Abstract
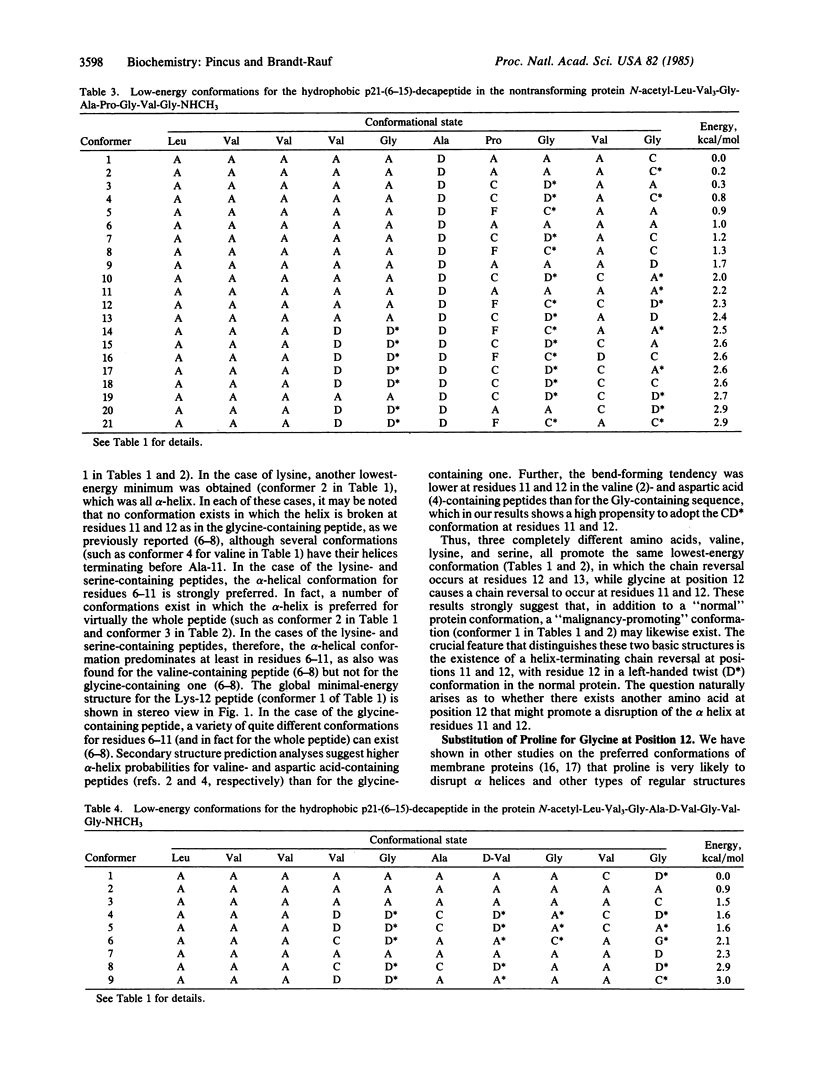
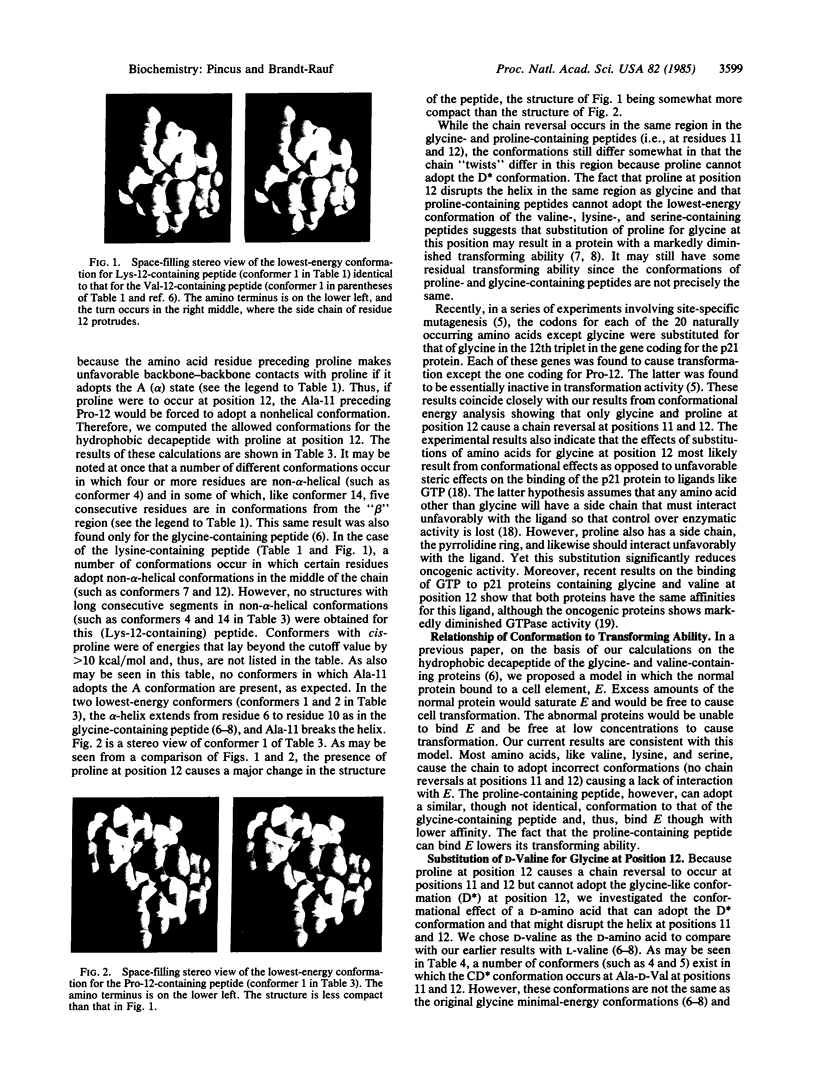
The conformational effects of different amino acid substitutions (lysine, serine, proline, and D-valine) for glycine at position 12 in the p21 oncogene-encoded proteins have been investigated by using conformational energy calculations. The normal cellular gene codes for a glycine at position 12 in the amino acid sequence, in the middle of a hydrophobic p21-(6-15)-decapepetide from Leu-6 to Gly-15. Mutations that cause amino acid substitutions for Gly-12 result in a protein product that produces malignant transformation of cells. We now find that not only are the preferred structures for the lysine- and serine-containing peptides more restricted and more helical than those for the glycine-containing peptide, but the lowest-energy structure for each substituted peptide is exactly the same as that previously found for the peptide with Val-12, suggesting the existence of a "malignancy-causing" conformation. None of the preferred conformations for the valine-, lysine-, and serine-containing peptides contain chain reversals at positions 11 and 12. However, we find that proline, unlike these residues but like glycine, at position 12 causes helix termination at positions 11 and 12, a result that suggests that the p21 protein product with proline at position 12 may exhibit lowered transforming potential, in agreement with the results of recent genetic recombination experiments.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang E. H., Furth M. E., Scolnick E. M., Lowy D. R. Tumorigenic transformation of mammalian cells induced by a normal human gene homologous to the oncogene of Harvey murine sarcoma virus. Nature. 1982 Jun 10;297(5866):479–483. doi: 10.1038/297479a0. [DOI] [PubMed] [Google Scholar]
- Dygert M., Gō N., Scheraga H. A. Use of a symmetry condition to compute the conformation of gramicidin S1. Macromolecules. 1975 Nov-Dec;8(6):750–761. doi: 10.1021/ma60048a016. [DOI] [PubMed] [Google Scholar]
- McGrath J. P., Capon D. J., Goeddel D. V., Levinson A. D. Comparative biochemical properties of normal and activated human ras p21 protein. Nature. 1984 Aug 23;310(5979):644–649. doi: 10.1038/310644a0. [DOI] [PubMed] [Google Scholar]
- Pincus M. R., Klausner R. D. Prediction of the three-dimensional structure of the leader sequence of pre-kappa light chain, a hexadecapeptide. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3413–3417. doi: 10.1073/pnas.79.11.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus M. R., Klausner R. D., Scheraga H. A. Calculation of the three-dimensional structure of the membrane-bound portion of melittin from its amino acid sequence. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5107–5110. doi: 10.1073/pnas.79.16.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus M. R., van Renswoude J., Harford J. B., Chang E. H., Carty R. P., Klausner R. D. Prediction of the three-dimensional structure of the transforming region of the EJ/T24 human bladder oncogene product and its normal cellular homologue. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5253–5257. doi: 10.1073/pnas.80.17.5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy E. P., Reynolds R. K., Santos E., Barbacid M. A point mutation is responsible for the acquisition of transforming properties by the T24 human bladder carcinoma oncogene. Nature. 1982 Nov 11;300(5888):149–152. doi: 10.1038/300149a0. [DOI] [PubMed] [Google Scholar]
- Santos E., Reddy E. P., Pulciani S., Feldmann R. J., Barbacid M. Spontaneous activation of a human proto-oncogene. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4679–4683. doi: 10.1073/pnas.80.15.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg P. H., Colby W. W., Capon D. J., Goeddel D. V., Levinson A. D. Biological properties of human c-Ha-ras1 genes mutated at codon 12. Nature. 1984 Nov 1;312(5989):71–75. doi: 10.1038/312071a0. [DOI] [PubMed] [Google Scholar]
- Tabin C. J., Bradley S. M., Bargmann C. I., Weinberg R. A., Papageorge A. G., Scolnick E. M., Dhar R., Lowy D. R., Chang E. H. Mechanism of activation of a human oncogene. Nature. 1982 Nov 11;300(5888):143–149. doi: 10.1038/300143a0. [DOI] [PubMed] [Google Scholar]
- Wierenga R. K., Hol W. G. Predicted nucleotide-binding properties of p21 protein and its cancer-associated variant. Nature. 1983 Apr 28;302(5911):842–844. doi: 10.1038/302842a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. S., Pottle M. S., Némethy G., Scheraga H. A. Conformational analysis of the 20 naturally occurring amino acid residues using ECEPP. Macromolecules. 1977 Jan-Feb;10(1):1–9. doi: 10.1021/ma60055a001. [DOI] [PubMed] [Google Scholar]