Abstract
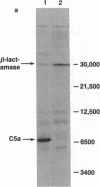
A gene coding for the C5a fragment of the fifth component of human complement has been chemically synthesized, cloned, and expressed in Escherichia coli. The 253-base-pair gene fragment was built through a two-step enzymic assembly of 16 oligonucleotides, the average length of each being 32 residues. The oligonucleotides were synthesized by using the phosphoramidite method. The gene was cloned in a pBR322-derivative plasmid downstream from the lac up-promoter mutant, UV5-D. The expression of C5a was detected and measured by immunoassay and a radioligand binding assay. C5a from E. coli was comparable to C5a purified from human serum in inhibiting binding of human 125I-labeled C5a to its putative receptor on polymorphonuclear leukocytes. Studies of smooth muscle contraction in isolated guinea pig ileum showed that the recombinant C5a was biologically active and produced cross-tachyphylaxis with human serum-derived C5a. The results demonstrate the feasibility of expressing C5a anaphylatoxin in bacteria and provide a system for mutagenesis of the C5a protein.
Full text
PDF
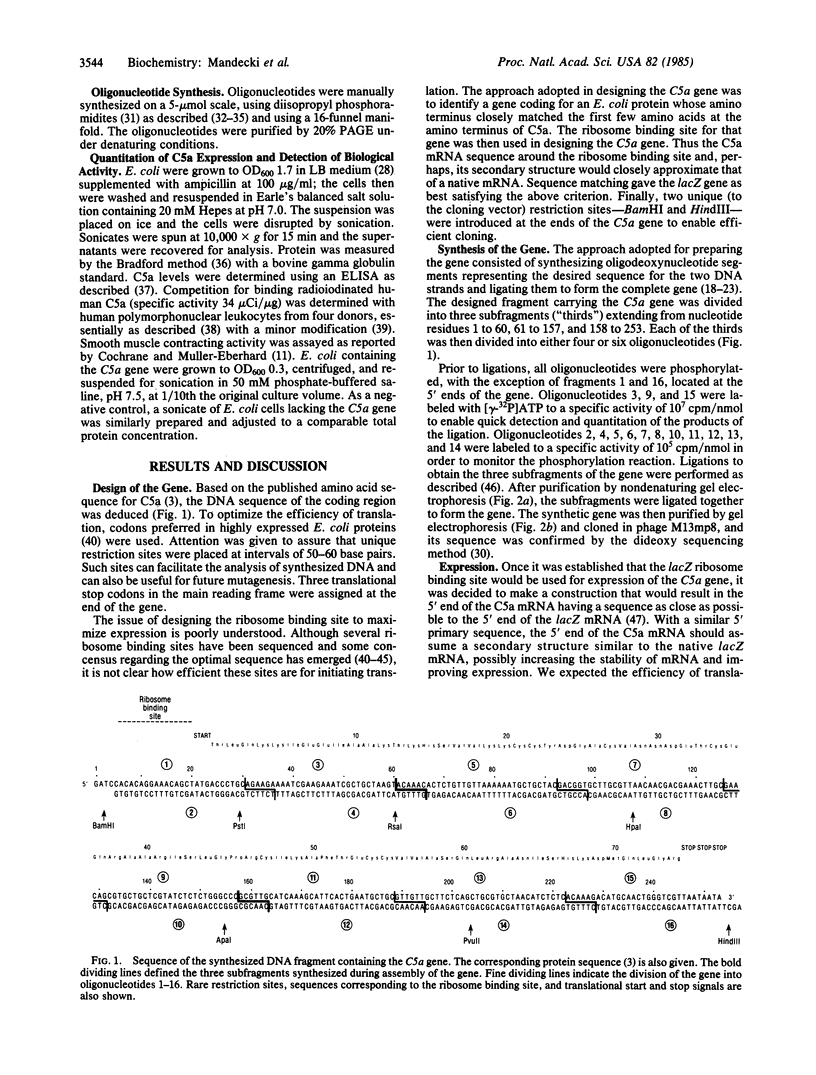


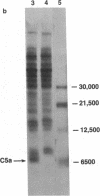
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bokisch V. A., Müller-Eberhard H. J. Anaphylatoxin inactivator of human plasma: its isolation and characterization as a carboxypeptidase. J Clin Invest. 1970 Dec;49(12):2427–2436. doi: 10.1172/JCI106462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Caruthers M. H., Beaucage S. L., Efcavitch J. W., Fisher E. F., Goldman R. A., deHaseth P. L., Mandecki W., Matteucci M. D., Rosendahl M. S., Stabinsky Y. Chemical synthesis and biological studies on mutated gene-control regions. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):411–418. doi: 10.1101/sqb.1983.047.01.048. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G., Müller-Eberhard H. J. The derivation of two distinct anaphylatoxin activities from the third and fifth components of human complement. J Exp Med. 1968 Feb 1;127(2):371–386. doi: 10.1084/jem.127.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock P. R., Hammerschmidt D., White J. G., Dalmosso A. P., Jacob H. S. Complement (C5-a)-induced granulocyte aggregation in vitro. A possible mechanism of complement-mediated leukostasis and leukopenia. J Clin Invest. 1977 Jul;60(1):260–264. doi: 10.1172/JCI108763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahinden C. A., Fehr J., Hugli T. E. Role of cell surface contact in the kinetics of superoxide production by granulocytes. J Clin Invest. 1983 Jul;72(1):113–121. doi: 10.1172/JCI110948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. W., Thomas M., Cameron J., St John T. P., Scherer S., Padgett R. A. Rapid DNA isolations for enzymatic and hybridization analysis. Methods Enzymol. 1980;65(1):404–411. doi: 10.1016/s0076-6879(80)65051-4. [DOI] [PubMed] [Google Scholar]
- Doel M. T., Eaton M., Cook E. A., Lewis H., Patel T., Carey N. H. The expression in E. coli of synthetic repeating polymeric genes coding for poly(L-aspartyl-L-phenylalanine). Nucleic Acids Res. 1980 Oct 24;8(20):4575–4592. doi: 10.1093/nar/8.20.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge M. D., Greene A. R., Heathcliffe G. R., Moore V. E., Faulkner N. J., Camble R., Petter N. N., Trueman P., Schuch W., Hennam J. Chemical synthesis of a human interferon-alpha 2 gene and its expression in Escherichia coli. Nucleic Acids Res. 1983 Sep 24;11(18):6419–6435. doi: 10.1093/nar/11.18.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez H. N., Henson P. M., Otani A., Hugli T. E. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978 Jan;120(1):109–115. [PubMed] [Google Scholar]
- Fernandez H. N., Hugli T. E. Partial characterization of human C5a anaphylatoxin. I. Chemical description of the carbohydrate and polypeptide prtions of human C5a. J Immunol. 1976 Nov;117(5 Pt 1):1688–1694. [PubMed] [Google Scholar]
- Fernandez H. N., Hugli T. E. Primary structural analysis of the polypeptide portion of human C5a anaphylatoxin. Polypeptide sequence determination and assignment of the oligosaccharide attachment site in C5a. J Biol Chem. 1978 Oct 10;253(19):6955–6964. [PubMed] [Google Scholar]
- Gerard C., Chenoweth D. E., Hugli T. E. Response of human neutrophils to C5a: a role for the oligosaccharide moiety of human C5ades Arg-74 but not of C5a in biologic activity. J Immunol. 1981 Nov;127(5):1978–1982. [PubMed] [Google Scholar]
- Gerard C., Hugli T. E. Amino acid sequence of the anaphylatoxin from the fifth component of porcine complement. J Biol Chem. 1980 May 25;255(10):4710–4715. [PubMed] [Google Scholar]
- Goeddel D. V., Kleid D. G., Bolivar F., Heyneker H. L., Yansura D. G., Crea R., Hirose T., Kraszewski A., Itakura K., Riggs A. D. Expression in Escherichia coli of chemically synthesized genes for human insulin. Proc Natl Acad Sci U S A. 1979 Jan;76(1):106–110. doi: 10.1073/pnas.76.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I., Hoffstein S., Gallin J., Weissmann G. Mechanisms of lysosomal enzyme release from human leukocytes: microtubule assembly and membrane fusion induced by a component of complement. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2916–2920. doi: 10.1073/pnas.70.10.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M. G., Chenoweth D. E., Weigle W. O. Potentiation of the primary humoral immune response in vitro by C5a anaphylatoxin. J Immunol. 1982 Jul;129(1):70–75. [PubMed] [Google Scholar]
- Gouy M., Gautier C. Codon usage in bacteria: correlation with gene expressivity. Nucleic Acids Res. 1982 Nov 25;10(22):7055–7074. doi: 10.1093/nar/10.22.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant J. A., Dupree E., Goldman A. S., Schultz D. R., Jackson A. L. Complement-mediated release of histamine from human leukocytes. J Immunol. 1975 Mar;114(3):1101–1106. [PubMed] [Google Scholar]
- Hugli T. E., Müller-Eberhard H. J. Anaphylatoxins: C3a and C5a. Adv Immunol. 1978;26:1–53. doi: 10.1016/s0065-2776(08)60228-x. [DOI] [PubMed] [Google Scholar]
- Hugli T. E. The structural basis for anaphylatoxin and chemotactic functions of C3a, C4a, and C5a. Crit Rev Immunol. 1981 Feb;1(4):321–366. [PubMed] [Google Scholar]
- Itakura K., Hirose T., Crea R., Riggs A. D., Heyneker H. L., Bolivar F., Boyer H. W. Expression in Escherichia coli of a chemically synthesized gene for the hormone somatostatin. Science. 1977 Dec 9;198(4321):1056–1063. doi: 10.1126/science.412251. [DOI] [PubMed] [Google Scholar]
- Jay G., Khoury G., Seth A. K., Jay E. Construction of a general vector for efficient expression of mammalian proteins in bacteria: use of a synthetic ribosome binding site. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5543–5548. doi: 10.1073/pnas.78.9.5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. R., Hugli T. E., Müller-Eberhard H. J. Release of histamine from rat mast cells by the complement peptides C3a and C5a. Immunology. 1975 Jun;28(6):1067–1080. [PMC free article] [PubMed] [Google Scholar]
- Johnson J. D., Hand W. L., Francis J. B., King-Thompson N., Corwin R. W. Antibiotic uptake by alveolar macrophages. J Lab Clin Med. 1980 Mar;95(3):429–439. [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel S. L., Manderino G. L., Marasco W., Kaercher K., Hirata A. A., Ward P. A. A specific enzyme-linked immunosorbent assay (ELISA) for the determination of human C5a antigen. J Immunol Methods. 1983 Sep 16;62(3):305–314. doi: 10.1016/0022-1759(83)90174-6. [DOI] [PubMed] [Google Scholar]
- Maizels N. M. The nucleotide sequence of the lactose messenger ribonucleic acid transcribed from the UV5 promoter mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3585–3589. doi: 10.1073/pnas.70.12.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandecki W., Caruthers M. H. Mutants of the lac promoter with large insertions and deletions between the CAP binding site and the -35 region. Gene. 1984 Nov;31(1-3):263–267. doi: 10.1016/0378-1119(84)90219-1. [DOI] [PubMed] [Google Scholar]
- Maquat L. E., Reznikoff W. S. In vitro analysis of the Escherichia coli RNA polymerase interaction with wild-type and mutant lactose promoters. J Mol Biol. 1978 Nov 15;125(4):467–490. doi: 10.1016/0022-2836(78)90311-x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson U. R., Mandle R. J., Jr, McConnell-Mapes J. A. Human C3 and C5: subunit structure and modifications by trypsin and C42-C423. J Immunol. 1975 Feb;114(2 Pt 2):815–822. [PubMed] [Google Scholar]
- Scherer G. F., Walkinshaw M. D., Arnott S., Morré D. J. The ribosome binding sites recognized by E. coli ribosomes have regions with signal character in both the leader and protein coding segments. Nucleic Acids Res. 1980 Sep 11;8(17):3895–3907. doi: 10.1093/nar/8.17.3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Smith J., Cook E., Fotheringham I., Pheby S., Derbyshire R., Eaton M. A., Doel M., Lilley D. M., Pardon J. F., Patel T. Chemical synthesis and cloning of a gene for human beta-urogastrone. Nucleic Acids Res. 1982 Aug 11;10(15):4467–4482. doi: 10.1093/nar/10.15.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Oshima T., Ohsuye K., Ono T., Mizono A., Ueno A., Nakazato H., Tsujimoto M., Higashi N., Noguchi T. Expression in Escherichia coli of chemically synthesized gene for the human immune interferon. Nucleic Acids Res. 1983 Mar 25;11(6):1707–1723. doi: 10.1093/nar/11.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt W. Activation, activities and pharmacologically active products of complement. Pharmacol Rev. 1974 Jun;26(2):125–169. [PubMed] [Google Scholar]
- Ward P. A., Newman L. J. A neutrophil chemotactic factor from human C'5. J Immunol. 1969 Jan;102(1):93–99. [PubMed] [Google Scholar]
- Webster R. O., Hong S. R., Johnston R. B., Jr, Henson P. M. Biologial effects of the human complement fragments C5a and C5ades Arg on neutrophil function. Immunopharmacology. 1980 Jun;2(3):201–219. doi: 10.1016/0162-3109(80)90050-8. [DOI] [PubMed] [Google Scholar]
- Wetzel R., Heyneker H. L., Goeddel D. V., Jhurani P., Shapiro J., Crea R., Low T. L., McClure J. E., Thurman G. B., Goldstein A. L. Production of biologically active N alpha-desacetylthymosin alpha 1 in Escherichia coli through expression of a chemically synthesized gene. Biochemistry. 1980 Dec 23;19(26):6096–6104. doi: 10.1021/bi00567a023. [DOI] [PubMed] [Google Scholar]