Abstract
OBJECTIVES:
Prognosis for patients with cirrhosis admitted to intensive care unit (ICU) is poor. ICU prognostic models are more accurate than liver-specific models. We identified predictors of mortality, developed a novel prognostic score (Royal Free Hospital (RFH) score), and tested it against established prognostic models and the yet unvalidated Chronic Liver Failure-Sequential Organ Failure Assessment (CLIF-SOFA) model.
METHODS:
Predictors of mortality were defined by logistic regression in a cohort of 635 consecutive patients with cirrhosis admitted to ICU (1989–2012). The RFH score was derived using a 75% training and 25% validation set. Predictive accuracy and calibration were evaluated using area under the receiver operating characteristic (AUROC) and goodness-of-fit χ2 for the RFH score, as well as for SOFA, Model for End-Stage Liver Disease (MELD), Acute Physiology and Chronic Health Evaluation (APACHE II), and Child-Pugh. CLIF-SOFA was applied to a recent subset (2005–2012) of patients.
RESULTS:
In-hospital mortality was 52.3%. Mortality improved over time but with a corresponding reduction in acuity of illness on admission. Predictors of mortality in training set, which constituted the RFH score, were the following: bilirubin, international normalized ratio, lactate, alveolar arterial partial pressure oxygen gradient, urea, while variceal bleeding as indication for admission conferred lesser risk. Classification accuracy was 73.4% in training and 76.7% in validation sample and did not change significantly across different eras of admission. The AUROC for the derived model was 0.83 and the goodness-of-fit χ2 was 3.74 (P=0.88). AUROC for SOFA was 0.81, MELD was 0.79, APACHE II was 0.78, and Child-Pugh was 0.67. In 2005–2012 cohort, AUROC was: SOFA: 0.74, CLIF-SOFA: 0.75, and RFH: 0.78. Goodness-of-fit χ2 was: SOFA: 6.21 (P=0.63), CLIF-SOFA: 9.18 (P=0.33), and RFH: 2.91 (P=0.94).
CONCLUSIONS:
RFH score demonstrated good discriminative ability and calibration. Internal validation supports its generalizability. CLIF-SOFA did not perform better than RFH and the original SOFA. External validation of our model should be undertaken to confirm its clinical utility.
INTRODUCTION
Patients with cirrhosis are admitted to intensive care units (ICUs) for complications of portal hypertension such as variceal bleeding or hepatic encephalopathy, or for sepsis resulting from spontaneous bacterial peritonitis, chest or urinary tract infections, culminating in multiple-organ failure in a large proportion of patients. Sepsis in the presence of cirrhosis is associated with poor prognosis; mortality rates increase with increasing number of failing organs (1,2). Despite some recent evidence suggesting improving outcomes in acutely ill patients with cirrhosis, in part due to the better understanding of disease processes and improving ICU care (3,4), the overall prognosis for patients with cirrhosis admitted to ICU remains poor with mortality rates ranging from 44 to 81% (5). Considering the high cost of adjunctive treatment modalities (6) and the limited availability of ICU beds, the task of identifying patients who are most likely to benefit from aggressive treatment is imperative, and poses great challenge for the clinicians involved in the care of these patients (5,7). Unfortunately, the quest for an accurate prognostic score applicable to these patients in clinical practice has remained elusive (5).
The Child-Pugh score and the Model for End-Stage Liver Disease (MELD) are widely utilized for grading of the severity of liver disease and for liver graft allocation for patients with decompensated cirrhosis. They are also used to assess prognosis for patients with cirrhosis admitted to ICU. However, general ICU prognostic scores, such as the Acute Physiology and Chronic Health Evaluation (APACHE) and the Sequential Organ Failure Assessment (SOFA) scores, have proven more accurate than the currently used liver-specific models in predicting mortality, despite the fact that they are not derived specifically from populations of patients with cirrhosis (3,8,9,10,11,12,13,14,15,16). This finding reinforces the contribution of multi-organ dysfunction in determining outcome, irrespective of the nature of underlying disease, and holds true even for patients with cirrhosis.
There are only three prognostic models (3,9,15) that have been developed from cohorts (n=111, 196, and 312) of ICU patients with cirrhosis. Some incorporate parameters such as serum sodium and lactate levels, which are highly predictive of outcome in the context of acute deterioration of chronic liver disease. Although most of these models demonstrate good discriminative ability, their calibration, i.e., the concordance between predicted and observed outcome, is modest at best. Therefore, to date none of the proposed models have been widely used.
Recently, a modification of SOFA, the Chronic Liver Failure-SOFA (CLIF-SOFA) score, has been proposed for patients with cirrhosis hospitalized for acute decompensation (17). According to this score, acute-on-chronic-liver-failure (ACLF) was defined, including three ACLF grades (ACLF 1–3). ACLF 1 includes (a) patients with single renal failure (creatinine≥177 μmol/l), (b) patients with single-organ failure and creatinine from 133 to 168 μmol/l and/or mild-to-moderate hepatic encephalopathy, or (c) patients with single-cerebral failure (hepatic encephalopathy grade 3 or 4) and creatinine from 133 to 168 μmol/l. ACLF 2 includes patients with two failing organs, and ACLF 3 patients with three or more failing organs. The 28-day mortality was 4.7% in those without ACLF, 22.1% in grade 1 ACLF, 32% in grade 2 ACLF, and 76.7% in grade 3 ACLF. The performance of CLIF-SOFA has not as yet been validated in cohorts other than the initial one from which it was derived.
The aims of our study were the following: (a) to identify predictors of mortality in a cohort of patients with cirrhosis admitted to ICU, (b) to generate a novel calibrated prognostic score for these patients (Royal Free Hospital (RFH) score), and (c) to compare the performance of the novel model to that of established liver-specific (Child-Pugh, MELD, and MELD-sodium), and general ICU prognostic models (APACHE II and SOFA) as well as the CLIF-SOFA score in more recent cohort (2005–2012).
METHODS
The study population included consecutive patients with cirrhosis admitted to ICU between 1989 and 2012 at the RFH, a tertiary referral center in the United Kingdom for liver diseases and liver transplantation. The diagnosis of cirrhosis was established by presence of portal hypertension (ascites, gastro-esophageal varices, hepatic encephalopathy, and so on), liver imaging studies, and liver biopsy if performed. Patients with acute liver failure, post-liver transplantation or other postoperative hepatobiliary admissions to ICU were excluded. All patients received optimal treatment according to local guidelines, including regular screening for infections according to local ICU protocols.
Admissions to ICU were divided into quartiles corresponding to four study periods: 1989–1996 (n=156), 1997–2004 (n=158), 2005–2008 (n=160), and 2009–2012 (n=161). Data on age, gender, etiology of liver disease, indication for ICU admission, length of ICU stay, and in-hospital mortality were available for all patients. Laboratory parameters recorded on the day of admission to the ICU included white blood cell count, platelet count, international normalized ratio (INR), urea, creatinine, sodium, potassium, albumin, bilirubin, lactate, pH, partial arterial pressure of oxygen (PaO2) and carbon dioxide (PaCO2), inspired oxygen concentration (FiO2), oxygenation index (FiO2/PaO2), and alveolar arterial partial pressure oxygen gradient (A-a gradient).
The severity of liver disease was graded by the Child-Pugh, MELD, and MELD-sodium scores, using parameters on the day of admission to the ICU. The acute physiology scores used were APACHE II and SOFA, as these two are consistently reported as the best prognostic scores for patients with cirrhosis admitted to ICU (5,18).
For the subset of patients admitted between 2005 and 2012, the CLIF-SOFA score was also calculated and patients were classified as ACLF 0–3. The number of failing organ systems (FOSs) was assessed using both the SOFA (SOFA≥3 for failing organs) and the CLIF-SOFA criteria as described previously (17) (FOS-SOFA and FOS-CLIF, respectively).
In-hospital mortality, rather than ICU mortality, was assessed, in order to include patients who died after discharge to the ward, i.e., patients for whom further aggressive treatment was withdrawn because of futility, and because of low chances of recovery.
Statistical analysis
Data were expressed as mean and s.d. for continuous and normally distributed variables, median and range for continuous variables without normal distribution, or frequencies (percentage) for categorical variables. We compared survivors with non-survivors with regard to demographic and laboratory variables, as well as liver-specific and acute physiology scores. For comparisons, the χ2-test was used for categorical variables; the Student's t-test and the Mann–Whitney test was used for continuous variables with or without normal distribution, respectively. For comparisons between more than two groups, the Kruskal–Wallis test was applied. Univariate analysis was used to identify parameters associated with in-hospital mortality. Multiple logistic regression (backward: likelihood ratio (LR) method) was used for multivariate analysis, and the coefficients derived were used to generate a prognostic model (RFH score). The RFH score was developed and validated using a training set (75% of the population) and a validation set (25%). The two sets were selected using a random number generator and checked for distribution of the year of admission. The performance of established prognostic scores, as well as the RFH score, was evaluated: the area under the receiver operating characteristic (AUROC) curve assessed the discriminative ability, whereas the Hosmer–Lemeshow goodness-of-fit χ2-test assessed the calibration of each model, with lower χ2 and higher P values indicating better calibration. The Youden index was used to identify the optimal cutoff point for each model, and the corresponding sensitivity, specificity, PPV (positive predictive value), NPV (negative predictive value), LR positive (LR+) and negative (LR−) were calculated. The level of statistical significance was set at P≤0.05. Statistical analysis was performed using the Statistical Package for Social Sciences, version 20 (SPSS, Chicago, IL).
RESULTS
Baseline characteristics, scoring, and outcomes
A total of 635 consecutive patients with cirrhosis were admitted to the RFH ICU between 1989 and 2012 (Supplementary Table S1 online). There were 395 men (62.4%) and, the mean age was 50.5±11.7 years (range 17–88 years). Alcoholic liver disease was the most common etiology of cirrhosis (63.3%), followed by chronic viral hepatitis B and C (16.2%). The majority of patients had advanced liver disease, as reflected by the median MELD score of 22 and Child-Pugh class distribution (B 18.4% and C 80.6%). The main indications for ICU admission were variceal bleeding (39.1%) and sepsis (23.9%). The mean length of ICU stay was 7.7±8 days. Three hundred and thirty-two patients (52.3%) died either in the ICU or after being discharged to the ward. ICU mortality was 30.2%.
Temporal change in outcomes and disease severity threshold for ICU admission between 1989 and 2012
In-hospital mortality significantly improved over time, from 71.8% in 1989–1996 to 60.8% in 1997–2004, 41.9% in 2005–2008, and 35.4% in 2009–2012 (P<0.0005; Table 1). However, the severity of illness threshold for admitting patients to the ICU decreased over time, as reflected by both less severe liver-specific scores and acute physiology scores at the time of admission to ICU in subsequent cohorts in the four quartiles between 1989 and 2012. ICU mortality did not change significantly over time.
Table 1. Time trends of in-hospital mortality and disease severity on admission to intensive care unit.
| 1989–1996 | 1997–2004 | 2005–2008 | 2009–2012 | P | |
|---|---|---|---|---|---|
| In-hospital mortality (%) | 112 (71.8) | 96 (60.8) | 67 (41.9) | 57 (35.4) | <0.0005a |
| Child-Pugh class (%) | |||||
| A | 1 (0.7) | 3 (2.1) | 0 (0) | 1 (1.1) | 0.448a |
| B | 28 (19.4) | 29 (20) | 23 (20) | 11 (12.2) | |
| C | 115 (79.9) | 113 (77.9) | 92 (80) | 78 (86.7) | |
| Child-Pugh score | 11.5 (6–15) | 11 (5–15) | 11 (7–15) | 12 (6–15) | 0.203b |
| MELD | 25.8 (9–40) | 24.2 (6–40) | 22.5 (8–40) | 17.9 (7–40) | <0.0005b |
| MELD-sodium | 28 (11–82) | 23.6 (1–40) | 21.7 (5–40) | 17.8 (3–74) | <0.0005b |
| SOFA | 12 (2–21) | 10 (0–19) | 8 (1–31) | 8 (0–17) | <0.0005b |
| APACHE II | 18 (2–41) | 19 (0–44) | 15 (6–42) | 14 (5–25) | <0.0005b |
APACHE, Acute Physiology and Chronic Health Evaluation; MELD, Model for End-Stage Liver Disease; SOFA, Sequential Organ Failure Assessment.
χ2-test.
The Kruskal–Wallis test.
Predictors of in-hospital mortality
Non-survivors were slightly older than survivors (median age 52 vs. 50) and were more commonly admitted with sepsis (30.7 vs. 16.6%), renal failure (14.8 vs. 4%), or multi-organ failure (9.9 vs. 1.7%), whereas survivors presented more often with variceal bleeding (55 vs. 24.7%). There was no significant difference in gender distribution and the length of ICU stay between survivors and non-survivors (all P<0.05; Table 2).
Table 2. Baseline characteristics of in-hospital survivors and non-survivors.
| Non-survivors (N=332) | Survivors (N=303) | P | |
|---|---|---|---|
| Age (years) | 52 (18–80) | 50 (17–88) | 0.046 |
| Gender (%) | |||
| Male | 198 (59.8) | 197 (65.2) | 0.186 |
| Female | 133 (40.2) | 105 (34.8) | |
| Liver disease (%) | |||
| Alcoholic liver disease | 207 (62.5) | 195 (64.4) | |
| Autoimmune hepatitis, primary sclerosing cholangitis, primary biliary cirrhosis, Wilson's disease | 27 (8.2) | 19 (6.3) | |
| Chronic hepatitis C | 39 (11.8) | 19 (6.3) | 0.04 |
| Chronic hepatitis B | 25 (7.6) | 20 (6.6) | |
| Cryptogenic cirrhosis | 9 (2.7) | 12 (4) | |
| Alcoholic liver disease and viral hepatitis | 10 (2.5) | 10 (3.3) | |
| Other | 14 (4.2) | 28 (9.2) | |
| Indication for ICU admission (%) | |||
| Respiratory failure | 14 (4.2) | 9 (3) | |
| Sepsis | 102 (30.7) | 50 (16.6) | |
| Renal failure | 49 (14.8) | 12 (4) | <0.0005 |
| Multiorgan failure | 33 (9.9) | 5 (1.7) | |
| Variceal bleeding | 82 (24.7) | 170 (56.3) | |
| Encephalopathy | 25 (7.5) | 20 (6.6) | |
| Other | 27 (8.1) | 36 (11.9) | |
| Length of ICU stay (days)a | 5 (0–42) | 5 (0–71) | 0.408 |
ICU, intensive care unit.
Median (range).
Survivors had significantly lower median Child-Pugh (11 vs. 12), MELD (18 vs. 26), and MELD-sodium score (19 vs. 28). The SOFA (8 vs. 12) and the APACHE II scores (14 vs. 19) were significantly higher among non-survivors (all P<0.05; Table 3).
Table 3. Characteristics of in-hospital survivors and non-survivors on the day of admission to intensive care unit.
| Survivors (N=303) | Non-survivors (N=332) | P | |
|---|---|---|---|
| Sodium (mmol/l) | 140 (104–178) | 137 (107–172) | 0.003 |
| Potasium (mmol/l) | 4.1 (2.3–8.7) | 4.2 (1.7–7.2) | 0.419 |
| Creatinine (μmol/l) | 78 (35–2759) | 126 (21–1252) | <0.0005 |
| Urea (μmol/l) | 8.1 (0.2–72) | 11.9 (0.6–52.5) | <0.0005 |
| Bilirubin (μmol/l) | 52 (5–667) | 125 (2–1058) | <0.0005 |
| Albumin (g/l) | 26 (8–58) | 27 (6–53) | 0.298 |
| White blood cells (×109/l) | 8.54 (0.84–64.37) | 11.2 (1.3–52) | <0.0005 |
| Platelets (×109/l) | 77 (11–824) | 73 (8–371) | 0.053 |
| INR | 1.8 (0.8–8) | 2.3 (1.09–10.2) | <0.0005 |
| Lactate (mmol/l) | 1.7 (0.14–18.3) | 3.28 (0.19–22.7) | <0.0005 |
| pH | 7.4 (7.1–7.59) | 7.36 (6.46–7.64) | <0.0005 |
| PaO2 (kPA) | 14.2 (3.49–59.76) | 13.19 (2.4–63.5) | 0.029 |
| PaCO2 (kPA) | 4.7 (2.74–8.8) | 4.8 (1.14–20.5) | 0.202 |
| FiO2 | 0.5 (0.1–1) | 0.6 (0.1–1) | <0.0005 |
| PaO2/FiO2 | 227 (44–910) | 187 (18–790) | <0.0005 |
| A-a gradient | 186 (−336 to 617) | 243 (−58 to 619) | <0.0005 |
| SOFA | 8 (0–31) | 12 (2–21) | <0.0005 |
| MELD | 18 (6–40) | 26 (9–40) | <0.0005 |
| MELD-sodium | 18.9 (1–74) | 28 (4–82) | <0.0005 |
| Child-Pugh score | 11 (5–15) | 12 (7–15) | <0.0005 |
| Child-Pugh class (%) | <0.0005 | ||
| A | 5 (2.2) | (0) | |
| B | 67 (29.6) | 24 (9) | |
| C | 154 (68.1) | 244 (91) | |
| APACHE II | 14 (0–31) | 19 (6–44) | <0.0005 |
A-a gradient, alveolar-arterial partial pressure oxygen gradient; APACHE, Acute Physiology and Chronic Health Evaluation; INR, international normalized ratio; MELD, Model for End-stage Liver Disease; SOFA, Sequential Organ Failure Assessment.
All values expressed as median (range).
On the day of admission to the ICU, non-survivors had significantly lower serum sodium, arterial pH, PaO2 and PaO2/FiO2, and higher white blood cell count, serum urea, creatinine and bilirubin, higher INR, arterial lactate, FiO2, and A-a gradient (all P<0.05; Table 3).
Parameters associated with in-hospital mortality in the univariate analysis were indication for ICU admission, serum sodium, urea, creatinine and bilirubin, INR, platelet, and white blood cell counts, arterial lactate, pH, PaCO2, FiO2, PaO2/FiO2, and A-a gradient ( Table 4).
Table 4. Predictors of in-hospital mortality (training sample).
| Univariate analysis |
Multivariate analysis—parameters included in the Royal Free Hospital score |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Indication | ||||||
| Sepsis | 1.199 | 0.780–1.843 | 0.407 | |||
| Variceal bleeding | 0.290 | 0.200–0.422 | <0.0005 | 0.369 | 0.222–0.615 | <0.0005 |
| Other | ||||||
| Sodium | 0.977 | 0.961–0.994 | 0.008 | |||
| Creatinine | 1.003 | 0.1.001–1.004 | <0.0005 | |||
| Urea | 1.048 | 1.028–1.096 | <0.0005 | 1.036 | 1.010–1.064 | 0.007 |
| Bilirubin | 1.005 | 1.004–1.007 | <0.0005 | 1.003 | 1.001–1.005 | 0.002 |
| White blood cells | 1.043 | 1.020–1.067 | <0.0005 | |||
| Platelets | 0.997 | 0.995–0.999 | 0.007 | |||
| INR | 2.135 | 1.73–2.634 | <0.0005 | 1.431 | 1.063–1.926 | 0.018 |
| Lactate | 1.250 | 1.168–1.339 | <0.0005 | 1.145 | 1.040–1.260 | 0.006 |
| PH | 0.016 | 0.04–0.64 | <0.0005 | |||
| PaCO2 | 1.172 | 1.034–1.328 | 0.013 | |||
| FiO2 | 11.939 | 5.045–28.256 | <0.0005 | |||
| PaO2/FiO2 | 0.997 | 0.996–0.999 | <0.0005 | |||
| A-a gradient | 1.004 | 1.002–1.005 | <0.0005 | 1.004 | 1.002–1.006 | <0.0005 |
A-a gradient, alveolar-arterial partial pressure oxygen gradient; CI, confidence interval; INR, international normalized ratio; OR, odds ratio.
However, in multivariate analysis, only indication for ICU admission, bilirubin, INR, lactate, urea, and A-a gradient were independent predictors of in-hospital mortality. The following score was generated using the 75% training sample: RFH score=−2.692−0.996*(variceal bleeding)+0.003*(bilirubin)+0.358*(INR)+0.136*(lactate)+0.004*(A-a gradient)+0.036*(urea).
Performance of prognostic models
The AUROC for the RFH score was 0.826. in the training set and 0.797 in the validation set. The goodness-of-fit χ2 was 3.747 (P=0.879) in the training set and 9.029 (P=0.340) in the validation set. The classification accuracy of the score was 73.4% in the training sample and 76.7% in the validation sample. The classification accuracy of the RFH score was assessed in the four different time periods: 82.6% in 1989–1996, 79.7% in 1997–2004, 75.8% in 2005–2008, and 75.2% in 2009–2012.
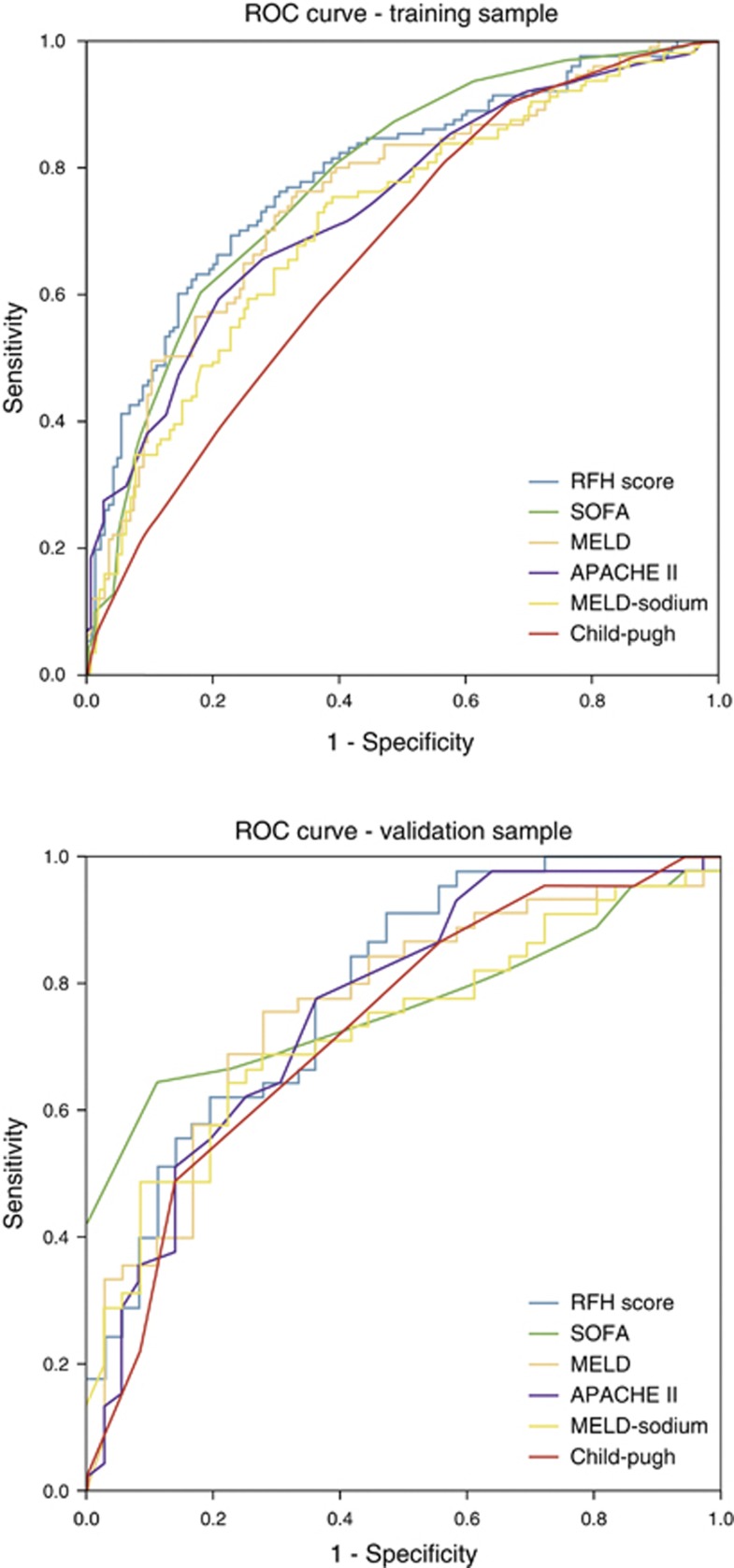
The AUROC and goodness-of-fit χ2 for the established prognostic models in the validation sample were, respectively, the following: SOFA: 0.785 and 9.255 (P=0.321), MELD: 0.749 and 7.672 (P=0.466), APACHE II: 0.736 and 11.133 (P=0.219), MELD-sodium: 0.716 and 10.598 (P=0.226), and Child-Pugh: 0.707 and 3.260 (P=0.660). The AUROC and goodness-of-fit χ2 for the different models in the training and validation set are displayed in Table 5. The ROC curves for the different prognostic models are in both the training and validation sample displayed in Figure 1.
Table 5. Predictive ability for mortality of different prognostic models for patients with cirrhosis admitted to intensive care unit (training and validation set).
|
Training set |
Validation set |
|||
|---|---|---|---|---|
| Prognostic model | AUROC | Goodness-of-fit χ2 (P value) | AUROC | Goodness-of-fit χ2 (P value) |
| RFH score | 0.826 | 3.747 (0.879) | 0.797 | 9.029 (0.340) |
| SOFA | 0.810 | 7.343 (0.500) | 0.785 | 9.255 (0.321) |
| MELD | 0.787 | 6.600 (0.580) | 0.749 | 7.672 (0.466) |
| APACHE II | 0.780 | 9.375 (0.312) | 0.736 | 11.133 (0.219) |
| MELD-sodium | 0.762 | 6.259 (0.618) | 0.716 | 10.598 (0.226) |
| Child-Pugh | 0.668 | 3.587 (0.610) | 0.707 | 3.260 (0.660) |
APACHE, Acute Physiology and Chronic Health Evaluation; AUROC, area under the receiver operating characteristic curve; MELD, Model for End-stage Liver Disease; RFH, Royal Free Hospital; SOFA, Sequential Organ Failure Assessment.
Figure 1.
Receiver operating characteristic curve for the different prognostic models in the training and validation sample.
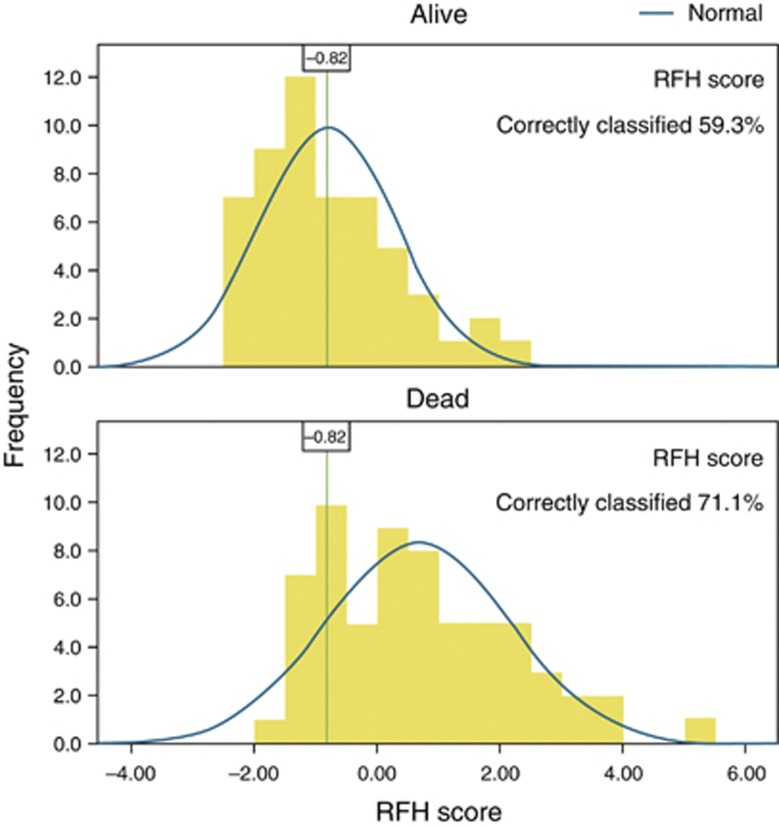
The optimal cutoff point according to best Youden index for each score, and corresponding sensitivity, specificity, PPV, NPV, LR+, and LR− are shown in Table 6. For the RFH score, the optimal cutoff of −0.82 conferred a sensitivity of 85.7%, specificity of 59.3%, PPV 0.71, NPV 0.78, LR+ 2.1, and LR− 0.24. The correctly classified cases using this cutoff point are shown graphically in Figure 2.
Table 6. Performance of different prognostic models in predicting mortality using the optimal cut-off point (validation set).
| Prognostic model | Cutoff point | Youden index | Sensitivity (%) | Specificity (%) | PPV | NPV | LR+ | LR− |
|---|---|---|---|---|---|---|---|---|
| RFH score | −0.82 | 0.45 | 85.7 | 59.3 | 0.71 | 0.78 | 2.1 | 0.24 |
| SOFA | 10.5 | 0.513 | 68 | 83.3 | 0.84 | 0.68 | 4.1 | 0.38 |
| MELD | 21 | 0.484 | 76.5 | 71.9 | 0.78 | 0.71 | 2.72 | 0.33 |
| APACHE II | 17.5 | 0.369 | 59.5 | 77.4 | 0.78 | 0.59 | 2.6 | 0.52 |
| MELD-sodium | 22.5 | 0.408 | 72.5 | 68.3 | 0.74 | 0.66 | 2.29 | 0.4 |
| Child-Pugh | 12.5 | 0.302 | 46.9 | 83.3 | 0.79 | 0.50 | 2.8 | 0.64 |
APACHE, Acute Physiology and Chronic Health Evaluation; LR+ likelihood ratio positive; LR−, likelihood ratio negative; MELD, Model for End-Stage Liver Disease; NPV, negative predictive value; PPV, positive predictive value; RFH, Royal Free Hospital; SOFA, Sequential Organ Failure Assessment.
Figure 2.
Performance of the Royal Free Hospital (RFH) score with the optimal cutoff point of −0.82 (validation sample).
Prognostic models in patients admitted between 2005 and 2012
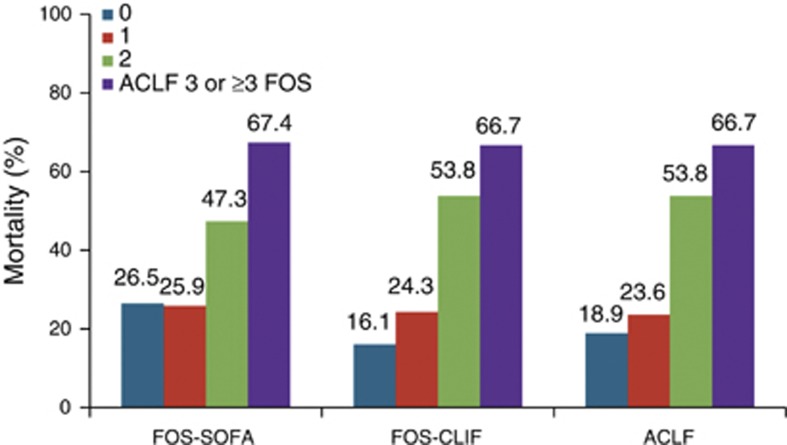
A subgroup analysis was performed for the 2005–2012 cohort, as this was the time period with the lowest in-hospital mortality compared with earlier time periods. Of the 306 patients admitted to ICU between 2005 and 2012, 74 (24.2%) had no ACLF, 89 (29.1%) had ACLF 1, 80 (26.1%) ACLF 2, and 63 (20.6%) ACLF 3. Mortality in those without ACLF was 18.9%, ACLF 1 23.6%, ACLF 2 53.8%, and ACLF 3 66.7% (Figure 3). The AUROC for the different scores was the following: Child-Pugh 0.68, MELD 0.73, MELD-sodium 0.71, APACHE II 0.73, SOFA 0.74, FOS-SOFA 0.66, CLIF-SOFA 0.75, FOS-CLIF 0.73, and RFH 0.78. The goodness-of-fit χ2 was the following: Child-Pugh 4.89 (P=0.43), MELD 2.81 (P=0.95), MELD-sodium 6.91 (P=0.55), APACHE II 11.26 (P=0.13), SOFA 6.21 (P=0.63), FOS-SOFA 3.33 (P=0.19), CLIF-SOFA 9.18 (P=0.33), FOS-CLIF 3.72 (P=0.29), and RFH 2.91 (P=0.94; Supplementary Table S2).
Figure 3.
Mortality according to number of failing organ system (FOS, according to SOFA and chronic liver failure-SOFA (CLIF-SOFA) criteria) and acute-on-chronic-liver-failure (ACLF) classification.
DISCUSSION
Prognosis for patients with cirrhosis admitted to the ICU is poor (7) and even worse than that in critically ill patients without cirrhosis (2). We developed a novel prognostic model, the RFH score, for critically ill patients with cirrhosis. Parameters included in this score reflect both hepatic and extrahepatic organ failure contributing to high mortality rates. The RFH score performed better than established and commonly used acute physiology and liver-specific scores in our cohort, and better than the recently proposed CLIF-SOFA score.
Several studies evaluated optimal prognostic scores for patients with cirrhosis admitted to ICU. Despite the unequivocal need for disease-specific scores, only a few studies have generated novel prognostic models from critically ill patients with cirrhosis (3,9,15), and even so these have not been widely endorsed. Zauner et al. (15) generated the “intensive care cirrhosis outcome score” (ICCO) from 196 patients with cirrhosis, which included bilirubin, cholesterol, creatinine clearance, and lactate (AUROC=0.9, but calibration not reported in the article) (15). The “mean arterial pressure, bilirubin, respiratory failure, and sepsis” (MBRS) score was derived from a study population (n=111) with very high mortality rate (81%) including mainly patients with hepatitis B and hepatocellular carcinoma (AUROC=0.9 for in-hospital mortality, P=0.268 for the goodness-of-fit χ2) (9). The original RFH score was developed from a cohort of patients (n=312) with cirrhosis admitted to the RFH ICU between 1989 and 2005, and included the number of failing organs, bilirubin, urea, FiO2, and lactate (AUROC=0.83, P=0.48 for the goodness-of-fit χ2) (3). The original RFH score was subsequently validated in a cohort of patients with cirrhosis admitted to a general ICU and was found to perform better than both acute physiology and liver-specific scores, indicating the potential utility of this score in clinical practice (19).
In the current updated RFH score, parameters included are bilirubin, INR, lactate, urea, A-a gradient, and variceal bleeding as the indication for ICU admission. Patients with variceal bleeding are often intubated only to protect the airway, and therefore have more favorable prognosis than patients with other indications for ICU admission. In addition, terlipressin and transjugular intrahepatic portosystemic shunts have significantly improved survival in these patients. Urea is an important surrogate of renal function and was included in the updated RFH model. Platelets did not improve the performance of the model and thus were not included. A-a gradient is a better marker of respiratory function than FiO2; thus, its inclusion in the final model improved performance. We did not include the number of failing organs in the updated RFH score, as we chose to use only simple, directly measurable parameters. Despite simplifying the model, the discriminative ability and calibration remained good. The classification accuracy of the RFH score remained good in the different eras of admission, although somewhat less good in more recent years, likely due to lower number of events-deaths in the later time frame.
The performance of already established scores—both liver-specific and acute physiology scores—has been assessed extensively (3,8,10,11,12,13,14,16,20,21,22,23,24,25,26,27,28). Published studies consistently showed that general ICU scores perform better than liver-specific scores. SOFA yielded the best predictive accuracy, whereas Child-Pugh had the worst accuracy. SOFA was usually more accurate than MELD score. In our study, SOFA had the best predictive accuracy, followed by MELD, APACHE II, and MELD-sodium. Child-Pugh was the least accurate, probably because it does not incorporate parameters of renal function. With regard to calibration, MELD showed the best goodness-of-fit P value. When we compared the updated RFH score with the above-established models, we found that it had better predictive accuracy, even better than SOFA and much better than MELD. When we validated our model in the 25% validation sample, the predictive accuracy was inferior (overfitting in the training sample from which it derived), but still better than the rest of the scores. MELD and Child-Pugh showed the best calibration in the validation sample.
The CLIF-SOFA, a modification of SOFA for patients with cirrhosis, and the ACLF classification have been recently proposed for patients with cirrhosis presenting with acute decompensation. A significant proportion of patients was already in ICU or they were admitted shortly thereafter (a total of 23.9%). In the subset of patients admitted to ICU between 2005 and 2012, 75% met the criteria of ACLF but 25% did not. Mortality was significantly higher in those with ACLF grade 2 and 3. Nevertheless, the RFH score performed slightly better than the CLIF-SOFA and SOFA, which had similar performance.
The severity of the underlying liver disease is a major contributor to the outcome. The majority of our patients had advanced liver disease on admission with median MELD score of 22. Non-survivors had more severe liver disease, 91% being classified as Child-Pugh C, with median MELD score of 26, while 68% of survivors were Child-Pugh class C, with median MELD score of 18. Indices of liver dysfunction, such as bilirubin and INR, were included in the RFH score. Albumin was not a significant predictor of mortality in our study. Intravenous albumin may have been administered before ICU admission for indications such as hepatorenal syndrome or large volume paracentesis, which may have accounted for the lack of association with mortality.
Extrahepatic organ failure is another major predictor of mortality. The number of FOS has been strongly associated with mortality in ICU patients with cirrhosis (3,13,21,23). Mortality exceeds 90% with more than three FOS (3,21). In our 2005–2012 cohort, mortality among patients with more than three FOS, according to both SOFA and CLIF-SOFA criteria, was 67%. Among different organs, renal failure has the most profound impact on survival (11,20,29,30). Indices of renal (urea and creatinine) and respiratory function (FiO2, PaO2/FiO2, and A-a gradient) were significantly worse in non-survivors on admission to the ICU, with A-a gradients and urea being incorporated in our prognostic model. Urea is also a surrogate of intravascular volume depletion, which may account for its inclusion in the model rather than creatinine, which can be “falsely low” in malnourished patients.
Lactate is a component of prognostic scores for acute liver failure, but it is also an important indicator of systemic derangement related to sepsis and circulatory failure. In patients with acute deterioration of chronic liver disease, high lactate levels might be due to the precipitating event, such as sepsis, respiratory, or cardiac failure. Following resuscitation, persistent high lactate levels might reflect the severity of the underlying liver disease. Thus, the dual role of lactate as a surrogate marker of both hepatic and extrahepatic organ failure may account for its high prognostic value (31).
Although outcomes have improved over time (3) mortality rates for critically ill patients with cirrhosis remain high (8,9,14,16,20,21,22,23,29,32,33). In our unit, in-hospital mortality decreased from 72% in 1989–1996 to 42% in 2005–2008 and 35% in 2009–2012. The improvement in survival may be and the advent of novel therapeutic modalities, such as terlipressin and transjugular intrahepatic portosystemic shunts, which are highly effective in treating complications of portal hypertension, in particular variceal bleeding (34). Galbois et al. (4) showed that mortality improved in 2005–2008 compared with 1995–1998, although patients admitted to ICU between 2005 and 2008 had significantly higher Child-Pugh, MELD, and SOFA scores (4). This was not the case in our study, as we showed that the threshold for admitting patients with cirrhosis to ICU has significantly decreased in our unit; thus, aggressive treatment was initiated at an earlier stage, which may also account for improving overall in-hospital survival in our cohort. Nevertheless, the cost of current therapeutic modalities is high (6), which further underlies the need for risk stratification and identification of patients who would mostly benefit from them.
We developed a prognostic score, the RFH score, for patients with cirrhosis admitted to ICU that incorporates few easily measurable parameters, and combines very good discriminative ability, comparable to SOFA, with good calibration. Our study included a large number of patients admitted to ICU over a long period of time during which medical practice and indications for ICU admission have changed. The validation of our model using training and validation cohorts, as well as in the different time frames, supports its generalizability. However, external validation in other cohorts of patients with cirrhosis in the ICU is needed. Such a model could serve as an important adjunct to clinical judgment in order to identify patients with cirrhosis who are highly unlikely to benefit from initiating aggressive treatment or continuing treatment in an ICU, especially in the context of prioritization for ICU bed allocation and the high cost of current treatment. In the “real world”, patients with cirrhosis admitted to ICU for indications other than variceal bleeding, with high bilirubin, INR, lactate, urea, and A-a gradient, on admission to ICU have very low chances of survival. Finally, the CLIF-SOFA and the ACLF classification, although derived from a large cohort of patients with acute deterioration, do not seem to perform better than the original SOFA and the commonly used MELD score in patients with cirrhosis admitted to ICU.
Study Highlights
Guarantor of the article: Andrew K. Burroughs, FRCP, FMedSci.
Specific author contributions: Statistical analysis, literature search, drafting the initial manuscript, final manuscript approval: Eleni Theocharidou; data collection, literature search, manuscript review, final manuscript approval: Giulia Pieri, Ali Omar Mohammad, Michelle Cheung, Evangelos Cholongitas; conceptualizing the article, critical review and revision, and final manuscript approval: Banwari Agarwal, Andrew K. Burroughs.
Financial support: None.
Potential competing interests: None.
Supplementary Material
References
- Arvaniti V, D'Amico G, Fede G, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246–1256. doi: 10.1053/j.gastro.2010.06.019. [DOI] [PubMed] [Google Scholar]
- O'Brien AJ, Welch CA, Singer M, et al. Prevalence and outcome of cirrhosis patients admitted to UK intensive care: a comparison against dialysis-dependent chronic renal failure patients. Intensive Care Med. 2012;38:991–1000. doi: 10.1007/s00134-012-2523-2. [DOI] [PubMed] [Google Scholar]
- Cholongitas E, Senzolo M, Patch D, et al. Risk factors, sequential organ failure assessment and model for end-stage liver disease scores for predicting short term mortality in cirrhotic patients admitted to intensive care unit. Aliment Pharmacol Ther. 2006;23:883–893. doi: 10.1111/j.1365-2036.2006.02842.x. [DOI] [PubMed] [Google Scholar]
- Galbois A, Trompette Ml, Das V, et al. Improvement in the prognosis of cirrhotic patients admitted to an intensive care unit, a retrospective study. Eur J Gastroenterol Hepatol. 2012;24:897–904. doi: 10.1097/MEG.0b013e3283544816. [DOI] [PubMed] [Google Scholar]
- Cholongitas E, Senzolo M, Patch D, et al. Review article: scoring systems for assessing prognosis in critically ill adult cirrhotics. Aliment Pharmacol Ther. 2006;24:453–464. doi: 10.1111/j.1365-2036.2006.02998.x. [DOI] [PubMed] [Google Scholar]
- Shawcross DL, Austin MJ, Abeles RD, et al. The impact of organ dysfunction in cirrhosis: survival at a cost. J Hepatol. 2012;56:1054–1062. doi: 10.1016/j.jhep.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Olson JC, Wendon JA, Kramer DJ, et al. Intensive care of the patient with cirrhosis. Hepatology. 2011;54:1864–1872. doi: 10.1002/hep.24622. [DOI] [PubMed] [Google Scholar]
- Chen YC, Tian YC, Liu NJ, et al. Prospective cohort study comparing sequential organ failure assessment and acute physiology, age, chronic health evaluation iii scoring systems for hospital mortality prediction in critically ill cirrhotic patients. Int J Clin Pract. 2006;60:160–166. doi: 10.1111/j.1742-1241.2005.00634.x. [DOI] [PubMed] [Google Scholar]
- Fang JT, Tsai MH, Tian YC, et al. outcome predictors and new score of critically ill cirrhotic patients with acute renal failure. Nephrol Dial Transplant. 2008;23:1961–1969. doi: 10.1093/ndt/gfm914. [DOI] [PubMed] [Google Scholar]
- Filloux B, Chagneau-Derrode C, Ragot S, et al. Short-term and long-term vital outcomes of cirrhotic patients admitted to an intensive care unit. Eur J Gastroenterol Hepatol. 2010;22:1474–1480. doi: 10.1097/MEG.0b013e32834059cd. [DOI] [PubMed] [Google Scholar]
- Juneja D, Gopal PB, Kapoor D, et al. Outcome of patients with liver cirrhosis admitted to a specialty liver intensive care unit in India. J Crit Care. 2009;24:387–393. doi: 10.1016/j.jcrc.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Singh N, Gayowski T, Wagener MM, et al. Outcome of patients with cirrhosis requiring intensive care unit support: prospective assessment of predictors of mortality. J Gastroenterol. 1998;33:73–79. doi: 10.1007/s005350050047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MH, Peng YS, Lien JM, et al. Multiple organ system failure in critically ill cirrhotic patients. A comparison of two multiple organ dysfunction/failure scoring systems. Digestion. 2004;69:190–200. doi: 10.1159/000078789. [DOI] [PubMed] [Google Scholar]
- Wehler M, Kokoska J, Reulbach U, et al. Short-term prognosis in critically ill patients with cirrhosis assessed by prognostic scoring systems. Hepatology. 2001;34:255–261. doi: 10.1053/jhep.2001.26522. [DOI] [PubMed] [Google Scholar]
- Zauner C, Schneeweiss B, Schneider B, et al. Short-term prognosis in critically ill patients with liver cirrhosis: an evaluation of a new scoring system. Eur J Gastroenterol Hepatol. 2000;12:517–522. doi: 10.1097/00042737-200012050-00007. [DOI] [PubMed] [Google Scholar]
- Zimmerman JE, Wagner DP, Seneff MG, et al. Intensive care unit admissions with cirrhosis: risk-stratifying patient groups and predicting individual survival. Hepatology. 1996;23:1393–1401. doi: 10.1002/hep.510230615. [DOI] [PubMed] [Google Scholar]
- Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- Feltracco P, Brezzi M, Barbieri S, et al. Intensive care unit admission of decompensated cirrhotic patients: prognostic scoring systems. Transplant Proc. 2011;43:1079–1084. doi: 10.1016/j.transproceed.2011.01.153. [DOI] [PubMed] [Google Scholar]
- Thomson SJ, Moran C, Cowan Ml, et al. Outcomes of critically ill patients with cirrhosis admitted to intensive care: an important perspective from the non-transplant setting. Aliment Pharmacol Ther. 2010;32:233–243. doi: 10.1111/j.1365-2036.2010.04341.x. [DOI] [PubMed] [Google Scholar]
- Arabi Y, Ahmed QA, Haddad S, et al. Outcome predictors of cirrhosis patients admitted to the intensive care unit. Eur J Gastroenterol Hepatol. 2004;16:333–339. doi: 10.1097/00042737-200403000-00014. [DOI] [PubMed] [Google Scholar]
- Das V, Boelle PY, Galbois A, et al. Cirrhotic patients in the medical intensive care unit: early prognosis and long-term survival. Crit Care Med. 2010;38:2108–2116. doi: 10.1097/CCM.0b013e3181f3dea9. [DOI] [PubMed] [Google Scholar]
- Ho YP, Chen YC, Yang C, et al. Outcome prediction for critically ill cirrhotic patients: a comparison of APACHE II and Child-Pugh scoring systems. J Intensive Care Med. 2004;19:105–110. doi: 10.1177/0885066603261991. [DOI] [PubMed] [Google Scholar]
- Tsai MH, Chen YC, Ho YP, et al. Organ system failure scoring system can predict hospital mortality in critically ill cirrhotic patients. J Clin Gastroenterol. 2003;37:251–257. doi: 10.1097/00004836-200309000-00011. [DOI] [PubMed] [Google Scholar]
- Karvellas CJ, Pink F, Mcphail M, et al. Bacteremia, acute physiology and chronic health evaluation ii and modified end stage liver disease are independent predictors of mortality in critically ill nontransplanted patients with acute on chronic liver failure. Crit Care Med. 2010;38:121–126. doi: 10.1097/CCM.0b013e3181b42a1c. [DOI] [PubMed] [Google Scholar]
- Zauner CA, Apsner RC, Kranz A, et al. Outcome prediction for patients with cirrhosis of the liver in a medical ICU: a comparison of the APACHE scores and liver-specific scoringsystems. Intensive Care Med. 1996;22:559–563. doi: 10.1007/BF01708096. [DOI] [PubMed] [Google Scholar]
- Cavallazzi R, Awe OO, Vasu TS, et al. Model for end-stage liver disease score for predicting outcome in critically ill medical patients with liver cirrhosis. J Crit Care. 2012;27:424–426. doi: 10.1016/j.jcrc.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Kavli M, Strom T, Carlsson M, et al. The outcome of critical illness in decompensated alcoholic liver cirrhosis. Acta Anaesthesiol Scand. 2012;56:987–994. doi: 10.1111/j.1399-6576.2012.02692.x. [DOI] [PubMed] [Google Scholar]
- Olmez S, Gumurdulu Y, Tas A, et al. Prognostic markers in cirrhotic patients requiring intensive care: a comparative prospective study. Ann Hepatol. 2012;11:513–518. [PubMed] [Google Scholar]
- Tu KH, Jenq CC, Tsai MH, et al. Outcome scoring systems for short-term prognosis in critically ill cirrhotic patients. Shock. 2011;36:445–450. doi: 10.1097/SHK.0b013e31822fb7e2. [DOI] [PubMed] [Google Scholar]
- Cholongitas E, Calvaruso V, Senzolo M, et al. Rifle classification as predictive factor of mortality in patients with cirrhosis admitted to intensive care unit. J Gastroenterol Hepatol. 2009;24:1639–1647. doi: 10.1111/j.1440-1746.2009.05908.x. [DOI] [PubMed] [Google Scholar]
- Funk GC, Doberer D, Kneidinger N, et al. Acid-base disturbances in critically ill patients with cirrhosis. Liver Int. 2007;27:901–909. doi: 10.1111/j.1478-3231.2007.01510.x. [DOI] [PubMed] [Google Scholar]
- Levesque E, Hoti E, Azoulay D, et al. Prospective evaluation of the prognostic scores for cirrhotic patients admitted to an intensive care unit. J Hepatol. 2012;56:95–102. doi: 10.1016/j.jhep.2011.06.024. [DOI] [PubMed] [Google Scholar]
- Aggarwal A, Ong JP, Younossi ZM, et al. Predictors of mortality and resource utilization in cirrhotic patients admitted to the medical ICU. Chest. 2001;119:1489–1497. doi: 10.1378/chest.119.5.1489. [DOI] [PubMed] [Google Scholar]
- Garcia-Pagan JC, Caca K, Bureau C, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370–2379. doi: 10.1056/NEJMoa0910102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.