Abstract
The purpose of this study was to determine whether providing subsensory stochastic-resonance mechanical vibration to the foot soles of elderly walkers could decrease gait variability. In a randomized double-blind controlled trial, twenty nine (29) subjects engaged in treadmill walking while wearing sandals customized with three (3) actuators capable of producing stochastic-resonance mechanical vibration embedded in each sole. For each subject, we determined a subsensory level of vibration stimulation. After a 5-minute acclimation period of walking with the footwear, subjects were asked to walk on the treadmill for six (6) trials, each thirty (30) seconds long. Trials were pair-wise random: in three trials, actuators provided subsensory vibration; in the other trials, they did not. Subjects wore reflective markers to track body motion. Stochastic-resonance mechanical stimulation exhibited baseline-dependent effects on spatial stride-to-stride variability in gait, slightly increasing variability in subjects with least baseline variability and providing greater reductions in variability for subjects with greater baseline variability (p < .001). Thus, applying stochastic-resonance mechanical vibrations on the plantar surface of the foot reduces gait variability for subjects with more variable gait. Stochastic-resonance mechanical vibrations may provide an effective intervention for preventing falls in healthy elderly walkers.
Keywords: gait, aged, accidental falls, stochastic resonance
INTRODUCTION
Developing effective clinical treatments to prevent falls is a major challenge for rehabilitation medicine. Each year, one third of community-dwelling individuals over the age of 65 fall,1 and the risk for fall-related injury increases with age.2 The consequences of a fall include short-term injury such as contusions, fractures, and head injuries but also longer-term pain and disability.2 Loss of confidence and fear of falling may increase the likelihood of more sedentary lifestyles and even recurrent falls. Effective clinical interventions are needed not only for treating the sequelae of falls but also for preventing new falls.
Although falling can be attributed to a variety of intrinsic factors such as deficits in the visual, somatosensory, vestibular and musculoskeletal systems as well as to environment factors,3 loss of tactile sensation and proprioception strongly predicts the propensity to fall4—even according to multifactorial analyses.5 Age- and disease-related decreases in somatosensation (elevated sensory thresholds for mechanoreceptors) diminish the efficacy of the motor response for postural control in older adults, compromising balance.6 During both quiet and perturbed stance, disturbances in incoming information greatly affect postural stability in older adults whereas young adults can quickly and effectively recover from such disturbances.7—9 Increased postural sway is associated with increased fall risk.10 Age-related degradation of balance extends beyond quiet standing to increased fall risk during gait. Changes in gait patterns with aging include decreased speed, shorter step length, increased time spent in double-limb support, and increased stride-to-stride variability.11,12 This variability provides insight into motor control and may inform clinical evaluations and interventions.13 Although healthy walking exhibits some degree of stride-to-stride variability, the extent of variability beyond healthy ranges is the strongest predictor of falling for older adults.13—16
Restoring age- and disease-related somatosensation has proven to be a difficult challenge, but the use of stochastic resonance (SR) may be a potential solution. SR may improve stability and reduce fall risk by enhancing transmission of information through human tactile and proprioceptive sensory networks.17,18 The low-level noise sensitizes the system to otherwise undiscernibly small signals or stimuli.18 The presence of subsensory mechanical noise under the soles of the feet significantly reduces postural sway during quiet standing, manifesting in either a mean reduction in variability or a baseline-dependent reduction of variability, in which SR has greater stabilizing effect for those with more variable posture.19—21 Since a large percentage of older adults fall while walking,22 it is critical to determine if SR is effective during walking as well.
Research in gait has already shown that SR mechanical stimulation produces baseline-dependent reductions in variability of temporal gait parameters such as stride time, stance time, and swing time.23 However, spatial gait variability, defined by stride length and step width, may predict fall risk independently of or better than temporal gait variability.15,16,24,25 That is, spatial gait variability constitutes an important component of the predicting who may suffer falls and when they may do so. So, the efficacy of SR mechanical stimulation for preventing falls would be stronger in a test of spatial gait variability. The aim of this study was to test whether SR mechanical stimulation exerts a baseline-dependent reduction of spatial gait variability. We hypothesized that, similar to previous findings in standing,21 SR mechanical stimulation will produce a graded, baseline-dependent reduction in step-width and stride-length variability.
METHODS
Subjects
A total of 29 healthy elderly subjects consented to participate and completed the experiment (Table 1). Subjects had no history of musculoskeletal, neurological, or cardiopulmonary disorders, and were not taking medications that might alter sensation or alertness. The experimental protocol was approved by the University of Virginia Institutional Review Board for Health Science Research, and written informed consent was obtained from each subject before testing. All testing was completed in the Gait and Motion Analysis Laboratory in the Department of Physical Medicine and Rehabilitation at the University of Virginia.
Table 1.
Subject demographics
| Subjects | n | Age (years) | Height (cm) | Mass (kg) | Stride Length (cm) |
|---|---|---|---|---|---|
| Males | 16 | 72.6±3.8 | 175.0±6.8 | 78.3±11.1 | 80.3±8.6 |
| Females | 13 | 70.9±4.5 | 161.8±7.3 | 65.8±16.2 | 81.7±10.1 |
| Combined | 29 | 71.9±4.1 | 169.1±9.6 | 72.7±14.8 | 80.7±8.9 |
Protocol
Three electromagnetic actuators (0.31 inches thick, 1.2 inches in diameter; C-2 Actuators, Engineering Acoustics, Winter Park, FL, USA) were embedded into three locations in the soles (heel, first and fifth metatarsal) of several pairs of sandals. Low-voltage analog signals drove actuators to transmit a 0-100 Hz white-noise vibration to the skin surface through the sandal material. Actuators were connected to a computer and power amplifier by wires fed from the sandals through a harness on the subject’s waist. A LabVIEW (National Instruments, Austin, TX, USA) user interface allowed independent control of the analog signal level to each actuated sandal.
Each subject was fit with a pair of actuated sandals, available in integer sizes ranging from Women’s USA size 6 to Men’s USA size 13. To ensure subthreshold stimulation during both swing and stance phases, experimenters set independent sub-sensory stimulation thresholds for each foot under each of three possible leg positions: 1) normal standing, 2) weight-bearing limb in one-legged standing, and 3) non-weight-bearing limb in one-legged standing. Setting thresholds involved raising stimulation levels to maximum and then decreasing them in a modified 4-2-1 algorithm26 until the subject could no longer feel the vibration. Each sub-sensory threshold was verified by repeating this procedure twice for each phase. The mechanical stimulation used in the experiment was set to 90% of these threshold values, a level subsensory to the subject. These thresholds were then used for the subsequent trials.
Subjects had five minutes to acclimate to treadmill walking at 1.4 m/s, comparable to comfortable gait speed in the elderly.27 While walking, threshold values were varied according to the appropriate phase so as to maintain the desired subthreshold stimulation level throughout the gait cycle. For this purpose, the sandals were equipped with FlexiForce (Tekscan, Inc., South Boston, MA, USA) force sensors embedded under the heel and forefoot to register heel strikes and toe-offs and allow detection of the current phase throughout gait. Each subject was asked to maintain the constant speed of 1.4 m/s, walking normally for all trials. Both subject and experimenter were blind to the stimulation condition during the protocol. Participants were asked whether they could distinguish trials with stimulation from trials without stimulation, but they were unable to distinguish any such differences. Accuracy of data collection and protocol in this double-blind design was guaranteed by a number of precautions: extensive testing of equipment and piloting of the protocol before trials began, inspection of all equipment before and after each subject’s participation, as well as inspection of equipment during threshold-setting. Trials were pair-wise randomized – three null (no stimulation) and three with stimulation for a total of six 30-second trials in each session. Stimulation remained on for the entire 30 seconds of the active (stimulation on) trials and off for the entire null (no stimulation) trials.
Data Collection
Three-dimensional position data of the feet were collected using a 10-camera VICON 624 Motion Capture system (240 Hz; Vicon Peak, Lake Forest, CA, USA). Stride-by-stride spatial gait parameters—specifically, step width and step length–were calculated using the distance between the right and left ankle joint centers. Heel strikes were identified via vertical ground reaction force using an instrumented AMTI treadmill.
Data Analysis
Individual stride-by-stride gait parameters were obtained for 15 consecutive strides from each of the six total trials using in-house algorithms developed using LabVIEW. Trials were separated into two groups: experimental (SR stimulation on) and null (SR stimulation off). Variability was calculated as the coefficient of variation (CV = standard deviation/mean) of spatial gait parameters.16 Stride-to-stride CVs for both stride length and step width were calculated for each subject over all experimental trials and over all null trials. Data for all complete stimulation strides and all complete null strides were pooled for each individual’s CV.
Maximum likelihood (ML) linear mixed-effects modeling was used to test sample-general regularities in the face of significant individual differences.28 Similar to more conventional regression techniques, ML mixed-effect modeling treats a given dependent measure as the sum of independent predictors with different weightings, depending on their relative importance in changing the dependent measure. These weightings, called B-coefficients, demonstrate how each predictor affects the dependent measure: Predictors with bigger B-coefficients have stronger effects on the dependent measure, and whereas predictors with positive B-coefficients lead to increases in the dependent measure, predictors with negative B-coefficients lead to decreases in the dependent measures.
Two mixed-effect models tested each of two possible kinds of effects due to SR mechanical stimulation, above and beyond any differences between gait parameters. The use of a gait-parameter predictor allowed pooling of variability from both gait parameters in the same model, thereby permitting concise test for parameter differences in the same model while also testing more general effects across gait parameters. Model 1 tested for overall mean changes in CV due to SR mechanical stimulation. The dependent measure for Model 1 was CV, and there were three predictors: stimulation (Stim, coded 0 or 1 for absence or presence of stimulation, respectively) and gait parameter (GP, coded 0 or 1 for step width or stride length, respectively), and the interaction of Stim and GP. Model 2 tested whether the change in CV due to stimulation depended on baseline variability. So, the dependent measure was the difference between variability with stimulation and variability without it (i.e., DiffCV equaling CVStim-CVBaseline). Model 2 contained three predictors: baseline variability (CVBaseline), GP, and the interaction of CVBaseline and GP. It is important to emphasize that including the gait-parameter predictor GP guaranteed that any significant differences attributed to Stim or CVBaseline predictors would not be due to differences in gait-parameter.
RESULTS
Overall Mean Reduction in Stride-to-Stride Variability
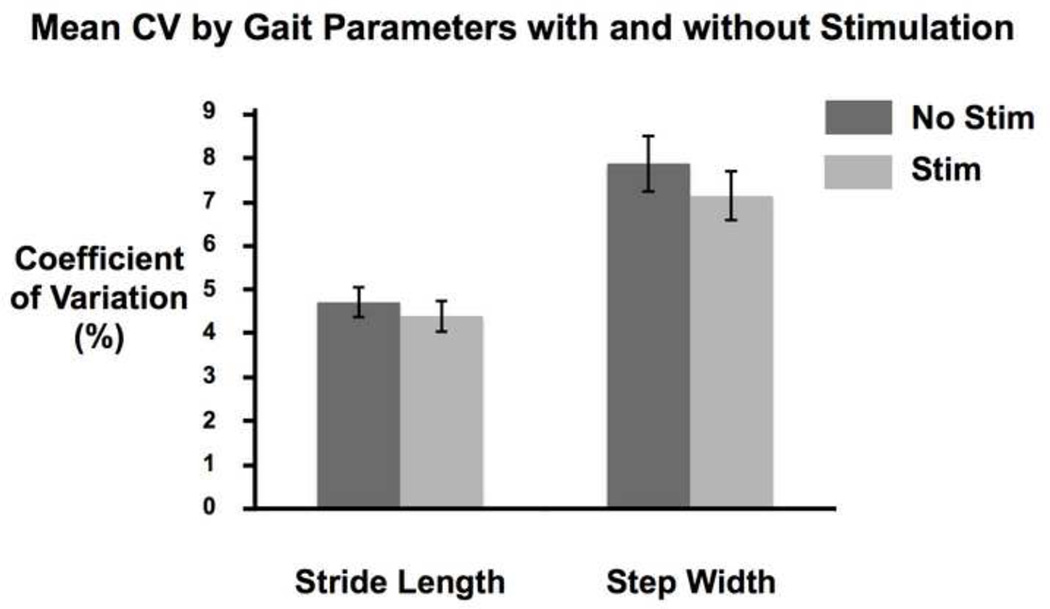
The average CV for both gait parameters decreased with stimulation (Figure 1; Table 2). However, Model 1 indicated that the negative effect of Stim (B = −.53, SE = .28, p = .20) was not significant (top of Table 3). Stride length was less variable than step width, as indicated by the negative effect of GP (B = −2.96, SE = .56, p < .0001).
Figure 1.
Plot of means and standard errors for coefficient of variation for stride length and step width, with and without stimulation.
Table 2.
Means and standard errors for coefficient of variation for step width and stride length without stimulation and with stimulation.
| Gait Parameter | Stimulation | M | SE |
|---|---|---|---|
| Stride Length | Off | 4.69 | .34 |
| On | 4.37 | .35 | |
| Step Width | Off | 7.86 | .64 |
| On | 7.12 | .56 |
Table 3.
Coefficients from Model 1 testing effects on CV and from Model 2 testing effects on DiffCV.
| Model | ||||
|---|---|---|---|---|
| 1 | Predictor | B | SE | p |
| Intercept | 7.86 | .49 | < .0001 | |
| Stim | −.73 | .56 | .20 | |
| GP | −3.17 | .56 | < .0001 | |
| Stim*GP | .41 | .80 | .61 | |
| 2 | Predictor | B | SE | p |
| Intercept | 2.60 | .83 | < .01 | |
| CVBaseline | −.42 | .10 | < .001 | |
| GP | −1.78 | 1.17 | .14 | |
| CVBaseline*GP | .18 | .20 | .37 | |
Baseline-Dependent Reduction in Stride-to-Stride Variability
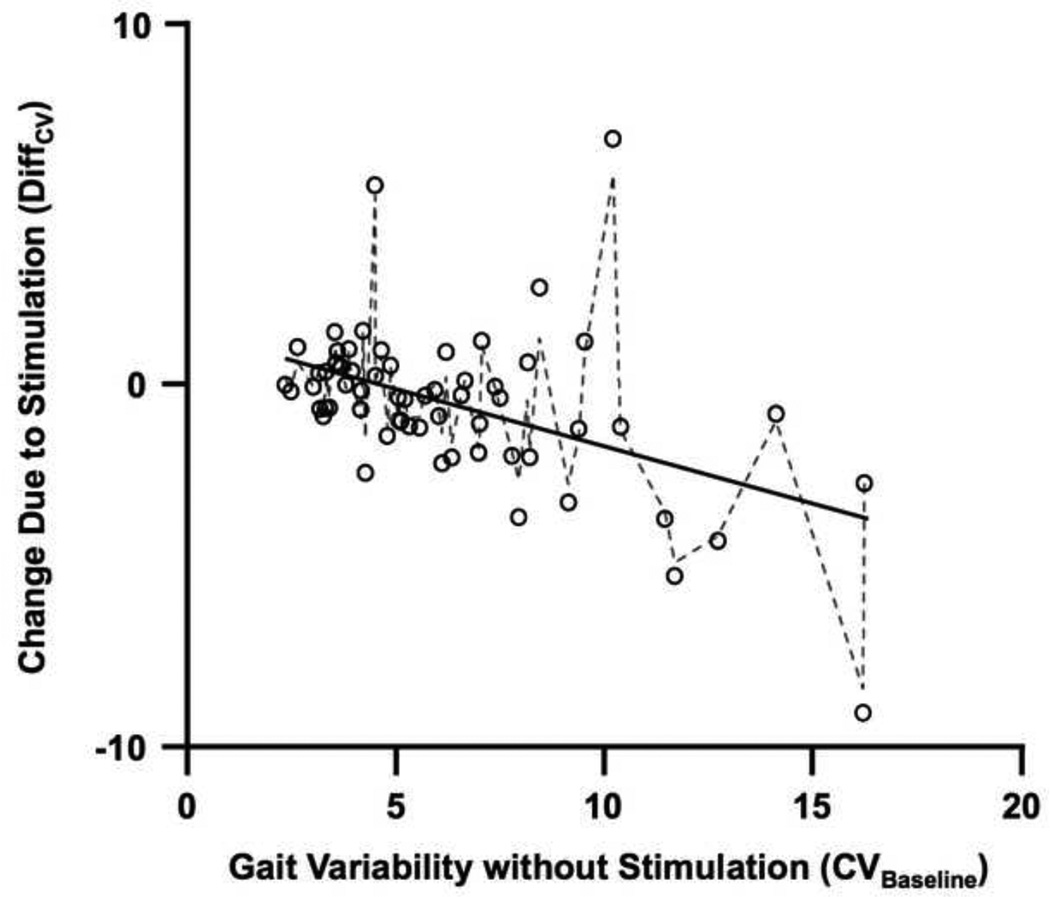
The change in variability due to stimulation (DiffCV) depended on baseline variability (CVBaseline) (Figure 2). The negative effect of CVBaseline (B = −.42, SE = .10, p < .001) indicated that DiffCV decreased linearly as CVBaseline increased (bottom of Table 3). The positive intercept (B = 1.75, SE = .80, p < .05) indicated that change in variability due to stimulation (DiffCV) was positive for subjects with least variable gait. So, stimulation increased CV for subjects with the least variable gait during control trials. For subjects with progressively more variable baseline gait, stimulation had a progressively reductive effect on gait variability. For baseline variability greater than 4.6%, predicted values of DiffCV become negative (see Figure 2). Model predictions of DiffCV from Model 2 correlated measured values of DiffCV correlated at r(27) = .80, p < .0001. The standardized effect size of the predictor DiffCV was large, Cohen’s ω2 =.37 and Cohen’s f = .77. The absence of a significant effect for GP indicated that the change due to stimulation did not differ across gait parameters. The absence of a significant interaction of GP and DiffCV indicates that the baseline-dependent change in variability with stimulation did not differ across gait parameters.
Figure 2.
Plot illustrating measured changes in stride-to-stride variability due to SR mechanical stimulation (DiffCV; circles) as well as values of DiffCV predicted by Model 2 (lines) along measured values of baseline stride-to-stride variability (CVBaseline). The solid line indicates values predicted by fixed effects only and the dashed line indicates the values predicted by the combination of fixed and random effects. Greater baseline stride-to-stride variability increases towards the right on the x-axis. Change in stride-to-stride variability is indicated by values of DiffCV below 0 on the y-axis. This plot pools information for both step-width and stride-length variability.
DISCUSSION
We hypothesized that SR mechanical stimulation would produce a graded, baseline-dependent reduction in spatial stride-to-stride variability of gait, indicated by a reduction in coefficients of variation for step width and stride length. The results of this study support this hypothesis. For elderly subjects with greater gait variability, the application of SR mechanical stimulation to the soles of the feet decreased stride-to-stride variability in step width and stride length. This effect was not different for the two spatial gait parameters. SR mechanical stimulation appeared to increase stride-to-stride variability slightly for subjects with the least variable gait parameters, with this increase diminishing to zero as baseline stride-to-stride variability increased to 4.6% coefficient of variation. As baseline stride-to-stride variability increased beyond 4.6% coefficient of variation, SR mechanical stimulation reduced spatial variability by progressively greater margins. These results are consistent with previous findings about the role of SR mechanical stimulation in clinical interventions. Baseline-dependent reductions in variability are consistent with previous findings that SR mechanical stimulation may reduce postural variability and that this reduction depends on baseline postural variability when there is no SR mechanical stimulation.19,20,21
The present results indicate that simple comparisons of mean variability with and without enhanced plantar-surface sensitivity are not sufficiently exhaustive for assessing the effect of SR mechanical stimulation. These comparisons of means may return null effects (e.g., Model 1) when the effect of SR mechanical stimulation depends on baseline variability (e.g., Model 2). This disparity reflects individual differences interfering with standard OLS modeling. The dependence on individual differences is brought into focus by the significant effect of baseline variability (CVBaseline) on the effect of SR mechanical stimulation (DiffCV; Model 2; Figure 2). The effect of individual differences is thus crucial for properly assessing the actual clinical efficacy of SR interventions; it may be equally important for fine-tuning the clinical application of SR mechanical stimulation on a patient-by-patient basis.
This investigation constitutes an early step towards understanding the potential benefit of SR mechanical stimulation and fall risk in the elderly. Although previous work had shown that SR mechanical stimulation can reduce variability in temporal gait parameters,23 temporal variability in gait has been a weaker predictor of fall risk than spatial variability in gait.24,25 Because spatial gait variability is a stronger predictor of falls,13—16,24,25 the present evidence suggests that SR mechanical stimulation may stabilize stride length and step width, crucial gait parameters for preventing falls in healthy elderly subjects. Whereas reduced temporal variability due to SR mechanical stimulation is simply suggestive of possible clinical relevance to gait, the present findings may provide more confidence in the clinical efficacy of SR mechanical stimulation in minimizing fall risk.
The baseline-dependent effect of SR mechanical stimulation on spatial gait parameters may be crucial for the clinical efficacy of SR in preventing falls. It is important to note that always reducing gait variability may actually be unsafe, and that fall risk is associated both with too much as well as too little spatial variability in gait.16 Variability can often be a signature of healthy, safe, effective motor function.13,29,30 So, although it might intuitively seem preferable for a clinical intervention preventing falls to minimize variability in all subjects, reducing variability in subjects who already exhibit the least variability might have the opposite effect of inducing rather than preventing falls. Perhaps to ensure safe, fall-free gait, the least variable walkers should be given a bit more variability, and the reductions of variability should be reserved for the more variable walkers. In this sense, the fact that SR mechanical stimulation appeared to increase variability for the least variable walkers and reduce variability for the more variable walkers is encouraging. That is, baseline-dependent changes in gait variability suggest clinical efficacy of SR far better than would simple overall-mean reduction in variability.
An important limitation to this study is that the gait data were collected on a treadmill at a fixed speed. The present experimental design allowed test of spatial parameters of gait while walking speed was constrained by the treadmill speed. It will be worth testing for spatial effects of SR mechanical stimulation during overground walking. Future studies might also involve a broader range of subjects. Subjects in this study were healthy, active older adults with unimpaired sensorimotor function. Priplata and colleagues21 reported that subjects with greater sensorimotor impairment displayed the most benefit from SR stimulation in standing balance experiment. The baseline-dependent effect of SR mechanical stimulation reported here may extend similarly into the range of baseline variability for subjects with sensorimotor impairment, including frail elderly and those with peripheral neuropathy. Future studies might also include younger age groups to determine whether the baseline-dependence reduction in variability by SR mechanical stimulation operates for young adults or only applies for the elderly.
In summary, SR mechanical stimulation provides baseline-dependent reductions in gait variability, suggesting clinical applications for preventing falls in the elderly as well as new research directions for SR effects.
Applying white-noise stimulation to the soles of the feet can stabilize posture. This stabilizing effect is greatest for people with most variable posture. We tested white-noise effects on spatial parameters of gait in elderly walkers. White-noise stabilized parameters for those walkers with more variable gait. Insoles applying white noise to the soles of the feet may reduce fall risk.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ganz DA, Bao Y, Shekelle PG, Rubenstein LZ. Will my patient fall? J Am Med Assoc. 2007;297:77–86. doi: 10.1001/jama.297.1.77. [DOI] [PubMed] [Google Scholar]
- 2.Kannus P, Sievanen H, Palvanen M, Jarvinen T, Pakkari J. Prevention of falls and consequent injuries in elderly people. Lancet. 2005;366:1885–1893. doi: 10.1016/S0140-6736(05)67604-0. [DOI] [PubMed] [Google Scholar]
- 3.Gillespie LD, Gillespie WJ, Robertson MC, Lamb SE, Cumming RG, Rowe BH. Interventions for preventing falls in elderly people. Cochrane Database of Systematic Reviews. 2003;(Issue 4) doi: 10.1002/14651858.CD000340. Art. No. CD000340. [DOI] [PubMed] [Google Scholar]
- 4.Richardson JK, Ashton-Miller JA. Peripheral neuropathy: an often-overlooked cause of falls in the elderly. Postgraduate Medicine. 1996;99:16172. [PubMed] [Google Scholar]
- 5.Delbaere K, Close JCT, Heim J, Sachdev PS, Brodaty H, Slavin MJ, Kochan NA. A multifactorial approach to understanding fall risk in older people. J Amer Geriatr Soc. 2010;58:1679–1685. doi: 10.1111/j.1532-5415.2010.03017.x. [DOI] [PubMed] [Google Scholar]
- 6.Judge JO, King MB, Whipple R, Clive J, Wolfson LI. Dynamic balance in older persons: effects of reduced visual and proprioceptive input. J Gerontology Series A: Biol Sci Med Sci. 1995;50:M263–M270. doi: 10.1093/gerona/50a.5.m263. [DOI] [PubMed] [Google Scholar]
- 7.Collins JJ, DeLuca CJ, Burrows A, Lipsitz LA. Age-related changes in open-loop and closed-loop postural control mechanisms. Exper Brain Res. 1995;104:480–492. doi: 10.1007/BF00231982. [DOI] [PubMed] [Google Scholar]
- 8.Teasdale N, Stelmach GE, Breunig A. Postural sway characteristics of the elderly under normal and altered visual and support surface conditions. J Gerontol. 1991;46A(6):B238–B244. doi: 10.1093/geronj/46.6.b238. [DOI] [PubMed] [Google Scholar]
- 9.Laughton CA, Slavin M, Katdare K, Nolan L, Bean JF, Kerrigan DC, Phillips E, Lipsitz LA, Collins JJ. Aging, muscle activity and balance control: physiologic changes associated with balance impairment. Gait Posture. 2003;18:101–108. doi: 10.1016/s0966-6362(02)00200-x. [DOI] [PubMed] [Google Scholar]
- 10.Campbell AJ, Borrie MJ, Spears GF. Risk factors for falls in a community-based prospective study of people 70 years and Older. J Gerontol. 1989;44:M112–M117. doi: 10.1093/geronj/44.4.m112. [DOI] [PubMed] [Google Scholar]
- 11.Lord SR, Lloyd DG, Li SK. Sensori-motor function, gait patterns and falls in community-dwelling women. Age Ageing. 1996;25:292–269. doi: 10.1093/ageing/25.4.292. [DOI] [PubMed] [Google Scholar]
- 12.Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82:1050–1055. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- 13.Hausdorff JM. Gait dynamics, fractals and falls: Finding meaning in the stride-to-stride fluctuations of human walking. Human Mov Sci. 2007;26:555–589. doi: 10.1016/j.humov.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guimaraes RM, Isaac B. Characteristics of the gait in old people who fall. International Rehabil Med. 1981;10:147–156. doi: 10.3109/09638288009163984. [DOI] [PubMed] [Google Scholar]
- 15.Owings TM, Grabiner MD. Step width variability, but not step length variability or step time variability, discriminates gait of healthy young and older adults during treadmill locomotion. J Biomech. 2004;37:935–938. doi: 10.1016/j.jbiomech.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Brach JS, Berlin JE, VanSweringen JM, Newman AB, Studenski SA. Too much or too little step width variability is associated with a fall history in older persons who walk at or near normal gait speed. J Neuroeng Rehabil. 2005;2:21. doi: 10.1186/1743-0003-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins JJ, Imhoff TT, Grigg P. Noise-enhanced information transmission in rat SA1 cutaneous mechanoreceptors via aperiodic stochastic resonance. J Neurophysiol. 1996;76:642–645. doi: 10.1152/jn.1996.76.1.642. [DOI] [PubMed] [Google Scholar]
- 18.Collins JJ, Imhoff TT, Grigg P. Noise-enhanced tactile sensation. Nature. 1996;383:770. doi: 10.1038/383770a0. [DOI] [PubMed] [Google Scholar]
- 19.Priplata A, Niemi J, Salen M, Harry J, Lipsitz LA, Collins JJ. Noise-enhanced human balance control. Physical Review Letters. 2002;89:238101–238104. doi: 10.1103/PhysRevLett.89.238101. [DOI] [PubMed] [Google Scholar]
- 20.Priplata AA, Niemi JB, Harry JD, Lipsitz LA, Collins JJ. Vibrating insoles and balance control in elderly people. Lancet. 2003;362:1123–1124. doi: 10.1016/S0140-6736(03)14470-4. [DOI] [PubMed] [Google Scholar]
- 21.Priplata AA, Patritti BL, Niemi JB, Hughes R, Gravelle DC, Lipsitz LA, Veves A, Stein J, Bonato P, Collins JJ. Noise-enhanced balance control in patients with diabetes and patients with stroke. Ann Neurol. 2006;59:4–12. doi: 10.1002/ana.20670. [DOI] [PubMed] [Google Scholar]
- 22.Menz HB, Lord SR, Fitzpatrick RC. Age-related differences in walking stability. Age Ageing. 2003;32:137–142. doi: 10.1093/ageing/32.2.137. [DOI] [PubMed] [Google Scholar]
- 23.Galica AM, Kang HG, Priplata AA, D’Andrea SE, Starobinets OV, Sorond FA, Cupples LA, Lipsitz LA. Subsensory vibrations to the feet reduce gait variability in elderly fallers. Gait Posture. 2009;30:383–387. doi: 10.1016/j.gaitpost.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brach JS, Studenski S, Perera S, VanSwearingen JM, Newman AB. Stance time and step width variability have unique contributing impairments in older persons. Gait Posture. 2008;27:431–439. doi: 10.1016/j.gaitpost.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear? J Am Geriatr Soc. 1997;45:313–320. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 26.Dyck PJ, O’Brien PC, Kosanke JL, Gillen DA, Karnes JL. A 4,2, and 1 stepping algorithm for quick and accurate estimation of cutaneous sensation threshold. Neurol. 1993;43:1508–1512. doi: 10.1212/wnl.43.8.1508. [DOI] [PubMed] [Google Scholar]
- 27.Bohannon RW. Comfortable and maximum walking speed of adults aged 20–79: Reference values and determinants. Age Ageing. 1997;26:15–19. doi: 10.1093/ageing/26.1.15. [DOI] [PubMed] [Google Scholar]
- 28.Singer JD, Willet JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 29.Hausdorff JM, Zemany J, Peng CK, Goldberger AL. Maturation of gait dynamics: Stride-to-stride variability and its temporal organization in children. J Appl Physiol. 1999;86:104–1047. doi: 10.1152/jappl.1999.86.3.1040. [DOI] [PubMed] [Google Scholar]
- 30.Stephen DG, Hajnal A. Transfer of calibration between hand and foot: Functional equivalence and fractal fluctuations. Attent Percept Psychophys. 2011;73:1302–1328. doi: 10.3758/s13414-011-0142-6. [DOI] [PubMed] [Google Scholar]