic drainage in a specified region. By modulating light fluence and photosensitizer dose, one can control the specificity of PDT to local lymphatic vessels versus blood vessels, as well as control the time for lymphatic drainage to be restored. Mechanisms of lymphatic occlusion and restoration need further investigation, but our studies suggest that PDT-blocked vessels become re-populated with LECs and pericytes before drainage is restored.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors are grateful to Hubert van den Bergh and Georges Wagnières for helpful advice and assistance, and Filip Bochner and Jeffrey Rice, Esra Güç and Marcela Rincon-Restrepo for technical assistance. This work was supported in part by grants from the European Research Commission (206653-2 to MAS), Swiss National Science Foundation (SNSF 205320-130518 to PNS), Polish-Swiss Research Programme (PSPB-057/2010 to JG and MAS), and the J. Jacobi Trust (to PNS). This work was also supported by a grant from the European Commission 7th Framework Programme: FP7-REGPOT-2012-CT2012-316254-BASTION. AM is recipient of the Polish L’Oréal-UNESCO Awards for Women in Science.
Conflict of interest
None.
Abbreviations
- BM
Basement membrane
- PDT
Photodynamic therapy
- PI
Propidium iodide
- SMC
Smooth muscle cell
- TRITC-dextran
Tetramethyl rhodamine iso-thiocyanate-dextran
Footnotes
Angelika Muchowicz and Malgorzata Wachowska contributed equally to this work.
Contributor Information
Witold W. Kilarski, Phone: +41-21-6931411, FAX: +41-21-6939670, Email: Witold.Kilarski@epfl.ch
Patrycja Nowak-Sliwinska, Phone: +41-21-3142981, FAX: +41-21-3142982, Email: Patrycja.Nowak-Sliwinska@chuv.ch.
References
- 1.Wiig H, Swartz MA. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev. 2012;92(3):1005–1060. doi: 10.1152/physrev.00037.2011. [DOI] [PubMed] [Google Scholar]
- 2.Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9(9):960–969. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- 3.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5(8):617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 4.Tammela T, Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 2010;140(4):460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 5.Skobe M, Hamberg LM, Hawighorst T, Schirner M, Wolf GL, Alitalo K, Detmar M. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am J Pathol. 2001;159(3):893–903. doi: 10.1016/S0002-9440(10)61765-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angeli V, Ginhoux F, Llodra J, Quemeneur L, Frenette PS, Skobe M, Jessberger R, Merad M, Randolph GJ. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24(2):203–215. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Halin C, Tobler NE, Vigl B, Brown LF, Detmar M. VEGF-A produced by chronically inflamed tissue induces lymphangiogenesis in draining lymph nodes. Blood. 2007;110(9):3158–3167. doi: 10.1182/blood-2007-01-066811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerjaschki D, Regele HM, Moosberger I, Nagy-Bojarski K, Watschinger B, Soleiman A, Birner P, Krieger S, Hovorka A, Silberhumer G, Laakkonen P, Petrova T, Langer B, Raab I. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol. 2004;15(3):603–612. doi: 10.1097/01.ASN.0000113316.52371.2E. [DOI] [PubMed] [Google Scholar]
- 9.Swartz MA, Hubbell JA, Reddy ST. Lymphatic drainage function and its immunological implications: from dendritic cell homing to vaccine design. Semin Immunol. 2008;20(2):147–156. doi: 10.1016/j.smim.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Lund AW, Duraes FV, Hirosue S, Raghavan VR, Nembrini C, Thomas SN, Issa A, Hugues S, Swartz MA. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep. 2012;1(3):191–199. doi: 10.1016/j.celrep.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Huggenberger R, Siddiqui SS, Brander D, Ullmann S, Zimmermann K, Antsiferova M, Werner S, Alitalo K, Detmar M. An important role of lymphatic vessel activation in limiting acute inflammation. Blood. 2011;117(17):4667–4678. doi: 10.1182/blood-2010-10-316356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ling S, Qi C, Li W, Xu J, Kuang W. Crucial role of corneal lymphangiogenesis for allograft rejection in alkali-burned cornea bed. Clin Experiment Ophthalmol. 2009;37(9):874–883. doi: 10.1111/j.1442-9071.2009.02178.x. [DOI] [PubMed] [Google Scholar]
- 13.Cursiefen C, Cao J, Chen L, Liu Y, Maruyama K, Jackson D, Kruse FE, Wiegand SJ, Dana MR, Streilein JW. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest Ophthalmol Visual Sci. 2004;45(8):2666–2673. doi: 10.1167/iovs.03-1380. [DOI] [PubMed] [Google Scholar]
- 14.Cursiefen C, Chen L, Dana MR, Streilein JW. Corneal lymphangiogenesis: evidence, mechanisms, and implications for corneal transplant immunology. Cornea. 2003;22(3):273–281. doi: 10.1097/00003226-200304000-00021. [DOI] [PubMed] [Google Scholar]
- 15.Yamagami S, Dana MR, Tsuru T. Draining lymph nodes play an essential role in alloimmunity generated in response to high-risk corneal transplantation. Cornea. 2002;21(4):405–409. doi: 10.1097/00003226-200205000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Yin N, Zhang N, Xu J, Shi Q, Ding Y, Bromberg JS. Targeting lymphangiogenesis after islet transplantation prolongs islet allograft survival. Transplantation. 2011;92(1):25–30. doi: 10.1097/TP.0b013e31821d2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldman J, Rutkowski JM, Shields JD, Pasquier M, Cui Y, Schmoekel HG, Wiley S, Hicklin DJ, Pytowski B, Swartz MA. Cooperative and redundant roles of VEGFR-2 and VEGFR-3 signaling in adult lymphangiogenesis. FASEB J. 2007;21:1003–1012. doi: 10.1096/fj.06-6656com. [DOI] [PubMed] [Google Scholar]
- 18.Pytowski B, Goldman J, Persaud K, Wu Y, Witte L, Hicklin DJ, Skobe M, Boardman KC, Swartz MA. Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. J Natl Cancer Inst. 2005;97(1):14–21. doi: 10.1093/jnci/dji003. [DOI] [PubMed] [Google Scholar]
- 19.Rutkowski JM, Boardman KC, Swartz MA. Characterization of lymphangiogenesis in a model of adult skin regeneration. Am J Physiol Heart Circ Physiol. 2006;291(3):H1402–H1410. doi: 10.1152/ajpheart.00038.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61(4):250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90(12):889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van den Bergh H, Ballini JP (2003) Photodynamic therapy: basic principle. In: FFaK S (ed) Lasers in ophthalmology—basic, diagnostic and surgical aspects. Kugler Publications, The Hague, pp 183–195
- 23.Dolmans D, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3(5):380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 24.van den Bergh H. Photodynamic therapy of age-related macular degeneration: history and principles. Semin Ophthalmol. 2001;16(4):181–200. doi: 10.1076/soph.16.4.181.10299. [DOI] [PubMed] [Google Scholar]
- 25.Nowak-Sliwinska P, Sickenberg M, van den Bergh H. Photodynamic therapy for polypoidal choroidal vasculopathy. In: Abdelkader M, editor. Photodynamic therapy: from theory to application. Cairo: Springer; 2013. [DOI] [PubMed] [Google Scholar]
- 26.Yoon KC, You IC, Kang IS, Im SK, Ahn JK, Park YG, Ahn KY. Photodynamic therapy with verteporfin for corneal neovascularization. Am J Ophthalmol. 2007;144(3):390–395. doi: 10.1016/j.ajo.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 27.You IC, Im SK, Lee SH, Yoon KC. Photodynamic therapy with verteporfin combined with subconjunctival injection of bevacizumab for corneal neovascularization. Cornea. 2011;30(1):30–33. doi: 10.1097/ICO.0b013e3181dc81a0. [DOI] [PubMed] [Google Scholar]
- 28.Augustin A. Triple therapy for age-related macular degeneration. Retina. 2009;29(6 Suppl):S8–S11. doi: 10.1097/IAE.0b013e3181ad253d. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt-Erfurth U, Hasan T. Mechanisms of action of photodynamic therapy with verteporfin for the treatment of age-related macular degeneration. Surv Ophthalmol. 2000;45(3):195–214. doi: 10.1016/S0039-6257(00)00158-2. [DOI] [PubMed] [Google Scholar]
- 30.Weiss A, van den Bergh H, Griffioen AW, Nowak-Sliwinska P. Angiogenesis inhibition for the improvement of photodynamic therapy: the revival of a promising idea. BBA Rev Cancer. 1826;1:53–70. doi: 10.1016/j.bbcan.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Tammela T, Saaristo A, Holopainen T, Yla-Herttuala S, Andersson LC, Virolainen S, Immonen I, Alitalo K (2011) Photodynamic ablation of lymphatic vessels and intralymphatic cancer cells prevents metastasis. Sci Transl Med 3(69):69ra11 [DOI] [PubMed]
- 32.Nowak-Sliwinska P, Weiss A, Beijnum JR, Wong TJ, Ballini JP, Lovisa B, van den Bergh H, Griffioen AW. Angiostatic kinase inhibitors to sustain photodynamic angio-occlusion. J Cell Mol Med. 2012;16(7):1553–1562. doi: 10.1111/j.1582-4934.2011.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kilarski WW, Guc E, Teo JC, Oliver SR, Lund AW, Swartz MA. Intravital immunofluorescence for visualizing the microcirculatory and immune microenvironments in the mouse ear dermis. PLoS ONE. 2013;8(2):e57135. doi: 10.1371/journal.pone.0057135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kilarski WW, Petersson L, Fuchs P, Zielinski M, Gerwins P. An in vivo neovascularization assay for screening regulators of angiogenesis and assessing their effects on pre-existing vessels. Angiogenesis. 2012;15(4):643–655. doi: 10.1007/s10456-012-9287-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muthuchamy M, Zawieja D. Molecular regulation of lymphatic contractility. Ann N Y Acad Sci. 2008;1131:89–99. doi: 10.1196/annals.1413.008. [DOI] [PubMed] [Google Scholar]
- 36.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3(5):349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 37.Kilarski WW, Gerwins P. A new mechanism of blood vessel growth—hope for new treatment strategies. Discov Med. 2009;8:23–27. [PubMed] [Google Scholar]
- 38.Mumprecht V, Detmar M (2009) Lymphangiogenesis and cancer metastasis. J Cell Mol Med 13(8A):1405–1416 [DOI] [PMC free article] [PubMed]
- 39.Barker CF, Billingham RE. The role of afferent lymphatics in the rejection of skin homografts. J Exp Med. 1968;128(1):197–221. doi: 10.1084/jem.128.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swartz MA, Lund AW. Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity. Nat Rev Cancer. 2012;12(3):210–219. doi: 10.1038/nrc3186. [DOI] [PubMed] [Google Scholar]
- 41.Bock F, Maruyama K, Regenfuss B, Hos D, Steven P, Heindl LM, Cursiefen C. Novel anti(lymph)angiogenic treatment strategies for corneal and ocular surface diseases. Prog Retin Eye Res. 2013;34:89–124. doi: 10.1016/j.preteyeres.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Alexiades-Armenakas M. Laser-mediated photodynamic therapy. Clin Dermatol. 2006;24(1):16–25. doi: 10.1016/j.clindermatol.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 43.Taylor MJ, Hoerauf A, Bockarie M. Lymphatic filariasis and onchocerciasis. Lancet. 2010;376(9747):1175–1185. doi: 10.1016/S0140-6736(10)60586-7. [DOI] [PubMed] [Google Scholar]
- 44.Padera TP, Kadambi A, di Tomaso E, Carreira CM, Brown EB, Boucher Y, Choi NC, Mathisen D, Wain J, Mark EJ, Munn LL, Jain RK. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296(5574):1883–1886. doi: 10.1126/science.1071420. [DOI] [PubMed] [Google Scholar]
- 45.Kilarski WW, Samolov B, Petersson L, Kvanta A, Gerwins P. Biomechanical regulation of blood vessel growth during tissue vascularization. Nat Med. 2009;15(6):657–664. doi: 10.1038/nm.1985. [DOI] [PubMed] [Google Scholar]
- 46.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7(4):452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.