Abstract
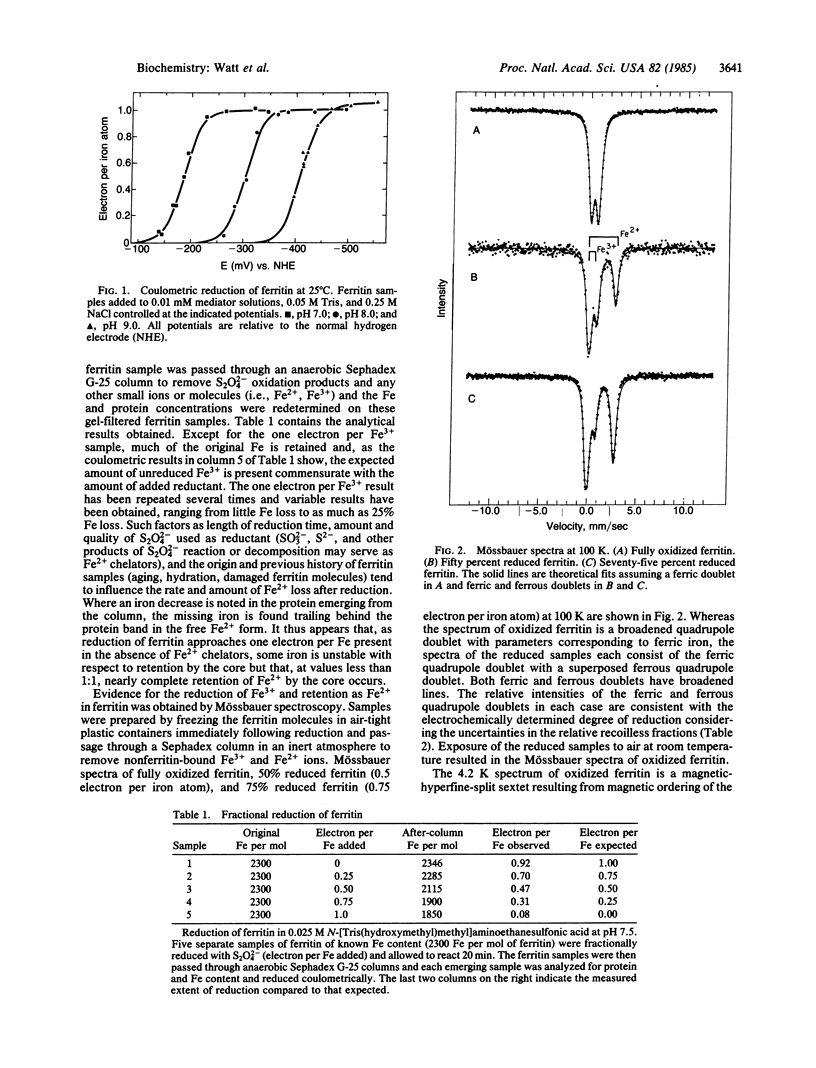
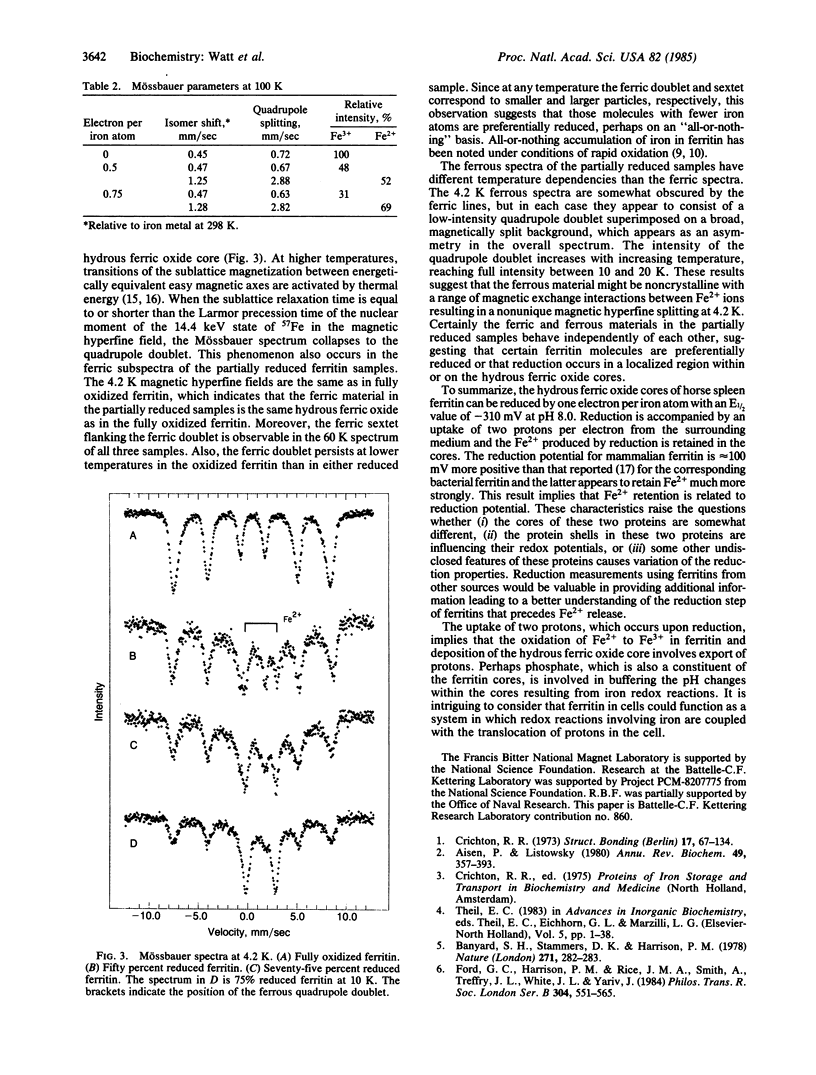
Mammalian ferritin from horse spleen undergoes an electrochemical or chemical reduction reaction in which each iron atom present is reduced by one electron (2300 electrons per ferritin molecule containing 2300 Fe3+ ions). Midpoint potentials of -190 mV, -310 mV, and -416 mV were determined at pH 7.0, 8.0, and 9.0. This variation of potential with pH indicates that approximately 2 H+ are transferred to the core for each Fe3+ reduced to Fe2+. Mössbauer measurements of partially reduced ferritin give spectra that consist of a ferric quadrupole doublet with a superposed ferrous quadrupole doublet. The relative intensities of these doublets are consistent with the electrochemically determined degree of reduction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Listowsky I. Iron transport and storage proteins. Annu Rev Biochem. 1980;49:357–393. doi: 10.1146/annurev.bi.49.070180.002041. [DOI] [PubMed] [Google Scholar]
- Banyard S. H., Stammers D. K., Harrison P. M. Electron density map of apoferritin at 2.8-A resolution. Nature. 1978 Jan 19;271(5642):282–284. doi: 10.1038/271282a0. [DOI] [PubMed] [Google Scholar]
- Dixon M. The acceptor specificity of flavins and flavoproteins. I. Techniques for anaerobic spectrophotometry. Biochim Biophys Acta. 1971 Mar 2;226(2):241–258. doi: 10.1016/0005-2728(71)90092-2. [DOI] [PubMed] [Google Scholar]
- Dognin J., Crichton R. R. Mobilisation of iron from ferritin fractions of defined iron content by biological reductants. FEBS Lett. 1975 Jun 15;54(2):234–236. doi: 10.1016/0014-5793(75)80081-0. [DOI] [PubMed] [Google Scholar]
- Ford G. C., Harrison P. M., Rice D. W., Smith J. M., Treffry A., White J. L., Yariv J. Ferritin: design and formation of an iron-storage molecule. Philos Trans R Soc Lond B Biol Sci. 1984 Feb 13;304(1121):551–565. doi: 10.1098/rstb.1984.0046. [DOI] [PubMed] [Google Scholar]
- Harrison P. M., Hoy T. G., Macara I. G., Hoare R. J. Ferritin iron uptake and release. Structure-function relationships. Biochem J. 1974 Nov;143(2):445–451. doi: 10.1042/bj1430445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T., Spencer R., Walsh C. Mechanism and kinetics of iron release from ferritin by dihydroflavins and dihydroflavin analogues. Biochemistry. 1978 Sep 19;17(19):4011–4017. doi: 10.1021/bi00612a021. [DOI] [PubMed] [Google Scholar]
- LOVENBERG W., BUCHANAN B. B., RABINOWITZ J. C. STUDIES ON THE CHEMICAL NATURE OF CLOSTRIDIAL FERREDOXIN. J Biol Chem. 1963 Dec;238:3899–3913. [PubMed] [Google Scholar]
- Macara I. G., Hoy T. G., Harrison P. M. The formation of ferritin from apoferritin. Kinetics and mechanism of iron uptake. Biochem J. 1972 Jan;126(1):151–162. doi: 10.1042/bj1260151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiefel E. I., Watt G. D. Azotobacter cytochrome b557.5 is a bacterioferritin. Nature. 1979 May 3;279(5708):81–83. doi: 10.1038/279081a0. [DOI] [PubMed] [Google Scholar]
- Watt G. D. An electrochemical method for measuring redox potentials of low potential proteins by microcoulometry at controlled potentials. Anal Biochem. 1979 Nov 1;99(2):399–407. doi: 10.1016/s0003-2697(79)80024-x. [DOI] [PubMed] [Google Scholar]
- Williams J. M., Danson D. P., Janot C. A Mossbauer determination of the iron core particle size distribution in ferritin. Phys Med Biol. 1978 Sep;23(5):835–851. doi: 10.1088/0031-9155/23/5/001. [DOI] [PubMed] [Google Scholar]



