Abstract
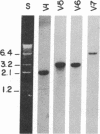
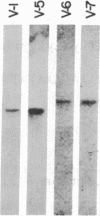
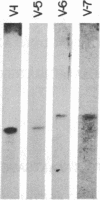
The human precursor gene for vasoactive intestinal peptide (VIP) and PHM-27, a peptide that has an NH2-terminal histidine and COOH-terminal methionine amide and is closely related in sequence and activity to VIP, was detected with synthetic oligodeoxynucleotide probes. These specific hybridization segments were constructed according to the neuroblastoma VIP cDNA sequence and contained up to 39 bases. The gene structure was partly deduced by hybridization to synthetic oligodeoxynucleotide probes and partly by direct chemical nucleotide sequencing. Four exons were discovered thus far; among them are two short exons separated by a 0.75-kilobase DNA stretch, one encoding PHM-27 and the second encoding VIP (exons 1 and 2). Each of these two exons encodes both the hormone amino acid residues as well as the post-translational processing signal sequences. The 3' splice sites of the two exons contain an identical stretch of nine nucleotides. At the cDNA level, the 3' splice sites contain the same stretch of six nucleotides, which are identically spliced. The occurrence of VIP and PHM-27 coding sequences on two separate exons of the human genome and the homology of their 3' splice site may allow alternative RNA processing as discussed below.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom S. R., Christofides N. D., Delamarter J., Buell G., Kawashima E., Polak J. M. Diarrhoea in vipoma patients associated with cosecretion of a second active peptide (peptide histidine isoleucine) explained by single coding gene. Lancet. 1983 Nov 19;2(8360):1163–1165. doi: 10.1016/s0140-6736(83)91215-1. [DOI] [PubMed] [Google Scholar]
- Dalbadie-McFarland G., Cohen L. W., Riggs A. D., Morin C., Itakura K., Richards J. H. Oligonucleotide-directed mutagenesis as a general and powerful method for studies of protein function. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6409–6413. doi: 10.1073/pnas.79.21.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixson A. F., Kendrick K. M., Blank M. A., Bloom S. R. Effects of tactile and electrical stimuli upon release of vasoactive intestinal polypeptide in the mammalian penis. J Endocrinol. 1984 Feb;100(2):249–252. doi: 10.1677/joe.0.1000249. [DOI] [PubMed] [Google Scholar]
- Eckenstein F., Baughman R. W. Two types of cholinergic innervation in cortex, one co-localized with vasoactive intestinal polypeptide. Nature. 1984 May 10;309(5964):153–155. doi: 10.1038/309153a0. [DOI] [PubMed] [Google Scholar]
- Gozes I., Bodner M., Shani Y., Fridkin M. Detection of mRNAs containing regulatory peptide coding sequences using synthetic oligodeoxynucleotides. J Cell Biochem. 1984;26(3):147–156. doi: 10.1002/jcb.240260303. [DOI] [PubMed] [Google Scholar]
- Gozes I., Bodner M., Shwartz H., Shani Y., Fridkin M. Studies toward the biosynthesis of vasoactive intestinal peptide (VIP). Peptides. 1984 Mar-Apr;5(2):161–166. doi: 10.1016/0196-9781(84)90200-6. [DOI] [PubMed] [Google Scholar]
- Hayakawa Y., Obata K., Itoh N., Yanaihara N., Okamoto H. Cyclic AMP regulation of pro-vasoactive intestinal polypeptide/PHM-27 synthesis in human neuroblastoma cells. J Biol Chem. 1984 Jul 25;259(14):9207–9211. [PubMed] [Google Scholar]
- Hökfelt T., Fahrenkrug J., Tatemoto K., Mutt V., Werner S., Hulting A. L., Terenius L., Chang K. J. The PHI (PHI-27)/corticotropin-releasing factor/enkephalin immunoreactive hypothalamic neuron: possible morphological basis for integrated control of prolactin, corticotropin, and growth hormone secretion. Proc Natl Acad Sci U S A. 1983 Feb;80(3):895–898. doi: 10.1073/pnas.80.3.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N., Obata K., Yanaihara N., Okamoto H. Human preprovasoactive intestinal polypeptide contains a novel PHI-27-like peptide, PHM-27. Nature. 1983 Aug 11;304(5926):547–549. doi: 10.1038/304547a0. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Lee T. J., Saito A., Berezin I. Vasoactive intestinal polypeptide-like substance: the potential transmitter for cerebral vasodilation. Science. 1984 May 25;224(4651):898–901. doi: 10.1126/science.6719122. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutt V., Said S. I. Structure of the porcine vasoactive intestinal octacosapeptide. The amino-acid sequence. Use of kallikrein in its determination. Eur J Biochem. 1974 Mar 1;42(2):581–589. doi: 10.1111/j.1432-1033.1974.tb03373.x. [DOI] [PubMed] [Google Scholar]
- Ottesen B., Wagner G., Virag R., Fahrenkrug J. Penile erection: possible role for vasoactive intestinal polypeptide as a neurotransmitter. Br Med J (Clin Res Ed) 1984 Jan 7;288(6410):9–11. doi: 10.1136/bmj.288.6410.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C. C. Phosphorylation of nucleic acid by an enzyme from T4 bacteriophage-infected Escherichia coli. Proc Natl Acad Sci U S A. 1965 Jul;54(1):158–165. doi: 10.1073/pnas.54.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M. G., Mermod J. J., Amara S. G., Swanson L. W., Sawchenko P. E., Rivier J., Vale W. W., Evans R. M. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature. 1983 Jul 14;304(5922):129–135. doi: 10.1038/304129a0. [DOI] [PubMed] [Google Scholar]
- Said S. I. Vasoactive intestinal polypeptide (VIP): current status. Peptides. 1984 Mar-Apr;5(2):143–150. doi: 10.1016/0196-9781(84)90197-9. [DOI] [PubMed] [Google Scholar]
- Verma I. M. The reverse transcriptase. Biochim Biophys Acta. 1977 Mar 21;473(1):1–38. doi: 10.1016/0304-419x(77)90005-1. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]