Interactions between cell adhesion molecules CRTAM and Cadm1 regulate the residency and maintenance of CD4+CD8+ and CD4+ T cells in the gut that can influence the immune response to infection.
Abstract
Retention of lymphocytes in the intestinal mucosa requires specialized chemokine receptors and adhesion molecules. We find that both CD4+CD8+ and CD4+ T cells in the intestinal epithelium, as well as CD8+ T cells in the intestinal mucosa and mesenteric lymph nodes, express the cell adhesion molecule class I–restricted T cell–associated molecule (Crtam) upon activation, whereas the ligand of Crtam, cell adhesion molecule 1 (Cadm1), is expressed on gut CD103+DCs. Lack of Crtam–Cadm1 interactions in Crtam−/− and Cadm1−/− mice results in loss of CD4+CD8+ T cells, which arise from mucosal CD4+ T cells that acquire a CD8 lineage expression profile. After acute oral infection with Toxoplasma gondii, both WT and Crtam−/− mice mounted a robust TH1 response, but markedly fewer TH17 cells were present in the intestinal mucosa of Crtam−/− mice. The almost exclusive TH1 response in Crtam−/− mice resulted in more efficient control of intestinal T. gondii infection. Thus, Crtam–Cadm1 interactions have a major impact on the residency and maintenance of CD4+CD8+ T cells in the gut mucosa in the steady state. During pathogenic infection, Crtam–Cadm1 interactions regulate the dynamic equilibrium between newly formed CD4+ T cells and their retention in the gut, thereby shaping representation of disparate CD4+ T cell subsets and the overall quality of the CD4+ T cell response.
Class I–restricted T cell–associated molecule (Crtam) is an Ig-like cell surface protein that was originally found on activated NKT cells (Kennedy et al., 2000), NK cells, and CD8+ T cells (Arase et al., 2005; Boles et al., 2005; Galibert et al., 2005) and shown to bind the cell adhesion molecule 1 (Cadm1, also known as Nectin like [Necl] 2; Arase et al., 2005; Boles et al., 2005; Galibert et al., 2005). Cadm1 is a cell surface molecule of the nectin and Necl families that is expressed on CD8α DCs (Galibert et al., 2005; Poulin et al., 2010), epithelial cells, neurons, and tumor cells (Sakisaka and Takai, 2004; Mizutani et al., 2011). Crtam–Cadm1 interactions strengthen NK cell and CD8+ T cell effector functions (Arase et al., 2005; Boles et al., 2005; Galibert et al., 2005; Murakami, 2005) and promote the retention of virus-specific CD8+ T cells within LNs (Takeuchi et al., 2009). One report proposed that Crtam is essential for the establishment of CD4+ T cell polarization after TCR engagement, a process which blocks CD4+ T cell division and induces the capacity to secrete IFN-γ, IL-17, and IL-22 (Yeh et al., 2008).
The immune system associated with the gastrointestinal mucosa comprises large numbers of dispersed lymphoid cells that reside in the epithelium and the underlying lamina propria. Intraepithelial lymphocytes (IELs) and lamina propria lymphocytes (LPLs) include antigen-experienced CD8+ and CD4+ T cells, γδ T cells, various subsets of innate lymphoid cells (ILCs), and IgA-secreting plasma cells (Jabri and Ebert, 2007; Cerutti, 2008; Cheroutre et al., 2011; Sheridan and Lefrançois, 2011; Spits et al., 2013). Homing and residency of IELs and LPLs in the mucosa requires specialized chemokine receptors, such as CCR9, CCR6, and CXCR6, which detect chemokines released by gut epithelial cells (CCL25, CCL20, and CXCL16, respectively; Johansson-Lindbom and Agace, 2007). Integrins, like CD103 (αE) and α4β7, also play an essential role in promoting homing and retention of IELs and LPLs in the mucosa by binding E-cadherin and MAdCAM-1 on epithelial cells and vascular endothelial cells, respectively (Johansson-Lindbom and Agace, 2007).
T cell acquisition of homing and adhesion molecules is induced by T cell interaction with DCs (Mora et al., 2008; Villablanca et al., 2011). Among the disparate subsets of DC in the intestinal lamina propria and mesenteric LNs (mLN), the CD103+ DC subset produces retinoic acid (RA), which induces the gut homing receptors CCR9 and α4β7 on lymphocytes (Coombes et al., 2007; Mora et al., 2008; Villablanca et al., 2011). Gut-associated CD103+ DCs also produce TGF-β, which induces the expression of CD103 on T cells (Coombes et al., 2007; Mora et al., 2008; Villablanca et al., 2011). In addition to imprinting gut-homing capacity on T cells, gut CD103+ DCs control the differentiation of CD4+ T cells by priming regulatory CD4+ T cells during the steady state (Mucida et al., 2007) and TH1 and TH17 cells during inflammation (DePaolo et al., 2011; Hall et al., 2011).
Here, we investigated the impact of Crtam–Cadm1 interaction in the intestinal immune system. We find that Crtam is expressed upon activation on all CD8+ T cells of the intestinal mucosa and mLN, intraepithelial CD4+ T cells, and intraepithelial CD4+CD8+ T cells, whereas Cadm1 is expressed on gut CD103+ DCs. Crtam–Cadm1 interactions have a major impact on the maintenance of intraepithelial CD4+CD8+ T cells and a limited influence on the presence of mucosal CD4+ and CD8+ T cells. Crtam−/− and Cadm1−/− mice almost completely lacked CD4+CD8+ T cells in the intestinal epithelium under steady-state conditions and had fewer CD4+ and CD8+ T cells in the intestinal mucosa than WT mice. CD4+CD8+ T cells arise from CD4+ T cells that acquire a CD8 T cell lineage gene expression profile upon reaching the intestinal mucosa (Mucida et al., 2013; Reis et al., 2013). Therefore, we further investigated the role of Crtam–Cadm1 interactions in governing CD4+ T cell homing and maintenance in the intestinal mucosa, and found that Crtam−/− CD4+ T cells reconstituted the intestinal CD4+ T cell and CD4+CD8+ T cell subsets less effectively than did WT CD4+ T cells after transfer into Rag1−/− mice. Moreover, fewer intestinal CD4+ T cells in Crtam−/− mice expressed gut homing, adhesion, and retention molecules typical of their counterparts in WT mice (including CCR9, CD103, and CD69), and hence adhered less firmly to the intestinal mucosa.
Crtam deficiency did not affect the intrinsic capacity of naive CD4+ T cells to differentiate into IFN-γ– or IL-17–producing CD4+ T cells in vitro. After acute oral infection with Toxoplasma gondii, both WT and Crtam−/− mice mounted a robust TH1 response and cleared the intestinal infection. However, a preferential reduction of TH17 cells was evident within the intestinal mucosa CD4+ T cells in the Crtam−/− mice. The almost exclusive TH1 response in Crtam−/− mice resulted in more efficient clearance of intestinal infection. Antibody blockade of IL-17 in WT mice orally infected with T. gondii recapitulated the enhanced host response of Crtam−/− mice. Thus, the defects in T cell gut homing and maintenance in Crtam−/− mice preferentially affected TH17, whereas TH1 were relatively unaffected. Because the differentiation of CD4+CD8+ T cells from CD4+ T cells skews CD4+ T cell cytokine production toward IFN-γ at the expenses of IL-17 production (Mucida et al., 2013; Reis et al., 2013), the need for continuous replacement of lost CD4+CD8+ T cells in Crtam−/− mice may result in a relative depletion of TH17 cells. Together, these results demonstrate that Crtam–Cadm1 interactions have a major impact on the residency and maintenance of CD4+CD8+ T cells in the gut mucosa in the steady-state. During mucosal responses against pathogenic infection, Crtam–Cadm1 interactions may be required to retain a balanced representation of disparate CD4+ T cell subsets, thereby influencing the overall quality of the CD4+ T cell response.
RESULTS AND DISCUSSION
Crtam−/− and Cadm1−/− mice have markedly fewer CD4+CD8+ T cells in the intestinal mucosa under steady-state conditions
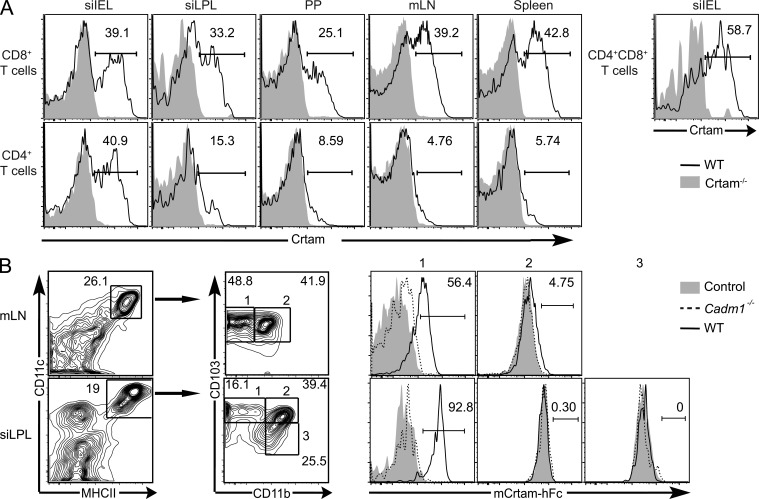
Previous studies showed that mouse Crtam is expressed on the surface of recently activated CD8+ T cells and NK cells (Arase et al., 2005; Boles et al., 2005; Takeuchi et al., 2009) as well as on a subset of activated CD4+ T cells (Yeh et al., 2008). We asked whether Crtam is expressed on T cells in the intestinal mucosa and mLN. All gut T cells expressed very low to undetectable amounts of Crtam ex vivo. Upon stimulation in vitro, robust expression of Crtam was detected on CD8+ T cells from all tissues examined, including intestinal epithelium and lamina propria, Peyer’s Patches, and mLN (Fig. 1 A). In contrast, only activated CD4+ T cells from the intestinal epithelium strongly expressed Crtam. Intraepithelial CD4+CD8+ T cells also expressed high levels of Crtam, consistent with the recent detection of Crtam mRNA in these cells (Mucida et al., 2013).
Figure 1.
CRTAM and its ligand are expressed in intestinal T cells and CD103+ DC, respectively. (A) Frequency of Crtam+ T cells from different tissues 14 h after stimulation with anti-CD3 and -CD28. (B) Frequency of mCRTAM-hFc+ cells within the CD103+ (1), the CD103+CD11b+ (2), and the CD11b+ (3) subsets of CD11c+MHCII+ DCs in the mLN and siLPL of WT and Cadm1−/− mice. All data are representative of two independent experiments.
Cadm1, the ligand of Crtam, is expressed on epithelial cells (Sakisaka and Takai, 2004; Murakami, 2005; Mizutani et al., 2011) and CD8α DCs (Galibert et al., 2005; Poulin et al., 2010), yet the distribution of Cadm1 in the intestinal mucosa has not been determined. To address this, we stained the LPLs from the small intestine and the mLNs with a fusion protein consisting of the extracellular domain of murine Crtam and the Fc portion of human IgG1 (mCrtam-hFc). We found that mCrtam-hFc bound CD11c+ MHCII+ DCs, particularly the CD103+ DC subset (Fig. 1 B). The binding of mCrtam-hFc to gut CD103+ DC was abrogated in Cadm1−/− mice (van der Weyden et al., 2006), indicating that Cadm1 is the bona fide ligand of Crtam in the intestine (Fig. 1 B). Although we observed no binding of mCrtam-hFc to intestinal epithelial cells (unpublished data), the amount of Cadm1 expressed on primary epithelial cells may be too low to be detected with an Fc fusion protein. It is also possible that binding of mCrtam-hFc to Cadm1 on epithelial cells is blocked by the homotypic or heterotypic interactions that Cadm1 establishes with other Nectins and Necls in cis and in trans (Masuda et al., 2002).
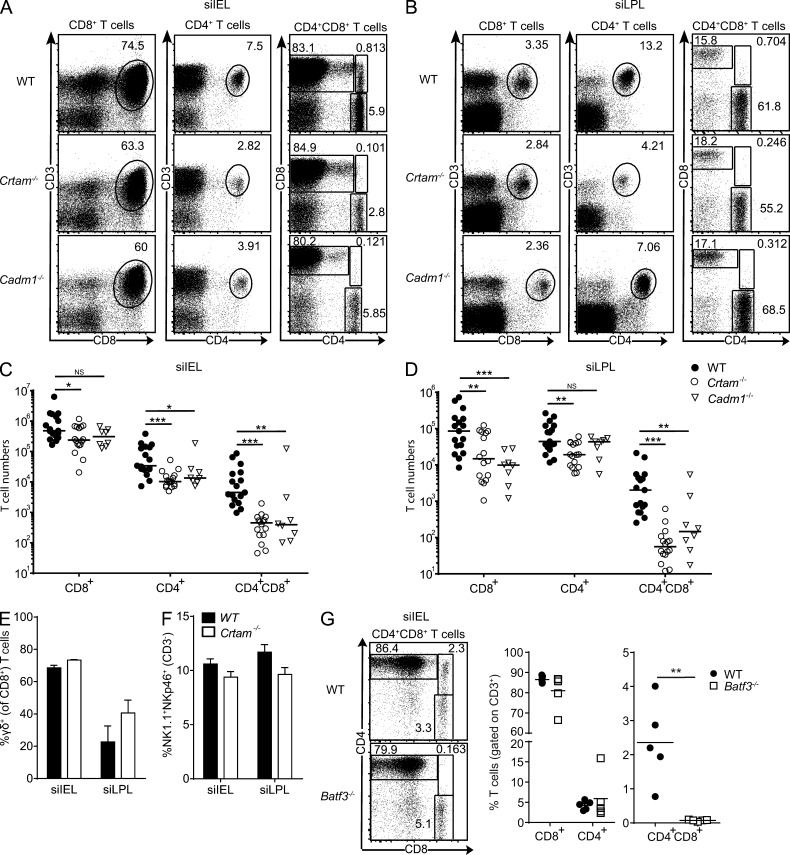
To investigate the functional impact of Crtam on intestinal mucosa T cells, we generated Crtam−/− mice (Fig. S1). Analysis of small intestine IELs and LPLs revealed a partial reduction of CD8+ T cells and CD4+ T cells in naive Crtam−/− mice compared with WT mice (Fig. 2, A–D). Furthermore, the lack of Crtam preferentially affected double-positive CD4+CD8+ T cells, which were almost absent, particularly in the epithelium (Fig. 2, A–D). Percentages of intestinal γδ T cells and intraepithelial ILC1 were similar in Crtam−/− and WT mice (Fig. 2, E and F). Crtam deficiency did not affect the presence of CD4+ or CD8+ T cells in the spleen (unpublished data), confirming previous data obtained with other Crtam−/− mouse strains (Yeh et al., 2008; Takeuchi et al., 2009). Altogether, these data suggest that Crtam is essential for the presence of mucosal CD4+CD8+ T cells and is partially required for intraepithelial and lamina propria CD8+ T cells and CD4+ T cells in the steady state.
Figure 2.
T cells in the small intestine are reduced in Crtam−/− and Cadm1−/− mice. (A–D) Representative plots and total cell numbers of CD8+ T cells (gated on CD45+ cells), CD4+ T cells (gated on CD45+ cells), and CD4+CD8+ T cells (gated on CD3+CD45+) from the siIEL (A and C) and siLPL (B and D) of WT, Crtam−/−, and Cadm1−/− mice. (E–F) Frequencies of γδ T cells (gated on CD8+CD3+; E) and ILC1 (gated on CD3−; F) in the siIEL and siLPL of WT and Crtam−/− mice. (G) Representative plots and frequencies of CD8+ T cells, CD4+ T cells, and CD4+CD8+ T cells from the siIEL of WT and Batf3−/− mice. Data are representative of pooled mice from at least four (A–D) and two (E–G) independent experiments. Statistical analysis was performed using Mann-Whitney test for cell numbers and Student’s t test for frequencies (ns = not significant; *, P < 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). Horizontal bars show medians. Error bars are SEM.
Analysis of IELs and LPLs from Cadm1−/− mice revealed a phenotype strikingly similar to that of Crtam−/− mice, distinguished by the partial reduction of CD8+ and CD4+ T cells and the near absence of CD4+CD8+ T cells (Fig. 2, A–D). Because Crtam is robustly induced on activated IEL and LPL T cells and Cadm1 is constitutively expressed on intestinal CD103+ DCs, we hypothesize that Crtam–Cadm1 interactions govern homing and/or retention of intestinal mucosa T cells. Supporting this notion, Batf3−/− mice, which lack CD103+ DC (Edelson et al., 2010), also showed a strong reduction of intraepithelial CD4+CD8+ T cells (Fig. 2 G). The strict expression pattern of Crtam only on intraepithelial CD4+ T cells, as well the dramatic reduction of CD4+CD8+ T cells, which are derived from CD4+ T cells upon arrival in the intestinal mucosa (Mucida et al., 2013; Reis et al., 2013), persuaded us to further study the role of Crtam specifically in these cells.
Crtam deficiency affects CD4+ T cell acquisition of molecules for homing and retention in the intestine
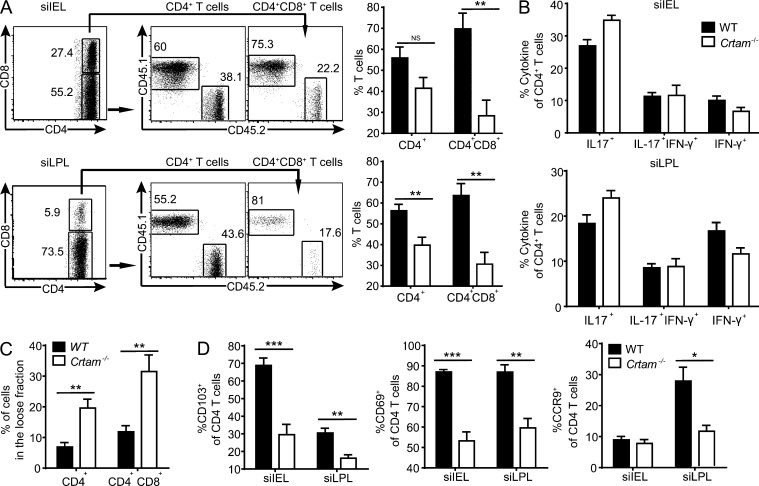
We asked whether Crtam–Cadm1 interaction imprints gut-homing capacity on CD4+ T cells. To test this hypothesis, we performed a competitive adoptive transfer in which purified T cells from WT (CD45.1) and Crtam−/− (CD45.2) mice were injected into Rag1−/− mice at a 1:1 ratio and given 8 wk to reconstitute the small intestine. WT T cells outcompeted Crtam−/− T cells in both the siIEL and siLPLs by frequencies of 60 to 40% for CD4+ T cells and 70 to 30% for CD4+CD8+ T cells (Fig. 3 A). To determine whether defective homing/retention is paralleled by defective acquisition of effector functions, we recovered siIEL and siLPL CD4+ T cells from reconstituted Rag1−/− mice and assessed their cytokine production. We detected no difference in the ability of CD4+ T cells to produce IL-17 or IFN-γ ex vivo, suggesting that there was no defect in T cell priming in these settings (Fig. 3 B).
Figure 3.
Crtam−/− CD4+ T cells have dysfunctional gut homing and retention properties but normal cytokine production. (A) Purified splenic T cells from WT (CD45.1) and Crtam−/− (CD45.2) mice were mixed at 1:1 ratio and transferred i.v. into Rag1−/− mice. Plots and bar graphs show the frequencies of CD45.2 versus CD45.1 within the CD4+ and CD4+CD8+ T cells (gated on CD45+CD3+ cells) in the siIEL and siLPL 8 wk after transfer. (B) Intracellular content of IL-17 and IFN-γ in the siIEL and siLPL recovered from the competitive adoptive transfer in A. (C) T cell adherence was analyzed in the siIEL of WT and Crtam−/− mice by a sequential gentle then stringent isolation. Percentages of CD4+ and CD4+CD8+ T cells in the loose fraction (gentle isolation) of the small intestine are shown. (D) Frequency of CD4+ T cells expressing CD103, CD69, and CCR9 in the siIEL and siLPL of WT and Crtam−/− mice. Data represent two (A and B) and at least three (C and D) independent experiments. Statistical analysis was performed using Student’s t test (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). Error bars are SEM.
To directly test the impact of Crtam on the retention of T cells in the gut, we performed a recently developed method for isolation that distinguishes IELs as either loosely or tightly attached to the intestinal mucosa (Zhang and Bevan, 2013). We found that the fraction of loosely attached CD4+ T cells and CD4+CD8+ T cells was significantly higher in Crtam−/− mice than WT mice (Fig. 3 C). Intraepithelial CD4+ T cells and CD4+CD8+ T cells express CD103, which facilitates their retention in the epithelium (Schön et al., 1999; Casey et al., 2012; Zhang and Bevan, 2013). They also highly express CD69, which is thought to prevent their exit from the mucosa (Shiow et al., 2006; Zhang and Bevan, 2013). Consistent with their loose interaction with the intestinal mucosa, intestinal CD4+ T cells isolated from Crtam−/− mice expressed much less CD103 and CD69 on the cell surface than did their counterparts in WT mice (Fig. 3 D). Moreover, Crtam−/− CD4+ T cells also expressed less CCR9 in LPLs, which is essential for homing in the gut.
It has been shown that CD103+ DCs imprint gut-homing capacity on T cells through the production of RA and TGF-β, which in turn induce CCR9 and CD103 on CD4+ T cells (Mora et al., 2008; Villablanca et al., 2011). In addition, exposure of CD4+ T cells to RA and TGF-β in the intestinal mucosa induces CD4+ T cells to down-regulate the CD4 T cell lineage profile and acquire a CD8 T cell lineage profile to become CD4+CD8+ T cells (Mucida et al., 2013; Reis et al., 2013). Thus, it is likely that Crtam–Cadm1 interactions promote expression of gut-homing molecules on CD4+ T cells as well as the generation of CD4+CD8+ T cells from CD4+ T cells by sustaining the exposure of CD4+ T cells to CD103+ DCs that produce RA and TGF-β. This mechanism is consistent with the overall reduced adhesiveness of Crtam-deficient T cells to other cells in the intestinal mucosa.
Crtam deficiency selectively impairs the presence of intestinal TH17 cells during T. gondii infection
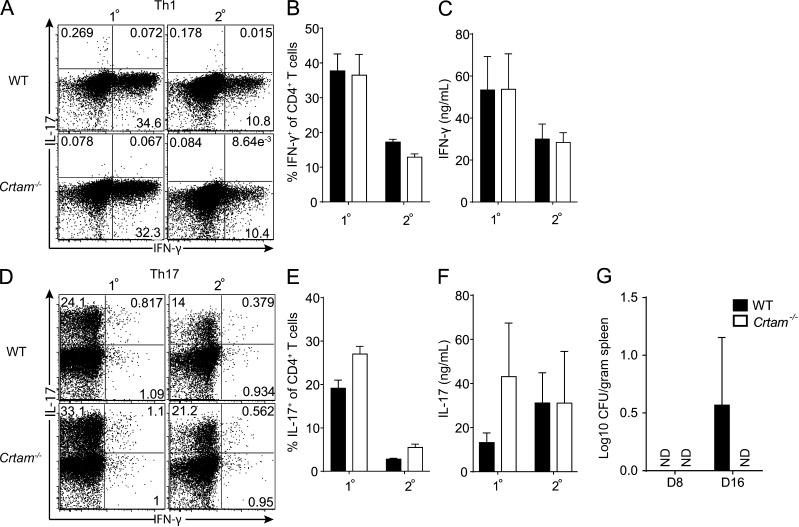
We sought to determine whether Crtam−/− T cells had defects in the capacity to secrete cytokines. A previous study reported that CD4+ T cells from Crtam−/− mice have an intrinsic defect in producing IFN-γ, IL-17, and IL-22 (Yeh et al., 2008). However, we found that naive Crtam−/− and WT CD4+ T cells stimulated through the TCR and exposed to polarizing cytokines produced equivalent amounts of IFN-γ and IL-17 (Fig. 4, A–F). Thus, Crtam−/− T cells have no intrinsic functional polarization defects in vitro. We next sought to determine whether Crtam−/− CD4+ T cells have in vivo functional defects that could impact host responses to pathogens. It was reported that Crtam−/− mice are susceptible to intestinal infections by Citrobacter rodentium (Yeh et al., 2008). This pathogen is primarily controlled through secretion of IL-22 by TH17, TH22, and type-3 ILC (Ouyang et al., 2008; Basu et al., 2012). However, we found that Crtam−/− and WT mice were equally resistant to C. rodentium infection (Fig. 4 G).
Figure 4.
Crtam−/− CD4+ T cells have normal TH1 and TH17 polarization in vitro. 2 × 105 purified CD4+ T cells from LNs of WT or Crtam−/− mice were stimulated in vitro under TH1 or TH17 polarizing conditions for 5 d (1°). For restimulation, T cells were recovered and 2 × 105 cells were plated for 2 additional days without polarizing cytokines (2°). IFN-γ and IL-17 production was assessed by intracellular staining: (A and D) representative plots; (B and E) quantification; and (C and F) cytometric bead array of culture supernatants. (G) WT and Crtam−/− mice were orally infected with 2 × 109 C. rodentium and splenic titers analyzed at days 8 and 16. The CFUs detected in WT at day 16 are insignificant. Data are representative of three (A and B) or two (C) independent experiments. Statistical analysis was performed using Student’s t test (ND, not detected). Error bars are SD.
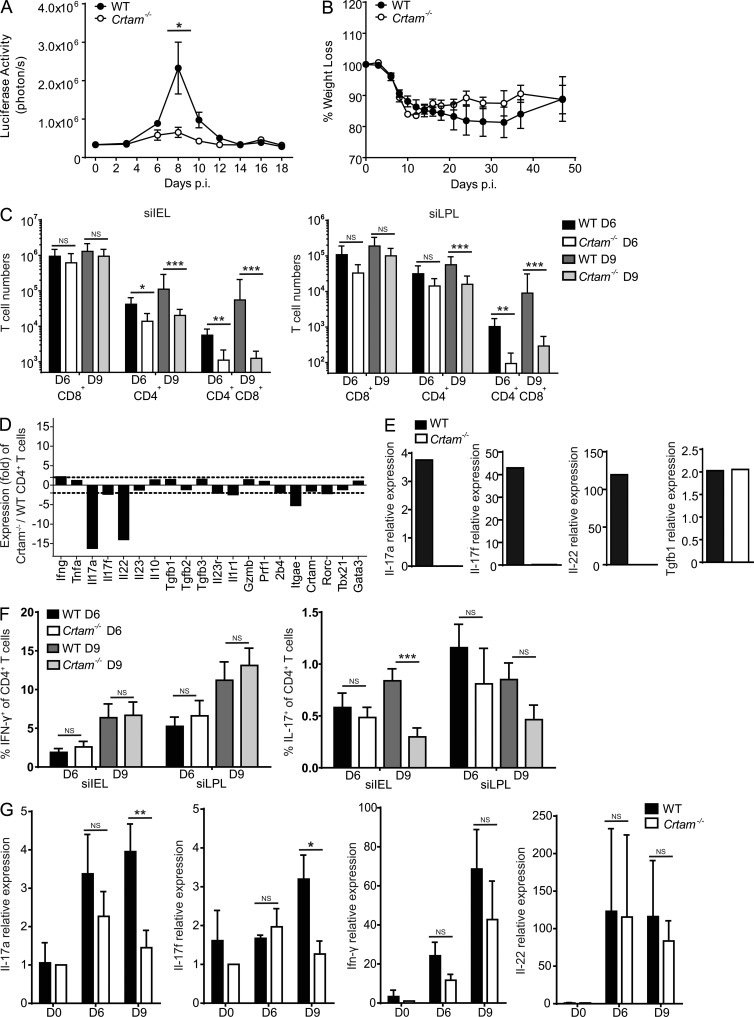
Because Crtam is expressed on several T cell sources of IFN-γ, such as CD8+ T cells, TH1, and CD4+CD8+ T cells, we investigated the impact of Crtam deficiency in the infection by T. gondii, an intracellular parasite which is highly sensitive to host IFN-γ (Suzuki et al., 1988; Gazzinelli et al., 1992; Sher et al., 2003; Goldszmid et al., 2012). We infected mice through the oral route using T. gondii tissue cysts to directly challenge the small intestine immune system. We used a type II avirulent Prugniaud strain of T. gondii expressing a firefly luciferase, so that parasitic replication could be monitored in live mice throughout the infection. After oral infection with T. gondii, WT mice had a peak of parasitic replication at day 8–9 post infection (p.i.) followed by clearance at day 14 p.i. (Fig. 5 A). WT mice lost ∼20% of their initial weight and started to recover weight 5 wk after infection (Fig. 5 B). In contrast, Crtam−/− mice showed minimal or no detectable T. gondii replication (Fig. 5 A) and lost less weight than WT mice during the initial 5 wk of infection (Fig. 5 B). Thus, Crtam−/− mice can control T. gondii intestinal infection more effectively than WT mice.
Figure 5.
Crtam−/− mice are more resistant to T. gondii oral infection due to reduced TH17 response. WT and Crtam−/− mice were orally infected with 5 tissue cysts of T. gondii (pru-luc). (A and B) Parasite burden (A) and weight loss (B) were monitored throughout the course of the infection. (C) Numbers of CD8+ T cells, CD4+ T cells, and CD4+ CD8+ T cells in the siIEL and siLPL of WT and Crtam−/− mice at days 6 and 9 after infection. (D) Gene expression microarray of siIEL CD4+ T cells (CD45+CD3+CD4+CD8−) from WT and Crtam−/− mice at day 8 p.i. The bar graph indicates fold differences in the expression of selected genes of interest between siIEL Crtam−/− and WT CD4+ T cells. The dashed lines are placed at 2.5-fold difference. (E) Quantitative PCR analysis of gene expression in siIEL CD4+ T cells (CD45+CD3+CD4+CD8−) 8 d p.i. (F) Intracellular stain for IFN-γ and IL-17 at days 6 and 9 p.i. (G) Quantitative PCR analysis of gene expression in ileum samples at days 6 and 9 p.i. Values are relative to mRNA expression in naive mice. Data (A–C and F–G) are representative of at least three independent experiments. Statistical analysis was performed using Mann-Whitney test for T cell numbers and Student’s t test for all others (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). Error bars are SEM.
We asked why Crtam−/− mice have a more effective intestinal immune response to T. gondii. Consistent with what we observed in the steady state, severely reduced numbers of CD4+CD8+ T cells and a partial reduction of CD4+ T cells were evident in the IELs and LPLs from the small intestine of Crtam−/− mice after infection with T. gondii (Fig. 5 C). Because the host response to T. gondii infection relies on IFN-γ (Suzuki et al., 1988; Gazzinelli et al., 1992; Sher et al., 2003; Goldszmid et al., 2012) and CD4+CD8+ T cells secrete IFN-γ, it seemed plausible that the reduction of CD4+CD8+ T cells in Crtam−/− mice would be compensated for by changes in other cytokines such that the overall immune response would be improved. Analysis of the transcriptional profile of total CD4+ T cells isolated from the intestine of WT and Crtam−/− mice after infection with T. gondii by microarray revealed a preferential reduction of IL-17A, IL-17F, and IL-22 in Crtam−/− CD4+ T cells, whereas IFN-γ, IL-10, and TGF-β were maintained (Fig. 5 D). Real-time PCR analysis confirmed the reduction in IL-17A, IL-17F, and IL-22, whereas IFN-γ, TGF-β, and IL-10 transcripts were not significantly different in WT and Crtam−/− CD4+ T cells (Fig. 5 E and not depicted). This data suggested a preferential reduction of the TH17 response during T. gondii infection.
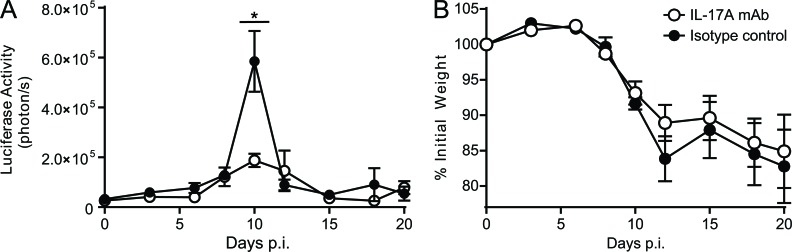
We further investigated the cytokine content of Crtam−/− and WT CD4+ T cells at days 6 and 9 after T. gondii infection by intracellular staining. Both Crtam−/− and WT mice had high frequencies of IFN-γ–producing CD4+ T cells (Fig. 5 F), consistent with the notion that T. gondii infection induces a robust TH1 cell response (Gazzinelli et al., 1992; Sher et al., 2003). IL-17–producing CD4+ T cells were less abundant than IFN-γ–producing CD4+ T cells and were selectively reduced in Crtam−/− mice (Fig. 5 F). The selective reduction of TH17 in the small intestine of Crtam−/− mice resulted in substantial attenuation of IL-17 mRNA in the whole ileum (Fig. 5 G). Ileal IL-22 mRNA was not noticeably reduced despite decreased IL-22 in the CD4+ T cell compartment, suggesting that other cellular sources of IL-22, such as type-3 ILC, may compensate at least in part for the functional defect of Crtam−/− CD4+ TH17 cells. In Crtam−/− mice, because no changes were noted in IFN-γ, which promotes resistance to T. gondii, or IL-10 and TGF-β, which modulate resistance to T. gondii (Mennechet et al., 2004; Jankovic et al., 2007), we hypothesized that the reduced IL-17 production in Crtam−/− mice was sufficient to explain the enhanced clearance of T. gondii. Indeed, antibody blockade of IL-17A (Fig. 6) or IL-17A and F (not depicted) in WT mice orally infected with T. gondii recapitulated the enhanced parasite clearance observed in Crtam−/− mice.
Figure 6.
IL-17 neutralization in WT mice reduces severity of T. gondii infection. WT mice were injected with isotype control or neutralizing antibodies to IL-17A at days −1, 5, and 10 of infection with T. gondii. (A and B) Parasite burden (A) and weight loss (B) were monitored throughout the course of the infection. Data represent two independent experiments. Statistical analysis was performed using Student’s t test (*, P ≤ 0.05). Similar results were obtained using a combination of neutralizing antibodies to IL-17A and IL-17F. Error bars are SEM.
Concluding remarks
Our study demonstrates that Crtam is highly expressed on activated CD4+ and CD4+CD8+ T cells of the intestinal epithelium and more broadly expressed on activated CD8+ T cells of the intestinal mucosa, Peyer’s Patches, and mLNs. Crtam is essential for residency of intraepithelial CD4+CD8+ T cells and is also partially required for mucosal CD8+ and CD4+ T cells under steady-state conditions. Crtam may mediate T cell residency by directly acting as an adhesion molecule that binds Cadm1 on gut CD103+ DCs. By strengthening adherence of T cells to CD103+ DCs, Crtam–Cadm1 interactions may also sustain exposure of T cells to RA and TGF-β, which induce acquisition of gut homing and adhesion molecules on T cells (Coombes et al., 2007; Mora et al., 2008; Villablanca et al., 2011). Moreover, because TGF-β and RA promote the conversion of CD4+ T cells into CD4+CD8+ T cells (Mucida et al., 2013; Reis et al., 2013), Crtam–Cadm1 interactions may also facilitate this conversion. Consistent with this hypothesis, CD4+CD8+ T cells were strongly reduced in the intestinal epithelium of Batf3−/− mice, which lack CD103+ DCs. Although we did not observe Cadm1 on intestinal epithelial cells, they may express low amounts of Cadm1 that, although not detectable with available tools, may help attract and hold T cells expressing Crtam within the epithelium, where they are exposed to TGF-β and RA produced by epithelial cells.
Our study also demonstrates that Crtam–Cadm1 interactions impact intestinal T cell responses during mucosal response against T. gondii infection. Crtam−/− mice mounted a protective TH1 response as robust as WT mice, despite the reduction of CD4+CD8+ T cells and CD4+ T cells. However, Crtam−/− mice had fewer TH17 cells than WT mice, which resulted in more efficient clearance of intestinal T. gondii infection. Antibody-mediated neutralization of IL-17A and IL-17F in WT mice confirmed that limiting IL-17 enhances the clearance of T. gondii. These results corroborate previous reports that IL-17 has deleterious effects on intestinal resistance to T. gondii, possibly by inducing recruitment and activation of neutrophils that damage the epithelial barrier (Guiton et al., 2010).
Because the differentiation of CD4+CD8+ T cells from CD4+ T cells skews CD4+ T cell cytokine production toward IFN-γ at the expense of IL-17 production (Mucida et al., 2013; Reis et al., 2013), the relative depletion of TH17 cells in Crtam−/− mice may reflect an ongoing replacement of lost CD4+CD8+ T cells. The selective impact of Crtam–Cadm1 interactions on intestinal TH17 during T. gondii infection may also depend on the dynamic equilibrium between newly formed T cells and their retention in the gut. Because T. gondii elicits a strong TH1 response, the robust generation of TH1 may be sufficient to compensate for the defective homing and retention of these cells in the intestine. Because of their low frequency during T. gondii infection, TH17 cells may be more affected by Crtam deficiency and defective maintenance in the gut. Further studies using more virulent T. gondii strains, such as ME-49, will further delineate the role of CRTAM in controlling intestinal inflammation. It is also possible that Crtam–Cadm1 interactions have more impact on TH17 than TH1 because TH1 cells express other adhesion molecules that compensate for lack of Crtam. Because T cells express multiple receptors for Nectins and Necls expressed in the gut, such as CD226, CD96, and Tigit (Fuchs and Colonna, 2006), it will be important to assess the contribution of these molecules to T cell residency in the steady state and during host responses to pathogens.
MATERIALS AND METHODS
Generation of Crtam−/− mice.
A targeting construct was designed to replace exon 1, which encodes the initial translation start site, with a floxed neomycin resistance cassette. The construct was electroporated into E14.1 (129P2Ola/Hsd) ES cells. One correctly targeted clone was injected into C57BL/6 blastocysts and chimeras bred to CMV-Cre tg mice to excise the neomycin resistance cassette. The Crtam mutation was backcrossed until the genetic background was >99% C57BL/6, facilitated and confirmed by genome-wide SSLP typing at 10-cM intervals. Heterozygote mice were intercrossed to generate Crtam−/− mice; homozygotes were born at the expected Mendelian frequency and were phenotypically normal. Both males and females were fertile and bred very similarly to C57BL/6 mice housed in the same room.
Mice.
Rag1−/− mice were obtained from The Jackson Laboratory. Cadm1−/− mice were generated by A. Bradley (Wellcome Trust Sanger Institute, Hinxton, England, UK; van der Weyden et al., 2006) and were backcrossed onto a C57BL/6 background until >99% C57BL/6, facilitated and confirmed by genome-wide SSLP typing at 10-cM intervals. Crtam−/−, Cadm1−/−, Rag1−/−, C57BL/6, and Batf3−/− on a 129S6/SvEv background and 129S6/SvEv mice were bred in a pathogen-free facility at Washington University. Age- and sex-matched animals were used throughout the experiments. All animal experiments were conducted according to USA Public Health Service Policy of Humane Care and Use of Laboratory Animals. All protocols were approved by the Institutional Animal Care and Use Committee (School of Medicine, Washington University, St. Louis, MO).
T. gondii infection.
The luciferase-expressing PRU-Luc-GFP type II of T. gondii strain was provided by J. Boothroyd (Stanford University, Palo Alto, CA). Tissue cysts were obtained from the brains of CD1 outbred mice (Charles River) after 3 mo of infection with 20 cysts by i.p. injection. Experimental mice were infected with 5 cysts of T. gondii PRU-Luc-GFP orally. Weight was monitored every 48 h for the first 20 d and twice a week until day 50. Parasite burden was analyzed by bioluminescence measurements. Mice were imaged every 48 h for 20 d by i.p. injection of 150 mg of luciferin D (Biosynth AG) per kilogram of body weight and using a Xenogen IVIS 100 (Caliper Life Sciences). Data were analyzed with the Living Image Software (Caliper Life Sciences) and is expressed in relative light units. Antibody blockade was performed using anti–IL-17A (clone 17F3; Bio X Cell), isotype control (clone MOPC-21, Bio X Cell), and, in some experiments, anti–IL-17A and anti–IL-17F antibodies (provided by W. Ouyen, Genentech, South San Francisco, CA). For those experiments, mice were injected i.p. with 350 µg antibodies at days −1, 5, and 10 of T. gondii infection.
Tissue isolation, flow cytometry, and cell sorting.
Small intestine IELs, small intestine LPLs, and Peyer’s Patches were prepared as described by Lefrancois and Lycke (2001). For flow cytometry, the following fluorophore-labeled mAbs were used: CD3 (145-2C11), CD8α (53–6.7), and CD69 (H1.2F3; all BD); CD4 (GK1.5), CD103 (2E7), and CCR9 (eBioCW-1.2; eBioscience); CD45 (30F11.1; Miltenyi Biotec); Crtam (Cr24.1; produced in our laboratory; Boles et al., 2005); and Crtam (11–5; BioLegend). For assessing the expression of CRTAM ligands, we used a mouse CRTAM-human Fc fusion protein (Boles et al., 2005) together with a mouse anti–human IgG (JDC-10; SouthernBiotech). Streptavidin conjugated to APC-Cy7 was from BD and streptavidin conjugated to APC was from Invitrogen. Samples were processed in a FACSCanto II (BD) and analyzed with FlowJo software (Tree Star). Flow cytometric cell sorting was performed using a FACSAria II (BD).
T cell stimulation.
To detect Crtam expression cells were stimulated for 14 h with plate-bound anti-CD3 and anti-CD28, followed by surface staining for T cell markers and Crtam. For intracellular staining, cells were stimulated with PMA and Ionomycin for 4 h in the presence of Golgi Plug (BD). Surface staining was performed, followed by fixation and permeabilization (Cytofix/Cytoperm Plus kit; BD). Intracellular cytokine contents were determined using the mAbs IL-17A (eBio17B7; eBioscience) and IFN-γ (XMG1.2; BD).
T cell adherence.
To analyze the percentage of loosely associated IEL fraction, we used a protocol recently described (Zhang and Bevan, 2013). In brief, a gentle dissociation of the small intestine consisting of stirring at 400 rpm in HBSS for 20 min was performed to obtain the loosely attached IEL before two vigorous extractions of stirring at 800 rpm in HBSS+ DTT for 20 min. The percentage of loose IEL was calculated using the absolute numbers of CD4+ and CD4+CD8+ T cells obtained in the loose and tight IEL fractions from each mouse.
Adoptive transfer.
Splenocytes from naive C57BL/6 (CD45.1) and Crtam−/− (CD45.2) mice were obtained and T cells purified with the Pan T cell isolation kit II (Miltenyi Biotec) and mixed at a 1:1 ratio, which was confirmed by flow cytometry. 106 cells were injected i.v. into Rag1−/− mice. 8 wk after transfer, frequencies of CD45.1 versus CD45.2 CD4+ and CD4+CD8+ T cells were analyzed in the small intestine of recipient mice.
In vitro T cell polarization.
LN CD4+ T cells from WT and Crtam−/− mice were enriched using a CD4+ T cell isolation kit (Miltenyi Biotec). 2 × 105 cells were activated with plate-bound anti-CD3 (10 µg/ml) and anti-CD28 (2 µg/ml) under polarizing TH1 conditions, i.e., recombinant IL-2 (PeproTech), mIL-12 (PeproTech), and anti–mouse IL-4 mAb (11B11), or TH17 conditions, i.e., recombinant mIL-6 (PeproTech), hTGFb, mIL-23, mAb anti-IL-4 (11B11), and anti–IFN-γ (RUGA2) for 5 d (primary stimulation). For the secondary stimulation, T cells were washed, counted, and 2 × 105 cells were restimulated with plate-bound anti-CD3 (10 µg/ml) and anti-CD28 (2 µg/ml) without added cytokines or antibodies for 2 more days. Cells were analyzed for cytokine production by intracellular staining either after 5-d stimulation or 2-d restimulation. Supernatants were also collected at these times, and IFN-γ and IL-17 concentrations determined by cytometric bead array (BD).
C. rodentium infection.
Experimental mice were made to fast for 8 h before intraoral inoculation of 2 × 109 C. rodentium strain DBS100 (American Type Culture Collection). Survival was monitored for 21 d. Spleens were weighed and homogenized and serial dilutions were plated for 24 h at 37°C onto MacConkey agar plates for measurement of colony-forming units.
RNA extraction and quantitative PCR.
RNA was extracted from cell-sorted populations of siIEL with an RNeasy micro kit and from ileum samples with an RNeasy Mini kit according to the manufacturer’s recommendations (QIAGEN). cDNA was synthesized from RNA with Superscript III first-strand synthesis system for RT-PCR (Invitrogen). RNA expression was analyzed by quantitative PCR using SYBR Green PCR Master Mix (Bio-Rad Laboratories) and an ABI7000 (Applied Biosystems). The following oligonucleotides were used: Tgfb1 forward, 5′-TTGCTTCAGCTCCACAGAGA-3′; Tgfb1 reverse, 5′-TGGTTGTAGAGGGCAAGGAC-3′; Il10 forward, 5′-TGGCCTTGTAGACACCTTGG-3′; Il10 reverse, 5′-AGCTGAAGACCCTCAGGATG-3′; Il17a forward, 5′-AGAGCTGCCCCTTCACTTTC-3′; Il17a reverse, 5′-TGGGGGTTTCTTAGGGGTCA-3′; Il17f forward, 5′-CTGGAGGATAACACTGTGAGAGT-3′; Il17f reverse, 5′-TGCTGAATGGCGACGGAGTTC-3′; Il22 forward, 5′-GCTCAGCTCCTGTCACATCA-3′; Il22 reverse, 5′-AGCTTCTTCTCGCTCAGACG-3′; ifng forward, 5′-ACAATGAACGCTACACACTGCAT-3′; ifng reverse, 5′-TGGCAGTAACAGCCAGAAACA-3′; tbp forward, 5′-CCTTCACCAATGACTCCTATGAC-3′; and tbp reverse, 5′-CAAGTTTACAGCCAAGATTCAC-3′. The expression of target mRNA was calculated and normalized to the expression of the house keeping gene tbp (tata-box binding protein) using the 2(−ΔΔCT) method.
Expression microarray analysis.
CD4+ T cells (CD45+CD3+CD4+CD8α−) from the siIEL of WT and Crtam−/− mice at day 8 after T. gondii infection were sorted. Total RNA was isolated from cells using RNeasy micro kit (QIAGEN). Mouse Gene 1.0 ST arrays were performed. RNA was amplified with the WT Expression kit (Invitrogen) and labeled, fragmented, and hybridized with the WT Terminal Labeling and Hybridization kit (Affymetrix). Data were processed using RMA (robust multichip average) quantile normalization, and expression values were modeled using ArrayStar software (DNASTAR).
Statistical analysis.
All statistical analyses were performed with Prism 5.0 (GraphPad Software). Cell numbers were analyzed by Mann-Whitney test; all other data were analyzed with a nonpaired Student’s t test. A p-value of <0.05 was considered significant.
Online supplemental material.
Figure S1 shows Crtam−/− mice. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20130904/DC1.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants 1U01AI095542, R01DE021255, and R21CA16719. V.S. Cortez was supported by the Infectious Disease Training Grant T32 AI 7172-34. L. Cervantes-Barragan was supported by Swiss National Science Foundation (PBSKP3-134332) and the Swiss Foundation for Medical-Biological Grants (PASMP3-145751). We thank the Genome Technology Access Center in the Department of Genetics at Washington University School of Medicine for help with genomic analysis. The Center is partially supported by NCI Cancer Center Support Grant no. P30 CA91842 to the Siteman Cancer Center and by ICTS/CTSA Grant no. UL1TR000448 from the National Center for Research Resources (NCRR), a component of the NIH, and NIH Roadmap for Medical Research. This publication is solely the responsibility of the authors and does not necessarily represent the official view of NCRR or NIH.
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- Cadm1
- cell adhesion molecule 1
- Crtam
- class I–restricted T cell–associated molecule
- IEL
- intraepithelial lymphocyte
- ILC
- innate lymphoid cell
- LPL
- lamina propria lymphocyte
- mLN
- mesenteric LN
- Necl
- Nectin like
- RA
- retinoic acid
References
- Arase N., Takeuchi A., Unno M., Hirano S., Yokosuka T., Arase H., Saito T. 2005. Heterotypic interaction of CRTAM with Necl2 induces cell adhesion on activated NK cells and CD8+ T cells. Int. Immunol. 17:1227–1237 10.1093/intimm/dxh299 [DOI] [PubMed] [Google Scholar]
- Basu R., O’Quinn D.B., Silberger D.J., Schoeb T.R., Fouser L., Ouyang W., Hatton R.D., Weaver C.T. 2012. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity. 37:1061–1075 10.1016/j.immuni.2012.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles K.S., Barchet W., Diacovo T., Cella M., Colonna M. 2005. The tumor suppressor TSLC1/NECL-2 triggers NK-cell and CD8+ T-cell responses through the cell-surface receptor CRTAM. Blood. 106:779–786 10.1182/blood-2005-02-0817 [DOI] [PubMed] [Google Scholar]
- Casey K.A., Fraser K.A., Schenkel J.M., Moran A., Abt M.C., Beura L.K., Lucas P.J., Artis D., Wherry E.J., Hogquist K., et al. 2012. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J. Immunol. 188:4866–4875 10.4049/jimmunol.1200402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti A. 2008. Location, location, location: B-cell differentiation in the gut lamina propria. Mucosal Immunol. 1:8–10 10.1038/mi.2007.8 [DOI] [PubMed] [Google Scholar]
- Cheroutre H., Lambolez F., Mucida D. 2011. The light and dark sides of intestinal intraepithelial lymphocytes. Nat. Rev. Immunol. 11:445–456 10.1038/nri3007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes J.L., Siddiqui K.R., Arancibia-Cárcamo C.V., Hall J., Sun C.M., Belkaid Y., Powrie F. 2007. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β– and retinoic acid–dependent mechanism. J. Exp. Med. 204:1757–1764 10.1084/jem.20070590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePaolo R.W., Abadie V., Tang F., Fehlner-Peach H., Hall J.A., Wang W., Marietta E.V., Kasarda D.D., Waldmann T.A., Murray J.A., et al. 2011. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature. 471:220–224 10.1038/nature09849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelson B.T., Kc W., Juang R., Kohyama M., Benoit L.A., Klekotka P.A., Moon C., Albring J.C., Ise W., Michael D.G., et al. 2010. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8α+ conventional dendritic cells. J. Exp. Med. 207:823–836 10.1084/jem.20091627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs A., Colonna M. 2006. The role of NK cell recognition of nectin and nectin-like proteins in tumor immunosurveillance. Semin. Cancer Biol. 16:359–366 10.1016/j.semcancer.2006.07.002 [DOI] [PubMed] [Google Scholar]
- Galibert L., Diemer G.S., Liu Z., Johnson R.S., Smith J.L., Walzer T., Comeau M.R., Rauch C.T., Wolfson M.F., Sorensen R.A., et al. 2005. Nectin-like protein 2 defines a subset of T-cell zone dendritic cells and is a ligand for class-I-restricted T-cell-associated molecule. J. Biol. Chem. 280:21955–21964 10.1074/jbc.M502095200 [DOI] [PubMed] [Google Scholar]
- Gazzinelli R., Xu Y., Hieny S., Cheever A., Sher A. 1992. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J. Immunol. 149:175–180 [PubMed] [Google Scholar]
- Goldszmid R.S., Caspar P., Rivollier A., White S., Dzutsev A., Hieny S., Kelsall B., Trinchieri G., Sher A. 2012. NK cell-derived interferon-γ orchestrates cellular dynamics and the differentiation of monocytes into dendritic cells at the site of infection. Immunity. 36:1047–1059 10.1016/j.immuni.2012.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiton R., Vasseur V., Charron S., Arias M.T., Van Langendonck N., Buzoni-Gatel D., Ryffel B., Dimier-Poisson I. 2010. Interleukin 17 receptor signaling is deleterious during Toxoplasma gondii infection in susceptible BL6 mice. J. Infect. Dis. 202:427–435 10.1086/653738 [DOI] [PubMed] [Google Scholar]
- Hall J.A., Grainger J.R., Spencer S.P., Belkaid Y. 2011. The role of retinoic acid in tolerance and immunity. Immunity. 35:13–22 10.1016/j.immuni.2011.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabri B., Ebert E. 2007. Human CD8+ intraepithelial lymphocytes: a unique model to study the regulation of effector cytotoxic T lymphocytes in tissue. Immunol. Rev. 215:202–214 10.1111/j.1600-065X.2006.00481.x [DOI] [PubMed] [Google Scholar]
- Jankovic D., Kullberg M.C., Feng C.G., Goldszmid R.S., Collazo C.M., Wilson M., Wynn T.A., Kamanaka M., Flavell R.A., Sher A. 2007. Conventional T-bet(+)Foxp3(-) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J. Exp. Med. 204:273–283 10.1084/jem.20062175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson-Lindbom B., Agace W.W. 2007. Generation of gut-homing T cells and their localization to the small intestinal mucosa. Immunol. Rev. 215:226–242 10.1111/j.1600-065X.2006.00482.x [DOI] [PubMed] [Google Scholar]
- Kennedy J., Vicari A.P., Saylor V., Zurawski S.M., Copeland N.G., Gilbert D.J., Jenkins N.A., Zlotnik A. 2000. A molecular analysis of NKT cells: identification of a class-I restricted T cell-associated molecule (CRTAM). J. Leukoc. Biol. 67:725–734 [DOI] [PubMed] [Google Scholar]
- Lefrançois L., Lycke N. 2001. Isolation of mouse small intestinal intraepithelial lymphocytes, Peyer’s patch, and lamina propria cells. Curr. Protoc. Immunol. Chapter 3:19. [DOI] [PubMed] [Google Scholar]
- Masuda M., Yageta M., Fukuhara H., Kuramochi M., Maruyama T., Nomoto A., Murakami Y. 2002. The tumor suppressor protein TSLC1 is involved in cell-cell adhesion. J. Biol. Chem. 277:31014–31019 10.1074/jbc.M203620200 [DOI] [PubMed] [Google Scholar]
- Mennechet F.J., Kasper L.H., Rachinel N., Minns L.A., Luangsay S., Vandewalle A., Buzoni-Gatel D. 2004. Intestinal intraepithelial lymphocytes prevent pathogen-driven inflammation and regulate the Smad/T-bet pathway of lamina propria CD4+ T cells. Eur. J. Immunol. 34:1059–1067 10.1002/eji.200324416 [DOI] [PubMed] [Google Scholar]
- Mizutani K., Kawano S., Minami A., Waseda M., Ikeda W., Takai Y. 2011. Interaction of nectin-like molecule 2 with integrin α6β4 and inhibition of disassembly of integrin α6β4 from hemidesmosomes. J. Biol. Chem. 286:36667–36676 10.1074/jbc.M110.200535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora J.R., Iwata M., von Andrian U.H. 2008. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat. Rev. Immunol. 8:685–698 10.1038/nri2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D., Park Y., Kim G., Turovskaya O., Scott I., Kronenberg M., Cheroutre H. 2007. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 317:256–260 10.1126/science.1145697 [DOI] [PubMed] [Google Scholar]
- Mucida D., Husain M.M., Muroi S., van Wijk F., Shinnakasu R., Naoe Y., Reis B.S., Huang Y., Lambolez F., Docherty M., et al. 2013. Transcriptional reprogramming of mature CD4+ helper T cells generates distinct MHC class II-restricted cytotoxic T lymphocytes. Nat. Immunol. 14:281–289 10.1038/ni.2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y. 2005. Involvement of a cell adhesion molecule, TSLC1/IGSF4, in human oncogenesis. Cancer Sci. 96:543–552 10.1111/j.1349-7006.2005.00089.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W., Kolls J.K., Zheng Y. 2008. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 28:454–467 10.1016/j.immuni.2008.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin L.F., Salio M., Griessinger E., Anjos-Afonso F., Craciun L., Chen J.L., Keller A.M., Joffre O., Zelenay S., Nye E., et al. 2010. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8α+ dendritic cells. J. Exp. Med. 207:1261–1271 10.1084/jem.20092618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis B.S., Rogoz A., Costa-Pinto F.A., Taniuchi I., Mucida D. 2013. Mutual expression of the transcription factors Runx3 and ThPOK regulates intestinal CD4+ T cell immunity. Nat. Immunol. 14:271–280 10.1038/ni.2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakisaka T., Takai Y. 2004. Biology and pathology of nectins and nectin-like molecules. Curr. Opin. Cell Biol. 16:513–521 10.1016/j.ceb.2004.07.007 [DOI] [PubMed] [Google Scholar]
- Schön M.P., Arya A., Murphy E.A., Adams C.M., Strauch U.G., Agace W.W., Marsal J., Donohue J.P., Her H., Beier D.R., et al. 1999. Mucosal T lymphocyte numbers are selectively reduced in integrin αE (CD103)-deficient mice. J. Immunol. 162:6641–6649 [PubMed] [Google Scholar]
- Sher A., Collazzo C., Scanga C., Jankovic D., Yap G., Aliberti J. 2003. Induction and regulation of IL-12-dependent host resistance to Toxoplasma gondii. Immunol. Res. 27:521–528 10.1385/IR:27:2-3:521 [DOI] [PubMed] [Google Scholar]
- Sheridan B.S., Lefrançois L. 2011. Regional and mucosal memory T cells. Nat. Immunol. 12:485–491 10.1038/ni.2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiow L.R., Rosen D.B., Brdicková N., Xu Y., An J., Lanier L.L., Cyster J.G., Matloubian M. 2006. CD69 acts downstream of interferon-α/β to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 440:540–544 10.1038/nature04606 [DOI] [PubMed] [Google Scholar]
- Spits H., Artis D., Colonna M., Diefenbach A., Di Santo J.P., Eberl G., Koyasu S., Locksley R.M., McKenzie A.N., Mebius R.E., et al. 2013. Innate lymphoid cells—a proposal for uniform nomenclature. Nat. Rev. Immunol. 13:145–149 10.1038/nri3365 [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Orellana M.A., Schreiber R.D., Remington J.S. 1988. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 240:516–518 10.1126/science.3128869 [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Itoh Y., Takumi A., Ishihara C., Arase N., Yokosuka T., Koseki H., Yamasaki S., Takai Y., Miyoshi J., et al. 2009. CRTAM confers late-stage activation of CD8+ T cells to regulate retention within lymph node. J. Immunol. 183:4220–4228 10.4049/jimmunol.0901248 [DOI] [PubMed] [Google Scholar]
- van der Weyden L., Arends M.J., Chausiaux O.E., Ellis P.J., Lange U.C., Surani M.A., Affara N., Murakami Y., Adams D.J., Bradley A. 2006. Loss of TSLC1 causes male infertility due to a defect at the spermatid stage of spermatogenesis. Mol. Cell. Biol. 26:3595–3609 10.1128/MCB.26.9.3595-3609.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villablanca E.J., Cassani B., von Andrian U.H., Mora J.R. 2011. Blocking lymphocyte localization to the gastrointestinal mucosa as a therapeutic strategy for inflammatory bowel diseases. Gastroenterology. 140:1776–1784: 1784.e5 10.1053/j.gastro.2011.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J.H., Sidhu S.S., Chan A.C. 2008. Regulation of a late phase of T cell polarity and effector functions by Crtam. Cell. 132:846–859 10.1016/j.cell.2008.01.013 [DOI] [PubMed] [Google Scholar]
- Zhang N., Bevan M.J. 2013. Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity. 39:687–696 10.1016/j.immuni.2013.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.