Abstract
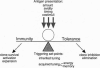
Immunological self-tolerance is ensured by eliminating or inhibiting self-reactive lymphocyte clones, creating physical or functional holes in the B- and T-lymphocyte antigen receptor repertoires. The nature and size of these gaps in our immune defenses must be balanced against the necessity of mounting rapid immune responses to an everchanging array of foreign pathogens. To achieve this balance, only a fraction of particularly hazardous self-reactive clones appears to be physically eliminated from the repertoire in a manner that fully prevents their recruitment into an antimicrobial immune response. Many self-reactive cells are retained with a variety of conditional and potentially flexible restraints: (i) their ability to be triggered by antigen is diminished by mechanisms that tune down signaling by their antigen receptors, (ii) their ability to carry out inflammatory effector functions can be inhibited, and (iii) their capacity to migrate and persist is constrained. This balance between tolerance and immunity can be shifted, altering susceptibility to autoimmune disease and to infection by genetic or environmental differences either in the way antigens are presented, in the tuning molecules that adjust triggering set points for lymphocyte responses to antigen, or in the effector molecules that eliminate, retain, or expand particular clones.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein S., Pritchard-Briscoe H., Anderson T. A., Crosbie J., Gammon G., Loblay R. H., Basten A., Goodnow C. C. Induction of self-tolerance in T cells but not B cells of transgenic mice expressing little self antigen. Science. 1991 Mar 8;251(4998):1223–1225. doi: 10.1126/science.1900950. [DOI] [PubMed] [Google Scholar]
- Ahearn J. M., Fearon D. T. Structure and function of the complement receptors, CR1 (CD35) and CR2 (CD21). Adv Immunol. 1989;46:183–219. doi: 10.1016/s0065-2776(08)60654-9. [DOI] [PubMed] [Google Scholar]
- Alkalay I., Yaron A., Hatzubai A., Jung S., Avraham A., Gerlitz O., Pashut-Lavon I., Ben-Neriah Y. In vivo stimulation of I kappa B phosphorylation is not sufficient to activate NF-kappa B. Mol Cell Biol. 1995 Mar;15(3):1294–1301. doi: 10.1128/mcb.15.3.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alters S. E., Shizuru J. A., Ackerman J., Grossman D., Seydel K. B., Fathman C. G. Anti-CD4 mediates clonal anergy during transplantation tolerance induction. J Exp Med. 1991 Feb 1;173(2):491–494. doi: 10.1084/jem.173.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J., Coutinho A., Lernhardt W., Melchers F. Clonal growth and maturation to immunoglobulin secretion in vitro of every growth-inducible B lymphocyte. Cell. 1977 Jan;10(1):27–34. doi: 10.1016/0092-8674(77)90136-2. [DOI] [PubMed] [Google Scholar]
- Andersson J., Coutinho A., Melchers F. Stimulation of murine B lymphocytes to IgG synthesis and secretion by the mitogens lipopolysaccharide and lipoprotein and its inhibition by anti-immunoglobulin antibodies. Eur J Immunol. 1978 May;8(5):336–343. doi: 10.1002/eji.1830080509. [DOI] [PubMed] [Google Scholar]
- Antonia S. J., Geiger T., Miller J., Flavell R. A. Mechanisms of immune tolerance induction through the thymic expression of a peripheral tissue-specific protein. Int Immunol. 1995 May;7(5):715–725. doi: 10.1093/intimm/7.5.715. [DOI] [PubMed] [Google Scholar]
- Ashton-Rickardt P. G., Bandeira A., Delaney J. R., Van Kaer L., Pircher H. P., Zinkernagel R. M., Tonegawa S. Evidence for a differential avidity model of T cell selection in the thymus. Cell. 1994 Feb 25;76(4):651–663. doi: 10.1016/0092-8674(94)90505-3. [DOI] [PubMed] [Google Scholar]
- Bachmann M. F., Rohrer U. H., Kündig T. M., Bürki K., Hengartner H., Zinkernagel R. M. The influence of antigen organization on B cell responsiveness. Science. 1993 Nov 26;262(5138):1448–1451. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Bazan F., Blanchard D., Brière F., Galizzi J. P., van Kooten C., Liu Y. J., Rousset F., Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- Beckman E. M., Porcelli S. A., Morita C. T., Behar S. M., Furlong S. T., Brenner M. B. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature. 1994 Dec 15;372(6507):691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- Bell S. E., Goodnow C. C. A selective defect in IgM antigen receptor synthesis and transport causes loss of cell surface IgM expression on tolerant B lymphocytes. EMBO J. 1994 Feb 15;13(4):816–826. doi: 10.1002/j.1460-2075.1994.tb06324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandoola A., Cho E. A., Yui K., Saragovi H. U., Greene M. I., Quill H. Reduced CD3-mediated protein tyrosine phosphorylation in anergic CD4+ and CD8+ T cells. J Immunol. 1993 Sep 1;151(5):2355–2367. [PubMed] [Google Scholar]
- Bijsterbosch M. K., Klaus G. G. Crosslinking of surface immunoglobulin and Fc receptors on B lymphocytes inhibits stimulation of inositol phospholipid breakdown via the antigen receptors. J Exp Med. 1985 Dec 1;162(6):1825–1836. doi: 10.1084/jem.162.6.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman M. A., Burgert H. G., Gerhard-Burgert H., Woodland D. L., Palmer E., Kappler J. W., Marrack P. A role for clonal inactivation in T cell tolerance to Mls-1a. Nature. 1990 Jun 7;345(6275):540–542. doi: 10.1038/345540a0. [DOI] [PubMed] [Google Scholar]
- Blackman M. A., Finkel T. H., Kappler J., Cambier J., Marrack P. Altered antigen receptor signaling in anergic T cells from self-tolerant T-cell receptor beta-chain transgenic mice. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6682–6686. doi: 10.1073/pnas.88.15.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogen B., Dembic Z., Weiss S. Clonal deletion of specific thymocytes by an immunoglobulin idiotype. EMBO J. 1993 Jan;12(1):357–363. doi: 10.1002/j.1460-2075.1993.tb05664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boise L. H., Minn A. J., Noel P. J., June C. H., Accavitti M. A., Lindsten T., Thompson C. B. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995 Jul;3(1):87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- Boussiotis V. A., Barber D. L., Nakarai T., Freeman G. J., Gribben J. G., Bernstein G. M., D'Andrea A. D., Ritz J., Nadler L. M. Prevention of T cell anergy by signaling through the gamma c chain of the IL-2 receptor. Science. 1994 Nov 11;266(5187):1039–1042. doi: 10.1126/science.7973657. [DOI] [PubMed] [Google Scholar]
- Bretscher P., Cohn M. A theory of self-nonself discrimination. Science. 1970 Sep 11;169(3950):1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- Brunner T., Mogil R. J., LaFace D., Yoo N. J., Mahboubi A., Echeverri F., Martin S. J., Force W. R., Lynch D. H., Ware C. F. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995 Feb 2;373(6513):441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- Burkly L. C., Lo D., Kanagawa O., Brinster R. L., Flavell R. A. T-cell tolerance by clonal anergy in transgenic mice with nonlymphoid expression of MHC class II I-E. Nature. 1989 Nov 30;342(6249):564–566. doi: 10.1038/342564a0. [DOI] [PubMed] [Google Scholar]
- Burkly L., Hession C., Ogata L., Reilly C., Marconi L. A., Olson D., Tizard R., Cate R., Lo D. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature. 1995 Feb 9;373(6514):531–536. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- Böhme J., Haskins K., Stecha P., van Ewijk W., LeMeur M., Gerlinger P., Benoist C., Mathis D. Transgenic mice with I-A on islet cells are normoglycemic but immunologically intolerant. Science. 1989 Jun 9;244(4909):1179–1183. doi: 10.1126/science.2499048. [DOI] [PubMed] [Google Scholar]
- Böttger E. C., Hoffmann T., Hadding U., Bitter-Suermann D. Influence of genetically inherited complement deficiencies on humoral immune response in guinea pigs. J Immunol. 1985 Dec;135(6):4100–4107. [PubMed] [Google Scholar]
- Carter R. H., Fearon D. T. CD19: lowering the threshold for antigen receptor stimulation of B lymphocytes. Science. 1992 Apr 3;256(5053):105–107. doi: 10.1126/science.1373518. [DOI] [PubMed] [Google Scholar]
- Carter R. H., Spycher M. O., Ng Y. C., Hoffman R., Fearon D. T. Synergistic interaction between complement receptor type 2 and membrane IgM on B lymphocytes. J Immunol. 1988 Jul 15;141(2):457–463. [PubMed] [Google Scholar]
- Casciola-Rosen L. A., Anhalt G., Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994 Apr 1;179(4):1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion S., Imhof B. A., Savagner P., Thiery J. P. The embryonic thymus produces chemotactic peptides involved in the homing of hemopoietic precursors. Cell. 1986 Mar 14;44(5):781–790. doi: 10.1016/0092-8674(86)90844-5. [DOI] [PubMed] [Google Scholar]
- Chan E. Y., MacLennan I. C. Only a small proportion of splenic B cells in adults are short-lived virgin cells. Eur J Immunol. 1993 Feb;23(2):357–363. doi: 10.1002/eji.1830230209. [DOI] [PubMed] [Google Scholar]
- Chang T. L., Capraro G., Kleinman R. E., Abbas A. K. Anergy in immature B lymphocytes. Differential responses to receptor-mediated stimulation and T helper cells. J Immunol. 1991 Aug 1;147(3):750–756. [PubMed] [Google Scholar]
- Chen-Bettecken U., Wecker E., Schimpl A. IgM RNA switch from membrane to secretory form is prevented by adding antireceptor antibody to bacterial lipopolysaccharide-stimulated murine primary B-cell cultures. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7384–7388. doi: 10.1073/pnas.82.21.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Nagy Z., Radic M. Z., Hardy R. R., Huszar D., Camper S. A., Weigert M. The site and stage of anti-DNA B-cell deletion. Nature. 1995 Jan 19;373(6511):252–255. doi: 10.1038/373252a0. [DOI] [PubMed] [Google Scholar]
- Chen Y., Kuchroo V. K., Inobe J., Hafler D. A., Weiner H. L. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994 Aug 26;265(5176):1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- Cho E. A., Riley M. P., Sillman A. L., Quill H. Altered protein tyrosine phosphorylation in anergic Th1 cells. J Immunol. 1993 Jul 1;151(1):20–28. [PubMed] [Google Scholar]
- Cibotti R., Kanellopoulos J. M., Cabaniols J. P., Halle-Panenko O., Kosmatopoulos K., Sercarz E., Kourilsky P. Tolerance to a self-protein involves its immunodominant but does not involve its subdominant determinants. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):416–420. doi: 10.1073/pnas.89.1.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke M. P., Heath A. W., Shokat K. M., Zeng Y., Finkelman F. D., Linsley P. S., Howard M., Goodnow C. C. Immunoglobulin signal transduction guides the specificity of B cell-T cell interactions and is blocked in tolerant self-reactive B cells. J Exp Med. 1994 Feb 1;179(2):425–438. doi: 10.1084/jem.179.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchfield J. M., Racke M. K., Zúiga-Pflücker J. C., Cannella B., Raine C. S., Goverman J., Lenardo M. J. T cell deletion in high antigen dose therapy of autoimmune encephalomyelitis. Science. 1994 Feb 25;263(5150):1139–1143. doi: 10.1126/science.7509084. [DOI] [PubMed] [Google Scholar]
- Cyster J. G., Goodnow C. C. Antigen-induced exclusion from follicles and anergy are separate and complementary processes that influence peripheral B cell fate. Immunity. 1995 Dec;3(6):691–701. doi: 10.1016/1074-7613(95)90059-4. [DOI] [PubMed] [Google Scholar]
- Cyster J. G., Goodnow C. C. Protein tyrosine phosphatase 1C negatively regulates antigen receptor signaling in B lymphocytes and determines thresholds for negative selection. Immunity. 1995 Jan;2(1):13–24. doi: 10.1016/1074-7613(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Cyster J. G., Hartley S. B., Goodnow C. C. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 1994 Sep 29;371(6496):389–395. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio D., Hippen K. L., Minskoff S. A., Mellman I., Pani G., Siminovitch K. A., Cambier J. C. Recruitment and activation of PTP1C in negative regulation of antigen receptor signaling by Fc gamma RIIB1. Science. 1995 Apr 14;268(5208):293–297. doi: 10.1126/science.7716523. [DOI] [PubMed] [Google Scholar]
- DeFranco A. L. Molecular aspects of B-lymphocyte activation. Annu Rev Cell Biol. 1987;3:143–178. doi: 10.1146/annurev.cb.03.110187.001043. [DOI] [PubMed] [Google Scholar]
- Decker D. J., Kline G. H., Hayden T. A., Zaharevitz S. N., Klinman N. R. Heavy chain V gene-specific elimination of B cells during the pre-B cell to B cell transition. J Immunol. 1995 May 15;154(10):4924–4935. [PubMed] [Google Scholar]
- Dempsey P. W., Allison M. E., Akkaraju S., Goodnow C. C., Fearon D. T. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996 Jan 19;271(5247):348–350. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- Denis K. A., Timson L. K., Witte O. N. VH repertoire in progeny of long term lymphoid-cultured cells used to reconstitute immunodeficient mice. J Immunol. 1989 May 1;142(9):2981–2987. [PubMed] [Google Scholar]
- Dhein J., Walczak H., Bäumler C., Debatin K. M., Krammer P. H. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995 Feb 2;373(6513):438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- Diamond B., Katz J. B., Paul E., Aranow C., Lustgarten D., Scharff M. D. The role of somatic mutation in the pathogenic anti-DNA response. Annu Rev Immunol. 1992;10:731–757. doi: 10.1146/annurev.iy.10.040192.003503. [DOI] [PubMed] [Google Scholar]
- Diamond B., Scharff M. D. Somatic mutation of the T15 heavy chain gives rise to an antibody with autoantibody specificity. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5841–5844. doi: 10.1073/pnas.81.18.5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon S. R., MacKay V. L., Fink P. J. A functionally compromised intermediate in extrathymic CD8+ T cell deletion. Immunity. 1995 Sep;3(3):321–333. doi: 10.1016/1074-7613(95)90117-5. [DOI] [PubMed] [Google Scholar]
- Dintzis R. Z., Middleton M. H., Dintzis H. M. Studies on the immunogenicity and tolerogenicity of T-independent antigens. J Immunol. 1983 Nov;131(5):2196–2203. [PubMed] [Google Scholar]
- Doody G. M., Justement L. B., Delibrias C. C., Matthews R. J., Lin J., Thomas M. L., Fearon D. T. A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science. 1995 Jul 14;269(5221):242–244. doi: 10.1126/science.7618087. [DOI] [PubMed] [Google Scholar]
- Dresser D. W., Mitchison N. A. The mechanism of immunological paralysis. Adv Immunol. 1968;8:129–181. doi: 10.1016/s0065-2776(08)60466-6. [DOI] [PubMed] [Google Scholar]
- Dukor P., Hartmann K. U. Hypothesis. Bound C3 as the second signal for B-cell activation. Cell Immunol. 1973 Jun;7(3):349–356. doi: 10.1016/0008-8749(73)90199-8. [DOI] [PubMed] [Google Scholar]
- Engel P., Zhou L. J., Ord D. C., Sato S., Koller B., Tedder T. F. Abnormal B lymphocyte development, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity. 1995 Jul;3(1):39–50. doi: 10.1016/1074-7613(95)90157-4. [DOI] [PubMed] [Google Scholar]
- Erikson J., Radic M. Z., Camper S. A., Hardy R. R., Carmack C., Weigert M. Expression of anti-DNA immunoglobulin transgenes in non-autoimmune mice. Nature. 1991 Jan 24;349(6307):331–334. doi: 10.1038/349331a0. [DOI] [PubMed] [Google Scholar]
- Evavold B. D., Sloan-Lancaster J., Allen P. M. Tickling the TCR: selective T-cell functions stimulated by altered peptide ligands. Immunol Today. 1993 Dec;14(12):602–609. doi: 10.1016/0167-5699(93)90200-5. [DOI] [PubMed] [Google Scholar]
- Ferber I., Schönrich G., Schenkel J., Mellor A. L., Hämmerling G. J., Arnold B. Levels of peripheral T cell tolerance induced by different doses of tolerogen. Science. 1994 Feb 4;263(5147):674–676. doi: 10.1126/science.8303275. [DOI] [PubMed] [Google Scholar]
- Fields L. E., Loh D. Y. Organ injury associated with extrathymic induction of immune tolerance in doubly transgenic mice. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5730–5734. doi: 10.1073/pnas.89.13.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T. H., McDuffie M., Kappler J. W., Marrack P., Cambier J. C. Both immature and mature T cells mobilize Ca2+ in response to antigen receptor crosslinking. Nature. 1987 Nov 12;330(6144):179–181. doi: 10.1038/330179a0. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Holmes J. M., Dukhanina O. I., Morris S. C. Cross-linking of membrane immunoglobulin D, in the absence of T cell help, kills mature B cells in vivo. J Exp Med. 1995 Feb 1;181(2):515–525. doi: 10.1084/jem.181.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher G. H., Rosenberg F. J., Straus S. E., Dale J. K., Middleton L. A., Lin A. Y., Strober W., Lenardo M. J., Puck J. M. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995 Jun 16;81(6):935–946. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- Fowell D., Mason D. Evidence that the T cell repertoire of normal rats contains cells with the potential to cause diabetes. Characterization of the CD4+ T cell subset that inhibits this autoimmune potential. J Exp Med. 1993 Mar 1;177(3):627–636. doi: 10.1084/jem.177.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman G. J., Gray G. S., Gimmi C. D., Lombard D. B., Zhou L. J., White M., Fingeroth J. D., Gribben J. G., Nadler L. M. Structure, expression, and T cell costimulatory activity of the murine homologue of the human B lymphocyte activation antigen B7. J Exp Med. 1991 Sep 1;174(3):625–631. doi: 10.1084/jem.174.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas A. A., Andrade L., Lembezat M. P., Coutinho A. Selection of VH gene repertoires: differentiating B cells of adult bone marrow mimic fetal development. Int Immunol. 1990;2(1):15–23. doi: 10.1093/intimm/2.1.15. [DOI] [PubMed] [Google Scholar]
- Förster I., Hirose R., Arbeit J. M., Clausen B. E., Hanahan D. Limited capacity for tolerization of CD4+ T cells specific for a pancreatic beta cell neo-antigen. Immunity. 1995 Jun;2(6):573–585. doi: 10.1016/1074-7613(95)90002-0. [DOI] [PubMed] [Google Scholar]
- Gajewski T. F., Lancki D. W., Stack R., Fitch F. W. "Anergy" of TH0 helper T lymphocytes induces downregulation of TH1 characteristics and a transition to a TH2-like phenotype. J Exp Med. 1994 Feb 1;179(2):481–491. doi: 10.1084/jem.179.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski T. F., Qian D., Fields P., Fitch F. W. Anergic T-lymphocyte clones have altered inositol phosphate, calcium, and tyrosine kinase signaling pathways. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):38–42. doi: 10.1073/pnas.91.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay D., Saunders T., Camper S., Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med. 1993 Apr 1;177(4):999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger T., Gooding L. R., Flavell R. A. T-cell responsiveness to an oncogenic peripheral protein and spontaneous autoimmunity in transgenic mice. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2985–2989. doi: 10.1073/pnas.89.7.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand M. C., Elfenbein G. J., Frank M. M., Paul W. E. Ontogeny of B lymphocytes. II. Relative rates of appearance of lymphocytes bearing surface immunoglobulin and complement receptors. J Exp Med. 1974 May 1;139(5):1125–1141. doi: 10.1084/jem.139.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Palmer S. M., Rodrigues N. R., Cordell H. J., Hearne C. M., Cornall R. J., Prins J. B., McShane P., Lathrop G. M., Peterson L. B. Polygenic control of autoimmune diabetes in nonobese diabetic mice. Nat Genet. 1993 Aug;4(4):404–409. doi: 10.1038/ng0893-404. [DOI] [PubMed] [Google Scholar]
- Goodnow C. C., Brink R., Adams E. Breakdown of self-tolerance in anergic B lymphocytes. Nature. 1991 Aug 8;352(6335):532–536. doi: 10.1038/352532a0. [DOI] [PubMed] [Google Scholar]
- Goodnow C. C., Crosbie J., Adelstein S., Lavoie T. B., Smith-Gill S. J., Brink R. A., Pritchard-Briscoe H., Wotherspoon J. S., Loblay R. H., Raphael K. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988 Aug 25;334(6184):676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- Goodnow C. C., Crosbie J., Jorgensen H., Brink R. A., Basten A. Induction of self-tolerance in mature peripheral B lymphocytes. Nature. 1989 Nov 23;342(6248):385–391. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- Goodnow C. C., Cyster J. G., Hartley S. B., Bell S. E., Cooke M. P., Healy J. I., Akkaraju S., Rathmell J. C., Pogue S. L., Shokat K. P. Self-tolerance checkpoints in B lymphocyte development. Adv Immunol. 1995;59:279–368. doi: 10.1016/s0065-2776(08)60633-1. [DOI] [PubMed] [Google Scholar]
- Goverman J., Woods A., Larson L., Weiner L. P., Hood L., Zaller D. M. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell. 1993 Feb 26;72(4):551–560. doi: 10.1016/0092-8674(93)90074-z. [DOI] [PubMed] [Google Scholar]
- Gribben J. G., Freeman G. J., Boussiotis V. A., Rennert P., Jellis C. L., Greenfield E., Barber M., Restivo V. A., Jr, Ke X., Gray G. S. CTLA4 mediates antigen-specific apoptosis of human T cells. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):811–815. doi: 10.1073/pnas.92.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Tarlinton D., Müller W., Rajewsky K., Förster I. Most peripheral B cells in mice are ligand selected. J Exp Med. 1991 Jun 1;173(6):1357–1371. doi: 10.1084/jem.173.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerder S., Meyerhoff J., Flavell R. The role of the T cell costimulator B7-1 in autoimmunity and the induction and maintenance of tolerance to peripheral antigen. Immunity. 1994 May;1(2):155–166. doi: 10.1016/1074-7613(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Harding F. A., McArthur J. G., Gross J. A., Raulet D. H., Allison J. P. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992 Apr 16;356(6370):607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- Hartley S. B., Cooke M. P., Fulcher D. A., Harris A. W., Cory S., Basten A., Goodnow C. C. Elimination of self-reactive B lymphocytes proceeds in two stages: arrested development and cell death. Cell. 1993 Feb 12;72(3):325–335. doi: 10.1016/0092-8674(93)90111-3. [DOI] [PubMed] [Google Scholar]
- Hartley S. B., Crosbie J., Brink R., Kantor A. B., Basten A., Goodnow C. C. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991 Oct 24;353(6346):765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- Hartley S. B., Goodnow C. C. Censoring of self-reactive B cells with a range of receptor affinities in transgenic mice expressing heavy chains for a lysozyme-specific antibody. Int Immunol. 1994 Sep;6(9):1417–1425. doi: 10.1093/intimm/6.9.1417. [DOI] [PubMed] [Google Scholar]
- Hathcock K. S., Laszlo G., Dickler H. B., Bradshaw J., Linsley P., Hodes R. J. Identification of an alternative CTLA-4 ligand costimulatory for T cell activation. Science. 1993 Nov 5;262(5135):905–907. doi: 10.1126/science.7694361. [DOI] [PubMed] [Google Scholar]
- Heath W. R., Allison J., Hoffmann M. W., Schönrich G., Hämmerling G., Arnold B., Miller J. F. Autoimmune diabetes as a consequence of locally produced interleukin-2. Nature. 1992 Oct 8;359(6395):547–549. doi: 10.1038/359547a0. [DOI] [PubMed] [Google Scholar]
- Hebell T., Ahearn J. M., Fearon D. T. Suppression of the immune response by a soluble complement receptor of B lymphocytes. Science. 1991 Oct 4;254(5028):102–105. doi: 10.1126/science.1718035. [DOI] [PubMed] [Google Scholar]
- Heyman B., Pilström L., Shulman M. J. Complement activation is required for IgM-mediated enhancement of the antibody response. J Exp Med. 1988 Jun 1;167(6):1999–2004. doi: 10.1084/jem.167.6.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs M. L., Tarlinton D. M., Armes J., Grail D., Hodgson G., Maglitto R., Stacker S. A., Dunn A. R. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995 Oct 20;83(2):301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- Ho W. Y., Cooke M. P., Goodnow C. C., Davis M. M. Resting and anergic B cells are defective in CD28-dependent costimulation of naive CD4+ T cells. J Exp Med. 1994 May 1;179(5):1539–1549. doi: 10.1084/jem.179.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogquist K. A., Jameson S. C., Heath W. R., Howard J. L., Bevan M. J., Carbone F. R. T cell receptor antagonist peptides induce positive selection. Cell. 1994 Jan 14;76(1):17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Howard M., Paul W. E. Regulation of B-cell growth and differentiation by soluble factors. Annu Rev Immunol. 1983;1:307–333. doi: 10.1146/annurev.iy.01.040183.001515. [DOI] [PubMed] [Google Scholar]
- Huetz F., Carlsson L., Tornberg U. C., Holmberg D. V-region directed selection in differentiating B lymphocytes. EMBO J. 1993 May;12(5):1819–1826. doi: 10.1002/j.1460-2075.1993.tb05830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatowicz L., Kappler J., Marrack P. The effects of chronic infection with a superantigen-producing virus. J Exp Med. 1992 Apr 1;175(4):917–923. doi: 10.1084/jem.175.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Witmer-Pack M., Inaba M., Hathcock K. S., Sakuta H., Azuma M., Yagita H., Okumura K., Linsley P. S., Ikehara S. The tissue distribution of the B7-2 costimulator in mice: abundant expression on dendritic cells in situ and during maturation in vitro. J Exp Med. 1994 Nov 1;180(5):1849–1860. doi: 10.1084/jem.180.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob H. J., Pettersson A., Wilson D., Mao Y., Lernmark A., Lander E. S. Genetic dissection of autoimmune type I diabetes in the BB rat. Nat Genet. 1992 Sep;2(1):56–60. doi: 10.1038/ng0992-56. [DOI] [PubMed] [Google Scholar]
- Jacobson B. A., Panka D. J., Nguyen K. A., Erikson J., Abbas A. K., Marshak-Rothstein A. Anatomy of autoantibody production: dominant localization of antibody-producing cells to T cell zones in Fas-deficient mice. Immunity. 1995 Oct;3(4):509–519. doi: 10.1016/1074-7613(95)90179-5. [DOI] [PubMed] [Google Scholar]
- Jameson S. C., Hogquist K. A., Bevan M. J. Specificity and flexibility in thymic selection. Nature. 1994 Jun 30;369(6483):750–752. doi: 10.1038/369750a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M. K., Pardoll D. M., Mizuguchi J., Chused T. M., Schwartz R. H. Molecular events in the induction of a nonresponsive state in interleukin 2-producing helper T-lymphocyte clones. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5409–5413. doi: 10.1073/pnas.84.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M. K., Schwartz R. H. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987 Feb 1;165(2):302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. A., Chin L. T., Longo D. L., Kruisbeek A. M. Peripheral clonal elimination of functional T cells. Science. 1990 Dec 21;250(4988):1726–1729. doi: 10.1126/science.2125368. [DOI] [PubMed] [Google Scholar]
- Ju S. T., Cui H., Panka D. J., Ettinger R., Marshak-Rothstein A. Participation of target Fas protein in apoptosis pathway induced by CD4+ Th1 and CD8+ cytotoxic T cells. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4185–4189. doi: 10.1073/pnas.91.10.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju S. T., Panka D. J., Cui H., Ettinger R., el-Khatib M., Sherr D. H., Stanger B. Z., Marshak-Rothstein A. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995 Feb 2;373(6513):444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- Kang S. M., Beverly B., Tran A. C., Brorson K., Schwartz R. H., Lenardo M. J. Transactivation by AP-1 is a molecular target of T cell clonal anergy. Science. 1992 Aug 21;257(5073):1134–1138. doi: 10.1126/science.257.5073.1134. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Staerz U., White J., Marrack P. C. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature. 1988 Mar 3;332(6159):35–40. doi: 10.1038/332035a0. [DOI] [PubMed] [Google Scholar]
- Katz J. D., Wang B., Haskins K., Benoist C., Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993 Sep 24;74(6):1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- Kawai K., Ohashi P. S. Immunological function of a defined T-cell population tolerized to low-affinity self antigens. Nature. 1995 Mar 2;374(6517):68–69. doi: 10.1038/374068a0. [DOI] [PubMed] [Google Scholar]
- Kearney E. R., Pape K. A., Loh D. Y., Jenkins M. K. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994 Jul;1(4):327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Bartels J., Hamilton A. M., Lehuen A., Solvason N., Vakil M. Development and function of the early B cell repertoire. Int Rev Immunol. 1992;8(2-3):247–257. doi: 10.3109/08830189209055577. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Klein J., Bockman D. E., Cooper M. D., Lawton A. R. B cell differentiation induced by lipopolysaccharide. V. Suppression of plasma cell maturation by anti-mu: mode of action and characteristics of suppressed cells. J Immunol. 1978 Jan;120(1):158–166. [PubMed] [Google Scholar]
- Killeen N., Moriarty A., Teh H. S., Littman D. R. Requirement for CD8-major histocompatibility complex class I interaction in positive and negative selection of developing T cells. J Exp Med. 1992 Jul 1;176(1):89–97. doi: 10.1084/jem.176.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishihara K., Penninger J., Wallace V. A., Kündig T. M., Kawai K., Wakeham A., Timms E., Pfeffer K., Ohashi P. S., Thomas M. L. Normal B lymphocyte development but impaired T cell maturation in CD45-exon6 protein tyrosine phosphatase-deficient mice. Cell. 1993 Jul 16;74(1):143–156. doi: 10.1016/0092-8674(93)90302-7. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Blüthmann H., Staerz U. D., Steinmetz M., von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988 Jun 23;333(6175):742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Klaus G. G., Humphrey J. H. The generation of memory cells. I. The role of C3 in the generation of B memory cells. Immunology. 1977 Jul;33(1):31–40. [PMC free article] [PubMed] [Google Scholar]
- Kotloff D. B., Bosma M. J., Ruetsch N. R. V(D)J recombination in peritoneal B cells of leaky scid mice. J Exp Med. 1993 Dec 1;178(6):1981–1994. doi: 10.1084/jem.178.6.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummel M. F., Allison J. P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995 Aug 1;182(2):459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDERBERG J. Genes and antibodies. Science. 1959 Jun 19;129(3364):1649–1653. doi: 10.1126/science.129.3364.1649. [DOI] [PubMed] [Google Scholar]
- LaSalle J. M., Tolentino P. J., Freeman G. J., Nadler L. M., Hafler D. A. Early signaling defects in human T cells anergized by T cell presentation of autoantigen. J Exp Med. 1992 Jul 1;176(1):177–186. doi: 10.1084/jem.176.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty K. J., Cunningham A. J. A new analysis of allogeneic interactions. Aust J Exp Biol Med Sci. 1975 Feb;53(1):27–42. doi: 10.1038/icb.1975.3. [DOI] [PubMed] [Google Scholar]
- Larsen C. P., Ritchie S. C., Pearson T. C., Linsley P. S., Lowry R. P. Functional expression of the costimulatory molecule, B7/BB1, on murine dendritic cell populations. J Exp Med. 1992 Oct 1;176(4):1215–1220. doi: 10.1084/jem.176.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. H., Levinson A. I., Schumacher H. R., Jr Hypogammaglobulinemia and rheumatic disease. Semin Arthritis Rheum. 1993 Feb;22(4):252–264. doi: 10.1016/0049-0172(93)80073-o. [DOI] [PubMed] [Google Scholar]
- Lee N. A., Loh D. Y., Lacy E. CD8 surface levels alter the fate of alpha/beta T cell receptor-expressing thymocytes in transgenic mice. J Exp Med. 1992 Apr 1;175(4):1013–1025. doi: 10.1084/jem.175.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M. J. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991 Oct 31;353(6347):858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- Lenschow D. J., Sperling A. I., Cooke M. P., Freeman G., Rhee L., Decker D. C., Gray G., Nadler L. M., Goodnow C. C., Bluestone J. A. Differential up-regulation of the B7-1 and B7-2 costimulatory molecules after Ig receptor engagement by antigen. J Immunol. 1994 Sep 1;153(5):1990–1997. [PubMed] [Google Scholar]
- Lindstrom J., Shelton D., Fujii Y. Myasthenia gravis. Adv Immunol. 1988;42:233–284. doi: 10.1016/s0065-2776(08)60847-0. [DOI] [PubMed] [Google Scholar]
- Linsley P. S., Ledbetter J. A. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- Linton P. J., Rudie A., Klinman N. R. Tolerance susceptibility of newly generating memory B cells. J Immunol. 1991 Jun 15;146(12):4099–4104. [PubMed] [Google Scholar]
- Liu G. Y., Fairchild P. J., Smith R. M., Prowle J. R., Kioussis D., Wraith D. C. Low avidity recognition of self-antigen by T cells permits escape from central tolerance. Immunity. 1995 Oct;3(4):407–415. doi: 10.1016/1074-7613(95)90170-1. [DOI] [PubMed] [Google Scholar]
- Lortan J. E., Roobottom C. A., Oldfield S., MacLennan I. C. Newly produced virgin B cells migrate to secondary lymphoid organs but their capacity to enter follicles is restricted. Eur J Immunol. 1987 Sep;17(9):1311–1316. doi: 10.1002/eji.1830170914. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Schneider R., Lees R. K., Howe R. C., Acha-Orbea H., Festenstein H., Zinkernagel R. M., Hengartner H. T-cell receptor V beta use predicts reactivity and tolerance to Mlsa-encoded antigens. Nature. 1988 Mar 3;332(6159):40–45. doi: 10.1038/332040a0. [DOI] [PubMed] [Google Scholar]
- MacLennan I. C. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- MacLennan I. C., Gray D. Antigen-driven selection of virgin and memory B cells. Immunol Rev. 1986 Jun;91:61–85. doi: 10.1111/j.1600-065x.1986.tb01484.x. [DOI] [PubMed] [Google Scholar]
- Malynn B. A., Yancopoulos G. D., Barth J. E., Bona C. A., Alt F. W. Biased expression of JH-proximal VH genes occurs in the newly generated repertoire of neonatal and adult mice. J Exp Med. 1990 Mar 1;171(3):843–859. doi: 10.1084/jem.171.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell T. J., Nunez G., Platt F. M., Hockenberry D., London L., McKearn J. P., Korsmeyer S. J. Deregulated Bcl-2-immunoglobulin transgene expands a resting but responsive immunoglobulin M and D-expressing B-cell population. Mol Cell Biol. 1990 May;10(5):1901–1907. doi: 10.1128/mcb.10.5.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHeyzer-Williams M. G., Davis M. M. Antigen-specific development of primary and memory T cells in vivo. Science. 1995 Apr 7;268(5207):106–111. doi: 10.1126/science.7535476. [DOI] [PubMed] [Google Scholar]
- Merino R., Ding L., Veis D. J., Korsmeyer S. J., Nuñez G. Developmental regulation of the Bcl-2 protein and susceptibility to cell death in B lymphocytes. EMBO J. 1994 Feb 1;13(3):683–691. doi: 10.1002/j.1460-2075.1994.tb06307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskophidis D., Lechner F., Pircher H., Zinkernagel R. M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993 Apr 22;362(6422):758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- Moudgil K. D., Sercarz E. E. Dominant determinants in hen eggwhite lysozyme correspond to the cryptic determinants within its self-homologue, mouse lysozyme: implications in shaping of the T cell repertoire and autoimmunity. J Exp Med. 1993 Dec 1;178(6):2131–2138. doi: 10.1084/jem.178.6.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D. L., Chiodetti L., Bacon P. A., Schwartz R. H. Clonal anergy blocks the response to IL-4, as well as the production of IL-2, in dual-producing T helper cell clones. J Immunol. 1991 Dec 15;147(12):4118–4125. [PubMed] [Google Scholar]
- Mueller D. L., Jenkins M. K. Molecular mechanisms underlying functional T-cell unresponsiveness. Curr Opin Immunol. 1995 Jun;7(3):375–381. doi: 10.1016/0952-7915(95)80113-8. [DOI] [PubMed] [Google Scholar]
- Mueller D. L., Jenkins M. K., Schwartz R. H. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- Murakami M., Tsubata T., Okamoto M., Shimizu A., Kumagai S., Imura H., Honjo T. Antigen-induced apoptotic death of Ly-1 B cells responsible for autoimmune disease in transgenic mice. Nature. 1992 May 7;357(6373):77–80. doi: 10.1038/357077a0. [DOI] [PubMed] [Google Scholar]
- Murphy K. M., Heimberger A. B., Loh D. Y. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990 Dec 21;250(4988):1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- Murphy K. M., Weaver C. T., Elish M., Allen P. M., Loh D. Y. Peripheral tolerance to allogeneic class II histocompatibility antigens expressed in transgenic mice: evidence against a clonal-deletion mechanism. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10034–10038. doi: 10.1073/pnas.86.24.10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S., Golstein P. The Fas death factor. Science. 1995 Mar 10;267(5203):1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- Nakayama K., Nakayama K., Negishi I., Kuida K., Shinkai Y., Louie M. C., Fields L. E., Lucas P. J., Stewart V., Alt F. W. Disappearance of the lymphoid system in Bcl-2 homozygous mutant chimeric mice. Science. 1993 Sep 17;261(5128):1584–1588. doi: 10.1126/science.8372353. [DOI] [PubMed] [Google Scholar]
- Nemazee D. A., Bürki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989 Feb 9;337(6207):562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- Nemazee D., Russell D., Arnold B., Haemmerling G., Allison J., Miller J. F., Morahan G., Buerki K. Clonal deletion of autospecific B lymphocytes. Immunol Rev. 1991 Aug;122:117–132. doi: 10.1111/j.1600-065x.1991.tb00600.x. [DOI] [PubMed] [Google Scholar]
- Nishizumi H., Taniuchi I., Yamanashi Y., Kitamura D., Ilic D., Mori S., Watanabe T., Yamamoto T. Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn-deficient mice. Immunity. 1995 Nov;3(5):549–560. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- Nisitani S., Tsubata T., Murakami M., Okamoto M., Honjo T. The bcl-2 gene product inhibits clonal deletion of self-reactive B lymphocytes in the periphery but not in the bone marrow. J Exp Med. 1993 Oct 1;178(4):1247–1254. doi: 10.1084/jem.178.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J. Cellular mechanisms of immunologic tolerance. Annu Rev Immunol. 1983;1:33–62. doi: 10.1146/annurev.iy.01.040183.000341. [DOI] [PubMed] [Google Scholar]
- Novak T. J., Farber D., Leitenberg D., Hong S. C., Johnson P., Bottomly K. Isoforms of the transmembrane tyrosine phosphatase CD45 differentially affect T cell recognition. Immunity. 1994 May;1(2):109–119. doi: 10.1016/1074-7613(94)90104-x. [DOI] [PubMed] [Google Scholar]
- O'Garra A., Murphy K. Role of cytokines in determining T-lymphocyte function. Curr Opin Immunol. 1994 Jun;6(3):458–466. doi: 10.1016/0952-7915(94)90128-7. [DOI] [PubMed] [Google Scholar]
- Ohashi P. S., Oehen S., Buerki K., Pircher H., Ohashi C. T., Odermatt B., Malissen B., Zinkernagel R. M., Hengartner H. Ablation of "tolerance" and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991 Apr 19;65(2):305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- Ohsugi Y., Gershwin M. E., Ahmed A., Skelly R. R., Milich D. R. Studies of congenitally immunologic mutant New Zealand mice. VI. Spontaneous and induced autoantibodies to red cells and DNA occur in New Zealand X-linked immunodeficient (Xid) mice without phenotypic alternations of the Xid gene or generalized polyclonal B cell activation. J Immunol. 1982 May;128(5):2220–2227. [PubMed] [Google Scholar]
- Okamoto M., Murakami M., Shimizu A., Ozaki S., Tsubata T., Kumagai S., Honjo T. A transgenic model of autoimmune hemolytic anemia. J Exp Med. 1992 Jan 1;175(1):71–79. doi: 10.1084/jem.175.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura M., Thomas M. L. Regulation of immune function by protein tyrosine phosphatases. Curr Opin Immunol. 1995 Jun;7(3):312–319. doi: 10.1016/0952-7915(95)80104-9. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Nerenberg M., Southern P., Price J., Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991 Apr 19;65(2):319–331. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- Otten G. R., Germain R. N. Split anergy in a CD8+ T cell: receptor-dependent cytolysis in the absence of interleukin-2 production. Science. 1991 Mar 8;251(4998):1228–1231. doi: 10.1126/science.1900952. [DOI] [PubMed] [Google Scholar]
- Parish C. R., Liew F. Y. Immune response to chemically modified flagellin. 3. Enhanced cell-mediated immunity during high and low zone antibody tolerance to flagellin. J Exp Med. 1972 Feb 1;135(2):298–311. doi: 10.1084/jem.135.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penhale W. J., Irvine W. J., Inglis J. R., Farmer A. Thyroiditis in T cell-depleted rats: suppression of the autoallergic response by reconstitution with normal lymphoid cells. Clin Exp Immunol. 1976 Jul;25(1):6–16. [PMC free article] [PubMed] [Google Scholar]
- Pepys M. B. Role of complement in induction of antibody production in vivo. Effect of cobra factor and other C3-reactive agents on thymus-dependent and thymus-independent antibody responses. J Exp Med. 1974 Jul 1;140(1):126–145. doi: 10.1084/jem.140.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzella F., Tse A. G., Cordell J. L., Pulford K. A., Gatter K. C., Mason D. Y. Expression of the bcl-2 oncogene protein is not specific for the 14;18 chromosomal translocation. Am J Pathol. 1990 Aug;137(2):225–232. [PMC free article] [PubMed] [Google Scholar]
- Pike B. L., Boyd A. W., Nossal G. J. Clonal anergy: the universally anergic B lymphocyte. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2013–2017. doi: 10.1073/pnas.79.6.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F., Leach M. W., Mauze S., Menon S., Caddle L. B., Coffman R. L. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994 Oct;1(7):553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Powrie F., Mason D. OX-22high CD4+ T cells induce wasting disease with multiple organ pathology: prevention by the OX-22low subset. J Exp Med. 1990 Dec 1;172(6):1701–1708. doi: 10.1084/jem.172.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B., Kannourakis G., Nouri S., Smith K. G., Nossal G. J. Soluble antigen can cause enhanced apoptosis of germinal-centre B cells. Nature. 1995 May 25;375(6529):331–334. doi: 10.1038/375331a0. [DOI] [PubMed] [Google Scholar]
- Pullen A. M., Marrack P., Kappler J. W. The T-cell repertoire is heavily influenced by tolerance to polymorphic self-antigens. Nature. 1988 Oct 27;335(6193):796–801. doi: 10.1038/335796a0. [DOI] [PubMed] [Google Scholar]
- Quill H., Bhandoola A., Trinchieri G., Haluskey J., Peritt D. Induction of interleukin 12 responsiveness is impaired in anergic T lymphocytes. J Exp Med. 1994 Mar 1;179(3):1065–1070. doi: 10.1084/jem.179.3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radic M. Z., Erikson J., Litwin S., Weigert M. B lymphocytes may escape tolerance by revising their antigen receptors. J Exp Med. 1993 Apr 1;177(4):1165–1173. doi: 10.1084/jem.177.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahemtulla A., Fung-Leung W. P., Schilham M. W., Kündig T. M., Sambhara S. R., Narendran A., Arabian A., Wakeham A., Paige C. J., Zinkernagel R. M. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature. 1991 Sep 12;353(6340):180–184. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- Rammensee H. G., Kroschewski R., Frangoulis B. Clonal anergy induced in mature V beta 6+ T lymphocytes on immunizing Mls-1b mice with Mls-1a expressing cells. Nature. 1989 Jun 15;339(6225):541–544. doi: 10.1038/339541a0. [DOI] [PubMed] [Google Scholar]
- Ramsdell F., Lantz T., Fowlkes B. J. A nondeletional mechanism of thymic self tolerance. Science. 1989 Nov 24;246(4933):1038–1041. doi: 10.1126/science.2511629. [DOI] [PubMed] [Google Scholar]
- Rathmell J. C., Cooke M. P., Ho W. Y., Grein J., Townsend S. E., Davis M. M., Goodnow C. C. CD95 (Fas)-dependent elimination of self-reactive B cells upon interaction with CD4+ T cells. Nature. 1995 Jul 13;376(6536):181–184. doi: 10.1038/376181a0. [DOI] [PubMed] [Google Scholar]
- Rathmell J. C., Goodnow C. C. Autoimmunity. The Fas track. Curr Biol. 1995 Nov 1;5(11):1218–1221. doi: 10.1016/s0960-9822(95)00241-7. [DOI] [PubMed] [Google Scholar]
- Raulet D. H., Held W. Natural killer cell receptors: the offs and ons of NK cell recognition. Cell. 1995 Sep 8;82(5):697–700. doi: 10.1016/0092-8674(95)90466-2. [DOI] [PubMed] [Google Scholar]
- Rawlings D. J., Saffran D. C., Tsukada S., Largaespada D. A., Grimaldi J. C., Cohen L., Mohr R. N., Bazan J. F., Howard M., Copeland N. G. Mutation of unique region of Bruton's tyrosine kinase in immunodeficient XID mice. Science. 1993 Jul 16;261(5119):358–361. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- Rickert R. C., Rajewsky K., Roes J. Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature. 1995 Jul 27;376(6538):352–355. doi: 10.1038/376352a0. [DOI] [PubMed] [Google Scholar]
- Rieux-Laucat F., Le Deist F., Hivroz C., Roberts I. A., Debatin K. M., Fischer A., de Villartay J. P. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995 Jun 2;268(5215):1347–1349. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- Robey E. A., Ramsdell F., Kioussis D., Sha W., Loh D., Axel R., Fowlkes B. J. The level of CD8 expression can determine the outcome of thymic selection. Cell. 1992 Jun 26;69(7):1089–1096. doi: 10.1016/0092-8674(92)90631-l. [DOI] [PubMed] [Google Scholar]
- Rocha B., Dautigny N., Pereira P. Peripheral T lymphocytes: expansion potential and homeostatic regulation of pool sizes and CD4/CD8 ratios in vivo. Eur J Immunol. 1989 May;19(5):905–911. doi: 10.1002/eji.1830190518. [DOI] [PubMed] [Google Scholar]
- Rocha B., Tanchot C., Von Boehmer H. Clonal anergy blocks in vivo growth of mature T cells and can be reversed in the absence of antigen. J Exp Med. 1993 May 1;177(5):1517–1521. doi: 10.1084/jem.177.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha B., von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991 Mar 8;251(4998):1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- Rothstein T. L., Wang J. K., Panka D. J., Foote L. C., Wang Z., Stanger B., Cui H., Ju S. T., Marshak-Rothstein A. Protection against Fas-dependent Th1-mediated apoptosis by antigen receptor engagement in B cells. Nature. 1995 Mar 9;374(6518):163–165. doi: 10.1038/374163a0. [DOI] [PubMed] [Google Scholar]
- Russell J. H., Rush B., Weaver C., Wang R. Mature T cells of autoimmune lpr/lpr mice have a defect in antigen-stimulated suicide. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4409–4413. doi: 10.1073/pnas.90.10.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadlack B., Merz H., Schorle H., Schimpl A., Feller A. C., Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993 Oct 22;75(2):253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- Scher I. CBA/N immune defective mice; evidence for the failure of a B cell subpopulation to be expressed. Immunol Rev. 1982;64:117–136. doi: 10.1111/j.1600-065x.1982.tb00421.x. [DOI] [PubMed] [Google Scholar]
- Schönrich G., Kalinke U., Momburg F., Malissen M., Schmitt-Verhulst A. M., Malissen B., Hämmerling G. J., Arnold B. Down-regulation of T cell receptors on self-reactive T cells as a novel mechanism for extrathymic tolerance induction. Cell. 1991 Apr 19;65(2):293–304. doi: 10.1016/0092-8674(91)90163-s. [DOI] [PubMed] [Google Scholar]
- Scollay R. G., Butcher E. C., Weissman I. L. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur J Immunol. 1980 Mar;10(3):210–218. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- Scott B., Liblau R., Degermann S., Marconi L. A., Ogata L., Caton A. J., McDevitt H. O., Lo D. A role for non-MHC genetic polymorphism in susceptibility to spontaneous autoimmunity. Immunity. 1994 Apr;1(1):73–83. doi: 10.1016/1074-7613(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Sebzda E., Wallace V. A., Mayer J., Yeung R. S., Mak T. W., Ohashi P. S. Positive and negative thymocyte selection induced by different concentrations of a single peptide. Science. 1994 Mar 18;263(5153):1615–1618. doi: 10.1126/science.8128249. [DOI] [PubMed] [Google Scholar]
- Sha W. C., Nelson C. A., Newberry R. D., Kranz D. M., Russell J. H., Loh D. Y. Positive and negative selection of an antigen receptor on T cells in transgenic mice. Nature. 1988 Nov 3;336(6194):73–76. doi: 10.1038/336073a0. [DOI] [PubMed] [Google Scholar]
- Shahinian A., Pfeffer K., Lee K. P., Kündig T. M., Kishihara K., Wakeham A., Kawai K., Ohashi P. S., Thompson C. B., Mak T. W. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993 Jul 30;261(5121):609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- Shinohara N., Watanabe M., Sachs D. H., Hozumi N. Killing of antigen-reactive B cells by class II-restricted, soluble antigen-specific CD8+ cytolytic T lymphocytes. Nature. 1988 Dec 1;336(6198):481–484. doi: 10.1038/336481a0. [DOI] [PubMed] [Google Scholar]
- Shlomchik M. J., Aucoin A. H., Pisetsky D. S., Weigert M. G. Structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9150–9154. doi: 10.1073/pnas.84.24.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik M. J., Marshak-Rothstein A., Wolfowicz C. B., Rothstein T. L., Weigert M. G. The role of clonal selection and somatic mutation in autoimmunity. 1987 Aug 27-Sep 2Nature. 328(6133):805–811. doi: 10.1038/328805a0. [DOI] [PubMed] [Google Scholar]
- Shlomchik M., Mascelli M., Shan H., Radic M. Z., Pisetsky D., Marshak-Rothstein A., Weigert M. Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. J Exp Med. 1990 Jan 1;171(1):265–292. doi: 10.1084/jem.171.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokat K. M., Goodnow C. C. Antigen-induced B-cell death and elimination during germinal-centre immune responses. Nature. 1995 May 25;375(6529):334–338. doi: 10.1038/375334a0. [DOI] [PubMed] [Google Scholar]
- Shultz L. D., Schweitzer P. A., Rajan T. V., Yi T., Ihle J. N., Matthews R. J., Thomas M. L., Beier D. R. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell. 1993 Jul 2;73(7):1445–1454. doi: 10.1016/0092-8674(93)90369-2. [DOI] [PubMed] [Google Scholar]
- Sidman C. L., Shultz L. D., Hardy R. R., Hayakawa K., Herzenberg L. A. Production of immunoglobulin isotypes by Ly-1+ B cells in viable motheaten and normal mice. Science. 1986 Jun 13;232(4756):1423–1425. doi: 10.1126/science.3487115. [DOI] [PubMed] [Google Scholar]
- Sidman C. L., Unanue E. R. Receptor-mediated inactivation of early B lymphocytes. Nature. 1975 Sep 11;257(5522):149–151. doi: 10.1038/257149a0. [DOI] [PubMed] [Google Scholar]
- Silverman D. A., Rose N. R. Neonatal thymectomy increases the incidence of spontaneous and methylcholanthrene-enhanced thyroiditis in rats. Science. 1974 Apr 12;184(4133):162–163. doi: 10.1126/science.184.4133.162. [DOI] [PubMed] [Google Scholar]
- Sinclair N. R. How many signals are enough? Cell Immunol. 1990 Oct 1;130(1):204–235. doi: 10.1016/0008-8749(90)90174-p. [DOI] [PubMed] [Google Scholar]
- Singer G. G., Abbas A. K. The fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994 Aug;1(5):365–371. doi: 10.1016/1074-7613(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Siskind G. W., Benacerraf B. Cell selection by antigen in the immune response. Adv Immunol. 1969;10:1–50. doi: 10.1016/s0065-2776(08)60414-9. [DOI] [PubMed] [Google Scholar]
- Sloan-Lancaster J., Evavold B. D., Allen P. M. Induction of T-cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature. 1993 May 13;363(6425):156–159. doi: 10.1038/363156a0. [DOI] [PubMed] [Google Scholar]
- Sloan-Lancaster J., Evavold B. D., Allen P. M. Th2 cell clonal anergy as a consequence of partial activation. J Exp Med. 1994 Oct 1;180(4):1195–1205. doi: 10.1084/jem.180.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Williams G. T., Kingston R., Jenkinson E. J., Owen J. J. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature. 1989 Jan 12;337(6203):181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- Sprent J., Bruce J. Physiology of B cells in mice with X-linked immunodeficiency (xid). III. Disappearance of xid B cells in double bone marrow chimeras. J Exp Med. 1984 Sep 1;160(3):711–723. doi: 10.1084/jem.160.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J., Schaefer M., Hurd M., Surh C. D., Ron Y. Mature murine B and T cells transferred to SCID mice can survive indefinitely and many maintain a virgin phenotype. J Exp Med. 1991 Sep 1;174(3):717–728. doi: 10.1084/jem.174.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg B. J., Smathers P. A., Frederiksen K., Steinberg A. D. Ability of the xid gene to prevent autoimmunity in (NZB X NZW)F1 mice during the course of their natural history, after polyclonal stimulation, or following immunization with DNA. J Clin Invest. 1982 Sep;70(3):587–597. doi: 10.1172/JCI110651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A., Whittingham S., Vaux D. L., Bath M. L., Adams J. M., Cory S., Harris A. W. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8661–8665. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su B., Jacinto E., Hibi M., Kallunki T., Karin M., Ben-Neriah Y. JNK is involved in signal integration during costimulation of T lymphocytes. Cell. 1994 Jun 3;77(5):727–736. doi: 10.1016/0092-8674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kündig T. M., Furlonger C., Wakeham A., Timms E., Matsuyama T., Schmits R., Simard J. J., Ohashi P. S., Griesser H. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 1995 Jun 9;268(5216):1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Sano S., Nieves E., De Libero G., Rosa D., Modlin R. L., Brenner M. B., Bloom B. R., Morita C. T. Nonpeptide ligands for human gamma delta T cells. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8175–8179. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarakhovsky A., Turner M., Schaal S., Mee P. J., Duddy L. P., Rajewsky K., Tybulewicz V. L. Defective antigen receptor-mediated proliferation of B and T cells in the absence of Vav. Nature. 1995 Mar 30;374(6521):467–470. doi: 10.1038/374467a0. [DOI] [PubMed] [Google Scholar]
- Thomas J. D., Sideras P., Smith C. I., Vorechovský I., Chapman V., Paul W. E. Colocalization of X-linked agammaglobulinemia and X-linked immunodeficiency genes. Science. 1993 Jul 16;261(5119):355–358. doi: 10.1126/science.8332900. [DOI] [PubMed] [Google Scholar]
- Tiegs S. L., Russell D. M., Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993 Apr 1;177(4):1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tighe H., Heaphy P., Baird S., Weigle W. O., Carson D. A. Human immunoglobulin (IgG) induced deletion of IgM rheumatoid factor B cells in transgenic mice. J Exp Med. 1995 Feb 1;181(2):599–606. doi: 10.1084/jem.181.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tivol E. A., Borriello F., Schweitzer A. N., Lynch W. P., Bluestone J. A., Sharpe A. H. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995 Nov;3(5):541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- Todd J. A., Bell J. I., McDevitt H. O. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987 Oct 15;329(6140):599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- Tough D. F., Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med. 1994 Apr 1;179(4):1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao B. P., Chow A., Cheroutre H., Song Y. W., McGrath M. E., Kronenberg M. B cells are anergic in transgenic mice that express IgM anti-DNA antibodies. Eur J Immunol. 1993 Sep;23(9):2332–2339. doi: 10.1002/eji.1830230942. [DOI] [PubMed] [Google Scholar]
- Tsui H. W., Siminovitch K. A., de Souza L., Tsui F. W. Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nat Genet. 1993 Jun;4(2):124–129. doi: 10.1038/ng0693-124. [DOI] [PubMed] [Google Scholar]
- Tsukada S., Saffran D. C., Rawlings D. J., Parolini O., Allen R. C., Klisak I., Sparkes R. S., Kubagawa H., Mohandas T., Quan S. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993 Jan 29;72(2):279–290. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- Vasquez N. J., Kaye J., Hedrick S. M. In vivo and in vitro clonal deletion of double-positive thymocytes. J Exp Med. 1992 May 1;175(5):1307–1316. doi: 10.1084/jem.175.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veis D. J., Sorenson C. M., Shutter J. R., Korsmeyer S. J. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993 Oct 22;75(2):229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- Vella A. T., McCormack J. E., Linsley P. S., Kappler J. W., Marrack P. Lipopolysaccharide interferes with the induction of peripheral T cell death. Immunity. 1995 Mar;2(3):261–270. doi: 10.1016/1074-7613(95)90050-0. [DOI] [PubMed] [Google Scholar]
- Vetrie D., Vorechovský I., Sideras P., Holland J., Davies A., Flinter F., Hammarström L., Kinnon C., Levinsky R., Bobrow M. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993 Jan 21;361(6409):226–233. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- Vignaux F., Golstein P. Fas-based lymphocyte-mediated cytotoxicity against syngeneic activated lymphocytes: a regulatory pathway? Eur J Immunol. 1994 Apr;24(4):923–927. doi: 10.1002/eji.1830240421. [DOI] [PubMed] [Google Scholar]
- Wallace V. A., Rahemtulla A., Timms E., Penninger J., Mak T. W. CD4 expression is differentially required for deletion of MLS-1a-reactive T cells. J Exp Med. 1992 Nov 1;176(5):1459–1463. doi: 10.1084/jem.176.5.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walunas T. L., Lenschow D. J., Bakker C. Y., Linsley P. S., Freeman G. J., Green J. M., Thompson C. B., Bluestone J. A. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994 Aug;1(5):405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Wang C. R., Loveland B. E., Lindahl K. F. H-2M3 encodes the MHC class I molecule presenting the maternally transmitted antigen of the mouse. Cell. 1991 Jul 26;66(2):335–345. doi: 10.1016/0092-8674(91)90623-7. [DOI] [PubMed] [Google Scholar]
- Waterhouse P., Penninger J. M., Timms E., Wakeham A., Shahinian A., Lee K. P., Thompson C. B., Griesser H., Mak T. W. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995 Nov 10;270(5238):985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- Webb S., Morris C., Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990 Dec 21;63(6):1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- Weigle W. O. Analysis of autoimmunity through experimental models of thyroiditis and allergic encephalomyelitis. Adv Immunol. 1980;30:159–273. doi: 10.1016/s0065-2776(08)60196-0. [DOI] [PubMed] [Google Scholar]
- Weigle W. O. Immunological unresponsiveness. Adv Immunol. 1973;16:61–122. doi: 10.1016/s0065-2776(08)60296-5. [DOI] [PubMed] [Google Scholar]
- Weih F., Carrasco D., Durham S. K., Barton D. S., Rizzo C. A., Ryseck R. P., Lira S. A., Bravo R. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-kappa B/Rel family. Cell. 1995 Jan 27;80(2):331–340. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- Weiss A., Littman D. R. Signal transduction by lymphocyte antigen receptors. Cell. 1994 Jan 28;76(2):263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Wiersma E. J., Kinoshita T., Heyman B. Inhibition of immunological memory and T-independent humoral responses by monoclonal antibodies specific for murine complement receptors. Eur J Immunol. 1991 Oct;21(10):2501–2506. doi: 10.1002/eji.1830211029. [DOI] [PubMed] [Google Scholar]
- Willerford D. M., Chen J., Ferry J. A., Davidson L., Ma A., Alt F. W. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995 Oct;3(4):521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- Wilson H. A., Greenblatt D., Taylor C. W., Putney J. W., Tsien R. Y., Finkelman F. D., Chused T. M. The B lymphocyte calcium response to anti-Ig is diminished by membrane immunoglobulin cross-linkage to the Fc gamma receptor. J Immunol. 1987 Mar 15;138(6):1712–1718. [PubMed] [Google Scholar]
- Woodland R. T., Schmidt M. R., Riggs J. E., Korsmeyer S. J., Lussier A. M., Gravel K. A. Radiation-induced apoptosis is differentially regulated in primary B cells from normal mice and mice with the CBA/N X-linked immunodeficiency. J Immunol. 1995 Oct 1;155(7):3453–3463. [PubMed] [Google Scholar]
- Yancopoulos G. D., Desiderio S. V., Paskind M., Kearney J. F., Baltimore D., Alt F. W. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature. 1984 Oct 25;311(5988):727–733. doi: 10.1038/311727a0. [DOI] [PubMed] [Google Scholar]
- Yellen A. J., Glenn W., Sukhatme V. P., Cao X. M., Monroe J. G. Signaling through surface IgM in tolerance-susceptible immature murine B lymphocytes. Developmentally regulated differences in transmembrane signaling in splenic B cells from adult and neonatal mice. J Immunol. 1991 Mar 1;146(5):1446–1454. [PubMed] [Google Scholar]
- Young D., Kearney J. F. Sequence analysis and antigen binding characteristics of Ig SCID Ig+ mice. Int Immunol. 1995 May;7(5):807–819. doi: 10.1093/intimm/7.5.807. [DOI] [PubMed] [Google Scholar]
- Yui K., Ishida Y., Katsumata M., Komori S., Chused T. M., Abe R. Two separate mechanisms of T cell clonal anergy to Mls-1. J Immunol. 1993 Dec 1;151(11):6062–6075. [PubMed] [Google Scholar]
- Zal T., Volkmann A., Stockinger B. Mechanisms of tolerance induction in major histocompatibility complex class II-restricted T cells specific for a blood-borne self-antigen. J Exp Med. 1994 Dec 1;180(6):2089–2099. doi: 10.1084/jem.180.6.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanders E. D., Lamb J. R., Feldmann M., Green N., Beverley P. C. Tolerance of T-cell clones is associated with membrane antigen changes. Nature. 1983 Jun 16;303(5918):625–627. doi: 10.1038/303625a0. [DOI] [PubMed] [Google Scholar]
- Zhang R., Alt F. W., Davidson L., Orkin S. H., Swat W. Defective signalling through the T- and B-cell antigen receptors in lymphoid cells lacking the vav proto-oncogene. Nature. 1995 Mar 30;374(6521):470–473. doi: 10.1038/374470a0. [DOI] [PubMed] [Google Scholar]
- Zheng L., Fisher G., Miller R. E., Peschon J., Lynch D. H., Lenardo M. J. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995 Sep 28;377(6547):348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- von Herrath M. G., Dockter J., Nerenberg M., Gairin J. E., Oldstone M. B. Thymic selection and adaptability of cytotoxic T lymphocyte responses in transgenic mice expressing a viral protein in the thymus. J Exp Med. 1994 Nov 1;180(5):1901–1910. doi: 10.1084/jem.180.5.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]