Abstract
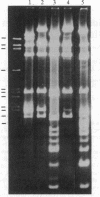
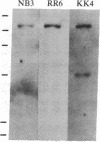
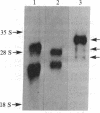
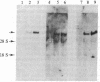
Screening of a human fibroblast cDNA library with a mouse type IV procollagen clone resulted in one 1.05-kilobase isolate that was used to identify a 1.7-kilobase clone overlapping the former by less than 150 nucleotides. EcoRII digestion revealed that the larger clone exhibited the pattern characteristic of DNA coding for a collagenous sequence. Blot hybridization to RNA from mouse F9 stem cells and from these cells treated with retinoic acid and N6, O2'-dibutyryl-cAMP showed induction of type IV mRNA. DNA sequencing and comparison of the derived amino acids with the reported protein data demonstrated that the clones encode part of the alpha 1 chain of human type IV procollagen. Alignment of alpha 1 (IV) with other human procollagens showed minimal but detectable homology. A small cluster of charged residues in the alpha chain is partially shared by type IV. In close proximity is an interruption in the alpha 1 (IV) Gly-Xaa-Yaa region corresponding to the 3' end of a unique proline-free, and therefore also less rigid, area in other collagen triple helices. Analysis of the carboxyl-terminal alpha 1 (IV) peptide showed a repeat symmetry possibly dictated by the six cysteines in each half of the structure. The position of five cysteines in addition to four tyrosine/tryptophan groups allowed a correlation to be drawn between the 3' noncollagenous type IV region and the larger, highly conserved carboxyl propeptides of other human procollagens. Such similarities in the different chains may define functional domains conserved throughout evolution.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aho S., Tate V., Boedtker H. Multiple 3' ends of the chicken pro alpha 2(I) collagen gene. Nucleic Acids Res. 1983 Aug 25;11(16):5443–5450. doi: 10.1093/nar/11.16.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babel W., Glanville R. W. Structure of human-basement-membrane (type IV) collagen. Complete amino-acid sequence of a 914-residue-long pepsin fragment from the alpha 1(IV) chain. Eur J Biochem. 1984 Sep 17;143(3):545–556. doi: 10.1111/j.1432-1033.1984.tb08404.x. [DOI] [PubMed] [Google Scholar]
- Barsh G. S., Roush C. L., Gelinas R. E. DNA and chromatin structure of the human alpha 1 (I) collagen gene. J Biol Chem. 1984 Dec 10;259(23):14906–14913. [PubMed] [Google Scholar]
- Bernard M. P., Chu M. L., Myers J. C., Ramirez F., Eikenberry E. F., Prockop D. J. Nucleotide sequences of complementary deoxyribonucleic acids for the pro alpha 1 chain of human type I procollagen. Statistical evaluation of structures that are conserved during evolution. Biochemistry. 1983 Oct 25;22(22):5213–5223. doi: 10.1021/bi00291a023. [DOI] [PubMed] [Google Scholar]
- Bernard M. P., Myers J. C., Chu M. L., Ramirez F., Eikenberry E. F., Prockop D. J. Structure of a cDNA for the pro alpha 2 chain of human type I procollagen. Comparison with chick cDNA for pro alpha 2(I) identifies structurally conserved features of the protein and the gene. Biochemistry. 1983 Mar 1;22(5):1139–1145. doi: 10.1021/bi00274a023. [DOI] [PubMed] [Google Scholar]
- Cooper A. R., Kurkinen M., Taylor A., Hogan B. L. Studies on the biosynthesis of laminin by murine parietal endoderm cells. Eur J Biochem. 1981 Sep;119(1):189–197. doi: 10.1111/j.1432-1033.1981.tb05593.x. [DOI] [PubMed] [Google Scholar]
- Crouch E., Sage H., Bornstein P. Structural basis for apparent heterogeneity of collagens in human basement membranes: type IV procollagen contains two distinct chains. Proc Natl Acad Sci U S A. 1980 Feb;77(2):745–749. doi: 10.1073/pnas.77.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fietzek P. P., Kühn K. The primary structure of collagen. Int Rev Connect Tissue Res. 1976;7:1–60. doi: 10.1016/b978-0-12-363707-9.50007-1. [DOI] [PubMed] [Google Scholar]
- Focht R. J., Adams S. L. Tissue specificity of type I collagen gene expression is determined at both transcriptional and post-transcriptional levels. Mol Cell Biol. 1984 Sep;4(9):1843–1852. doi: 10.1128/mcb.4.9.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller F., Boedtker H. Sequence determination and analysis of the 3' region of chicken pro-alpha 1(I) and pro-alpha 2(I) collagen messenger ribonucleic acids including the carboxy-terminal propeptide sequences. Biochemistry. 1981 Feb 17;20(4):996–1006. doi: 10.1021/bi00507a054. [DOI] [PubMed] [Google Scholar]
- Hofmann H., Voss T., Kühn K., Engel J. Localization of flexible sites in thread-like molecules from electron micrographs. Comparison of interstitial, basement membrane and intima collagens. J Mol Biol. 1984 Jan 25;172(3):325–343. doi: 10.1016/s0022-2836(84)80029-7. [DOI] [PubMed] [Google Scholar]
- Kefalides N. A., Alper R., Clark C. C. Biochemistry and metabolism of basement membranes. Int Rev Cytol. 1979;61:167–228. doi: 10.1016/s0074-7696(08)61998-1. [DOI] [PubMed] [Google Scholar]
- Kefalides N. A. Chemical properties of basement membranes. Int Rev Exp Pathol. 1971;10:1–39. [PubMed] [Google Scholar]
- Kresina T. F., Miller E. J. Isolation and characterization of basement membrane collagen from human placental tissue. Evidence for the presence of two genetically distinct collagen chains. Biochemistry. 1979 Jul 10;18(14):3089–3097. doi: 10.1021/bi00581a028. [DOI] [PubMed] [Google Scholar]
- Krieg T., Timpl R., Alitalo K., Kurkinen M., Vaheri A. Type III procollagen is the major collageneous component produced by a continuous rhabdomyosarcoma cell line. FEBS Lett. 1979 Aug 15;104(2):405–409. doi: 10.1016/0014-5793(79)80863-7. [DOI] [PubMed] [Google Scholar]
- Kurkinen M., Barlow D. P., Helfman D. M., Williams J. G., Hogan B. L. Isolation of cDNA clones for basal lamina components: type IV procollagen. Nucleic Acids Res. 1983 Sep 24;11(18):6199–6209. doi: 10.1093/nar/11.18.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loidl H. R., Brinker J. M., May M., Pihlajaniemi T., Morrow S., Rosenbloom J., Myers J. C. Molecular cloning and carboxyl-propeptide analysis of human type III procollagen. Nucleic Acids Res. 1984 Dec 21;12(24):9383–9394. doi: 10.1093/nar/12.24.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Minor R. R., Clark C. C., Strause E. L., Koszalka T. R., Brent R. L., Kefalides N. A. Basement membrane procollagen is not converted to collagen in organ cultures of parietal yolk sac endoderm. J Biol Chem. 1976 Mar 25;251(6):1789–1794. [PubMed] [Google Scholar]
- Myers J. C., Chu M. L., Faro S. H., Clark W. J., Prockop D. J., Ramirez F. Cloning a cDNA for the pro-alpha 2 chain of human type I collagen. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3516–3520. doi: 10.1073/pnas.78.6.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J. C., Dickson L. A., de Wet W. J., Bernard M. P., Chu M. L., Di Liberto M., Pepe G., Sangiorgi F. O., Ramirez F. Analysis of the 3' end of the human pro-alpha 2(I) collagen gene. Utilization of multiple polyadenylation sites in cultured fibroblasts. J Biol Chem. 1983 Aug 25;258(16):10128–10135. [PubMed] [Google Scholar]
- Myers J. C., Loidl H. R., Stolle C. A., Seyer J. M. Partial covalent structure of the human alpha 2 type V collagen chain. J Biol Chem. 1985 May 10;260(9):5533–5541. [PubMed] [Google Scholar]
- Sandell L. J., Prentice H. L., Kravis D., Upholt W. B. Structure and sequence of the chicken type II procollagen gene. Characterization of the region encoding the carboxyl-terminal telopeptide and propeptide. J Biol Chem. 1984 Jun 25;259(12):7826–7834. [PubMed] [Google Scholar]
- Schuppan D., Glanville R. W., Timpl R. Covalent structure of mouse type-IV collagen. Isolation, order and partial amino-acid sequence of cyanogen-bromide and tryptic peptides of pepsin fragment P1 from the alpha 1(IV) chain. Eur J Biochem. 1982 Apr;123(3):505–512. [PubMed] [Google Scholar]
- Schuppan D., Glanville R. W., Timpl R., Dixit S. N., Kang A. H. Sequence comparison of pepsin-resistant segments of basement-membrane collagen alpha 1(IV) chains from bovine lens capsule and mouse tumour. Biochem J. 1984 May 15;220(1):227–233. doi: 10.1042/bj2200227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuppan D., Timpl R., Glanville R. W. Discontinuities in the triple helical sequence Gly-X-Y of basement membrane (type IV) collagen. FEBS Lett. 1980 Jun 30;115(2):297–300. doi: 10.1016/0014-5793(80)81191-4. [DOI] [PubMed] [Google Scholar]
- Strickland S., Smith K. K., Marotti K. R. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980 Sep;21(2):347–355. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- Tryggvason K., Robey P. G., Martin G. R. Biosynthesis of type IV procollagens. Biochemistry. 1980 Apr 1;19(7):1284–1289. doi: 10.1021/bi00548a003. [DOI] [PubMed] [Google Scholar]
- Wang S. Y., Gudas L. J. Isolation of cDNA clones specific for collagen IV and laminin from mouse teratocarcinoma cells. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5880–5884. doi: 10.1073/pnas.80.19.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. J., Eyre D. R. Identification of hydroxypyridinium cross-linking sites in type II collagen of bovine articular cartilage. Biochemistry. 1984 Apr 10;23(8):1850–1857. doi: 10.1021/bi00303a041. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Avvedimento V. E., Mudryj M., Ohkubo H., Vogeli G., Irani M., Pastan I., de Crombrugghe B. The collagen gene: evidence for its evolutinary assembly by amplification of a DNA segment containing an exon of 54 bp. Cell. 1980 Dec;22(3):887–892. doi: 10.1016/0092-8674(80)90565-6. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Kühn K., de Crombrugghe B. A conserved nucleotide sequence, coding for a segment of the C-propeptide, is found at the same location in different collagen genes. Nucleic Acids Res. 1983 May 11;11(9):2733–2744. doi: 10.1093/nar/11.9.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet W. J., Pihlajaniemi T., Myers J., Kelly T. E., Prockop D. J. Synthesis of a shortened pro-alpha 2(I) chain and decreased synthesis of pro-alpha 2(I) chains in a proband with osteogenesis imperfecta. J Biol Chem. 1983 Jun 25;258(12):7721–7728. [PubMed] [Google Scholar]