Abstract
Theoretical analyses of targeting agent pharmacokinetics provides specific guidance with respect to desirable design objectives such as agent size, affinity, and target antigen. These analyses suggest that IgG-sized macromolecular constructs exhibit the most favorable balance between systemic clearance and vascular extravasation, resulting in maximal tumor uptake. Quantitative predictions of the effects of dose and binding affinity on tumor uptake and penetration are also provided. The single bolus dose required for saturation of xenografted tumors in mice can be predicted from knowledge of antigen expression level and metabolic half-life. The role of high binding affinity in tumor uptake can be summarized as: essential for small peptides, less important for antibodies, and negligible for nanoparticles.
1. Introduction
A powerful molecular toolbox is available to vary the size and binding affinity of tumor-targeting agents essentially at will. Such agents can vary in size from small peptidic scaffolds just a few nanometers across to nanoparticles hundreds of nanometers in diameter, and directed evolution can be used to engineer extremely tight binding to tumor-specific antigens. This impressive raw capability raises important questions with regard to the design criteria for a tumor-targeting agent. How does size variation affect the delivery of a drug payload to and throughout a tumor? Can ligand targeting alter nanoparticle biodistribution? How does binding affinity affect overall biodistribution and penetration throughout the tumor volume? What dosage is necessary to obtain uniform penetration of an antibody throughout a tumor? Recently completed analyses of the key rate processes and the quantitative balances among them in macromolecular pharmacokinetics lead to several recommendations and quantitative predictions.
2. What Molecular Size Is Best for Tumor Uptake?
We recently utilized a compartmental model to quantitatively analyze the effect of size on tumor uptake, using previously published data correlating macromolecular size with the key transport parameters (vascular permeability coefficient and the half-life for systemic clearance; Schmidt and Wittrup, 2009). The trend in tumor uptake with increasing size is shown in Fig. 10.1.
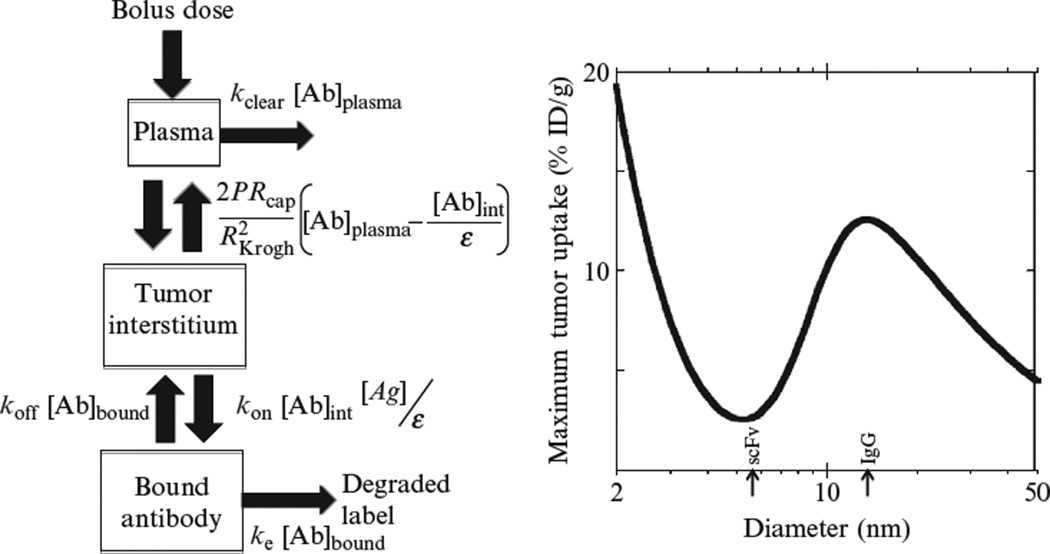
Figure 10.1.
The relationship between tumor uptake and size of the molecular targeting agent (Schmidt and Wittrup, 2009). On the left, a schematic diagram of a compartmental model for targeting biodistribution is shown. On the right, the predicted maximum tumor uptake is plotted as a function of the size of the targeting agent, with scFv and IgG sizes indicated with arrows. The parameters used were appropriate for HER2 binding molecules with Kd = 1nM and labeled with 99mTc, and the size effects on the plasma clearance rate constant kclear, tumor vascular permeability P, and tumor void fraction ε were correlated from published experimental measurements of these parameters.
A local optimum in tumor uptake is predicted for targeting agents approximately the size of an IgG immunoglobulin. (It should be noted that this prediction accounts only for the effect of passive clearance (e.g., renal); in fact, FcRn-mediated lifetime extension substantially further improves tumor uptake for antibodies.) Mathematically, an optimum such as this results from the balance between two opposing trends. The crux of the behavior is a trade-off between systemic clearance and extravasation. It has been shown experimentally that the vascular extravasation rate drops precipitously with increasing macromolecular size (Dreher et al., 2006)—however, this unfavorable effect is partially compensated for by the benefit of extended systemic circulation due to decreased renal filtration. It is remarkable to note that of the wide range of targeting agent sizes created by genetic and materials engineers, spanning two orders of magnitude, the best size for tumor uptake is in the range that natural selection already converged to for the primary targeting agents of the humoral immune system.
Antibody fragments such as scFvs reside in an apparent “death valley” for uptake versus size. This prediction dovetails with the widespread experimental observation that scFvs and Fabs deliver relatively anemic tumor localization by comparison to whole antibodies. It has often been wishfully proposed that the several fold smaller size of antibody fragments might provide advantageous extravasation and intratumoral penetration characteristics. However, the rapid renal clearance of these small proteins collapses the circulating concentration too rapidly to reap the benefits of their favorable transport parameters (Thurber et al., 2007). Consequently, scFvs, Fabs, diabodies, and the like essentially inhabit the worst of both worlds: too large for sufficiently rapid extravasation and too small to escape renal clearance.
Interestingly, agents with hydrodynamic diameters <5nm are predicted to exhibit increased improvements in tumor uptake with further reductions in molecular size. This is because in this size range, renal clearance is essentially first-pass and so there is little incremental cost to further shrinkage in size. However, the extravasation rate rises rapidly with decreasing size, and so even if the tumor-targeting agent only circulates once through the bloodstream before being renally cleared, more agent is taken up into the tumor as size decreases. Experimental evidence for the benefits of decreasing size in this range have been provided for tumor-targeted scaffold proteins of the affibody (Orlova et al., 2006) and DARPin (Zahnd et al., 2010) types. However, binding scaffolds in this size range are occasionally pushed into the trough of the curve in Fig. 10.1 by fusion to additional binding domains, thereby obviating the key advantage of their small size.
Plückthun and coworkers’ elegant and systematic study of the effect of size on DARPin uptake into tumors directly confirms the minimum in tumor uptake with respect to size shown in Fig. 10.1. A picomolar affinity anti-HER2 DARPin accumulated in xenografted mouse tumors to 8.1% ID/g at 24h. However, a larger heterodimer of this DARPin with a nonbinding DARPin accumulated to only 1.8% ID/g. Further increasing the size by addition of 20kDa PEG to a DARPin monomer raises the 24-h accumulation to 13% ID/g. Each of these three constructs had a single identical binding domain attached to varying amounts of additional macromolecular mass, allowing the effect of size alone to be determined. Tumor accumulation was therefore experimentally demonstrated to go through a minimum with respect to size, as predicted by the compartmental model (Fig. 10.1).
The overall recommendation arising from this analysis is that a tumor-targeting agent the size of an antibody, or slightly larger, should accumulate within tumors to the greatest extent. It is noteworthy that tumor uptake is predicted to continually decrease as a consequence of decreased extravasation as size increases above 20nm in radius—a significant dilemma for nanomedicine.
As an approximate prediction of the effects of size on tumor uptake, a relationship can be derived from the compartmental model of Schmidt and Wittrup (2009), for the high-affinity limit (Kd →0). This relationship is as follows:
where [Ab]tumor is the antibody concentration in the tumor, [Ab]plasma,t = 0 is the initial peak antibody concentration in the plasma, is the permeability coefficient times the vascular surface to tumor volume ratio (Rcap is the capillary radius; RKrogh is the radius of the cylinder of tissue supplied by the capillary), kclear is the effective first-order systemic clearance rate constant, and ke is the rate constant for endocytic turnover of the tumor surface-bound antibody. To estimate the predicted effect of size variation on systemic clearance in the mouse, the following sigmoidal curve fit to published pharmacokinetic data can be used (Schmidt and Wittrup, 2009):
where kclear is the clearance rate constant in units of h−1, and R is the hydrodynamic radius of the targeting agent, in units of nm.
In the absence of a specific measurement of the metabolic half-life for surface-bound antibody, a reasonable approximation (Mattes et al., 1994; Schmidt et al., 2008) would be to use a constitutive half-life of 12h, providing ke=0.06h−1.
An estimate of the dependence of the vascular permeability coefficient on size can be obtained from the following curve fit to published data, assuming a capillary radius of 8µm and RKrogh = 75µm (Dreher et al., 2006; Yuan et al., 1994, 1995):
in the range 1nm<R<100nm, with in units of h−1.
These relationships can be used to obtain a rough approximation of the expected tumor uptake time trajectory of a given targeting agent as a function of its size.
3. Will Targeting Increase Nanoparticle Accumulation in a Tumor?
It is a common strategy to add targeting ligands to the surface of nanoparticles, with the expressed intention of increasing tumor uptake. It is an unfortunate fact however that this approach does not succeed in that objective for agents 100nm in diameter or larger. This is because the loss of unbound nanoparticles from the tumor by intravasation is slower than constitutive internalization and consumption of nanoparticles within the tumor interstitium (Schmidt and Wittrup, 2009). There are nevertheless benefits to attaching ligands to nanoparticles, as tumor cell endocytic uptake effectively delivers nanoparticle payloads.
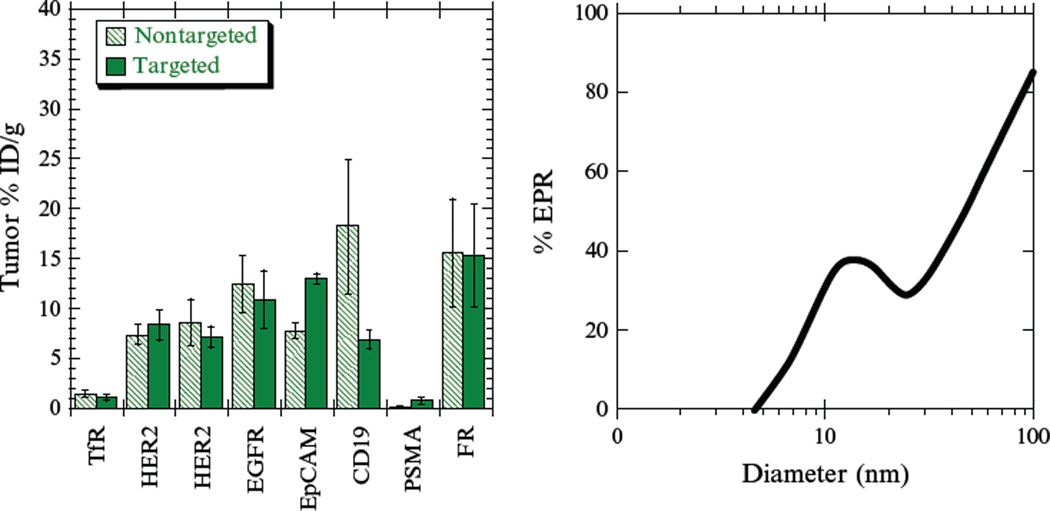
The impact (or lack thereof) of molecular targeting on biodistribution is only discernable when negative, nontargeted controls are included in studies. A survey of such biodistribution studies of targeted and untargeted nanoparticles is shown in Fig. 10.2. There is, in general, an insignificant difference in tumor uptake between targeted and untargeted nanoparticles, indicating that accumulation is predominantly via the passive enhanced permeability and retention (EPR) effect (Bartlett et al., 2007; Gabizon et al., 2003; Gu et al., 2008; Hussain et al., 2007; Kirpotin et al., 2006; Lopes de Menezes et al., 1998; Mamot et al., 2005). One might suspect that transport limitations might conceivably differ in qualitative respects in actual human tumors by comparison to these model xenografts. However, the presence of passive targeting has been directly demonstrated in humans, as untargeted stealth liposomes accumulate at significant levels in tumors (Harrington et al., 2001).
Figure 10.2.
Biodistribution of targeted and nontargeted nanoparticles for the antigens transferrin receptor (TfR; Bartlett et al., 2007), HER2 (Kirpotin et al., 2006; Lub-de Hooge et al., 2004), EGFR (Mamot et al., 2005; Ping Li et al., 2008), EpCAM (Goldrosen et al., 1990; Hussain et al., 2007), CD19 (Lopes de Menezes et al., 1998), PSMA (Gu et al., 2008; Smith-Jones et al., 2003), and the folate receptor (FR; Coliva et al., 2005; Gabizon et al., 2003). Data are for 24-h postinjection in all cases, and all of the nanoparticles were ~100nm in diameter. Negative controls for the nanoparticle studies are generally irrelevant ligands. In the panel on the right, a compartmental model is used to predict what proportion of observed tumor uptake at 24h could be attained via the enhanced permeability and retention (EPR) effect without the use of ligand targeting (Schmidt and Wittrup, 2009).
Nanoparticles that are sufficiently small (<50nm diameter) are predicted to distribute in a fashion more similar to proteins and can therefore exhibit targeting-mediated tumor accumulation (Fig. 10.2, right). This predicted size dependence is consistent with a number of published experimental observations. Dendrimers, which are generally <5nm in diameter, have been shown to accumulate in tumors to a greater extent when conjugated to a ligand (Kukowska-Latallo et al., 2005). Similarly, iron oxide nanoparticles under 20nm in diameter accumulate in tumors to a greater extent when conjugated to a specific antibody (DeNardo et al., 2005). Targeting peptide-conjugated cross-linked iron oxide particles under 40nm in diameter also exhibits improved tumor accumulation (Kelly et al., 2008).
Despite the fact that targeting does not significantly increase tumor accumulation of nanoparticles above 50nm in diameter, targeting can enhance therapeutic efficacy. These advantages are discernable when negative, nontargeted controls are included in studies. Targeting has been shown to increase tumor cell internalization of nanoparticles, whereas untargeted nanoparticles are consumed by reticuloendothelial cells such as tumor-associated macrophages (Kirpotin et al., 2006). Targeting to rapidly internalized antigens provides greater antitumor efficacy than targeting to a slowly internalized antigens (Sapra and Allen, 2002). Thus targeting-driven endocytosis within the tumor improves efficacy (Bartlett et al., 2007; Kirpotin et al., 2006), but targeting has a negligible effect at the whole organism biodistribution level where partitioning amongst clearance organs and the tumor occurs.
4. How Does Affinity Affect Biodistribution?
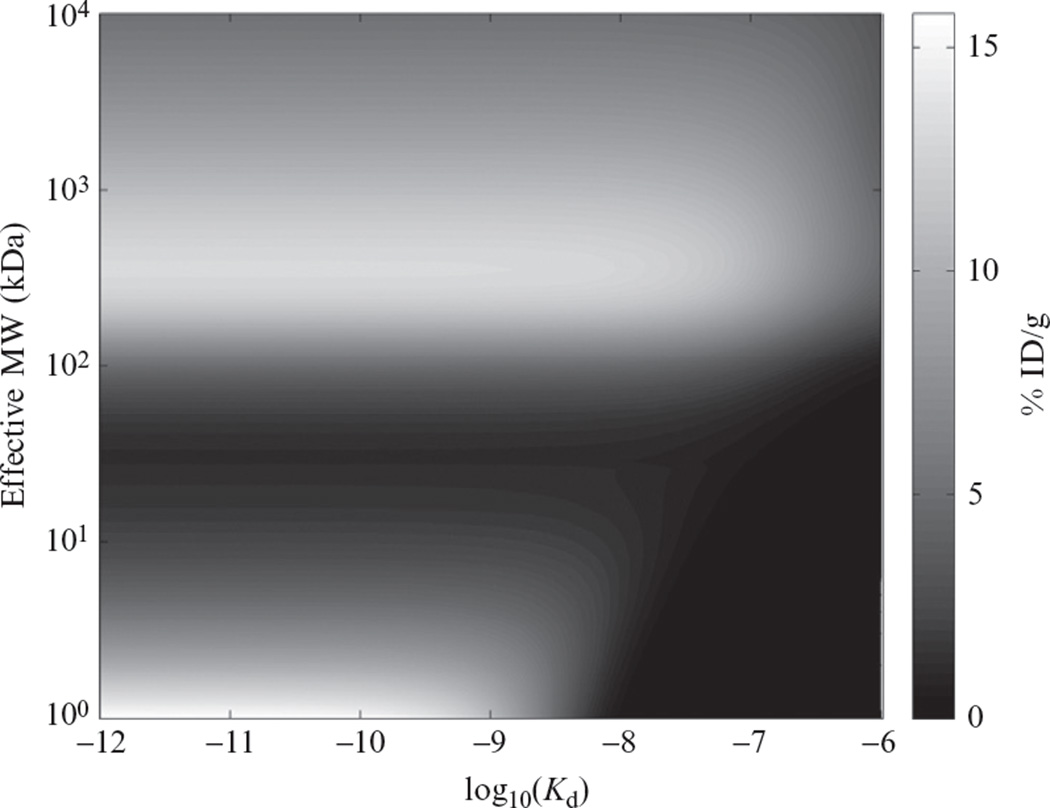
There is not a single answer to this question—the effect depends dramatically on the size of the targeting agent. As shown in Fig. 10.3, a compartmental model predicts that smaller targeting agents require higher affinity in order to be retained in the tumor, while the retention of larger agents does not depend on affinity to as great an extent. As emphasized in Section 1, intermediate-sized agents from ~10 to 100kDa in size reside in a “death valley” where tumor uptake is weak. Smaller peptide-sized targeting agents depend strongly on binding affinity to accumulate within the tumor, with subnanomolar affinity essentially required to obtain significant tumor retention.
Figure 10.3.
Topographical plot of the effect of size and binding affinity on tumor uptake 24h following a bolus dose of targeting agent (Schmidt and Wittrup, 2009).
The general features of this relationship were experimentally confirmed by Zahnd et al. (2010), with different size and affinity Darpins against HER2. Darpins, at 14.5kDa molecular weight, are expected to lie on the steep gradient of improving tumor uptake versus affinity at the bottom of Fig. 10.3. A series of anti-HER2 Darpins were engineered with increasing affinity (Zahnd et al., 2010): G3-HAVD (Kd = 270nM), G3-AVD(Kd = 10nM), G3-D (Kd = 1.5nM), and G3 (Kd = 0.091nM). The tumor uptake at 24h of technetium-labeled Darpins were: 0.57% ID/g (G3-HAVD), 2.4% ID/g (G3-AVD), 3.7% ID/g (G3-D), and 8.1% ID/g (G3). Clearly, for this series of small binding scaffolds, improving affinity directly improves tumor uptake, consistent with the steep gradient of increasing uptake versus affinity at the bottom of Fig. 10.3. Fascinatingly, the story changes when these same binders are conjugated to PEG20, giving them an apparent molecular weight >300kDa by size exclusion chromatography. This size places the PEGylated binders at the top of the plateau in uptake versus Kd and at sizes from 102 to 103kDa. G3-HAVD-PEG20 accumulates to 3.0% ID/g, G3-AVD-PEG20 to 9.2% ID/g, G3-D-PEG20 to 8.6% ID/g, and G3-PEG20 to 13% ID/g. Fully consistent with the model prediction, for these larger targeting agents, the dependence of tumor uptake on binding affinity is weak once Kd<100nM.
The affinity–uptake relationship predicted in Fig. 10.3 was further validated by a series of anti-HER2 antibodies of varying affinity (Rudnick et al., 2011). These antibodies differed only by point mutations, bound at the same HER2 epitope, and had very similar plasma clearance kinetics. The lowest affinity antibody used in this study (Kd = 270nM) is predicted to be on the edge of the plateau in Fig. 10.3, while the three higher affinity antibodies (K = 23, 7.3, and 0.56nM) are predicted to lie squarely on the plateau and therefore have similar accumulation. When biodistribution studies were performed with all four antibodies using nonresidualizing radioisotopes, tumor uptake of the 270-nM affinity antibody was significantly lower than the uptake of all the higher affinity antibodies. By contrast, the accumulation of the higher affinity antibodies did not differ significantly from each other.
The relationship in Fig. 10.3 only captures the bulk uptake as % ID/g, and does not provide any information on the pharmacodynamic effect of the targeting agent, or the microdistribution within the tumor (considered in the following section). For antitumor agents designed to block receptor/ligand or receptor/receptor interactions, clearly affinity will be a dominant variable in achieving efficacious tumor control. Further, when antibody-directed cellular cytotoxicity (ADCC) is the objective, it has been shown that higher affinity antibodies lead to more effective NK-mediated cell killing (Tang et al., 2007). Consequently, the plateau in Fig. 10.3 that extends above 10nM Kd for agents the size of antibodies should not be interpreted as the absence of potential therapeutic benefit for higher, pM affinity antibodies—it is simply a prediction that for Kd<10nM, bulk antibody uptake in tumors will not be a strong function of affinity.
5. What Dose Is Necessary in Order to Overcome the “Binding Site Barrier”?
It has been known since 1989 that high-affinity antibodies may accumulate around the vasculature and fail to distribute evenly throughout tumors, a phenomenon known by the term “binding site barrier” (Fujimori et al., 1989). The word “barrier” implies a rigidity and permanence that fails to appropriately capture the phenomenon, which is actually a dynamic moving front balancing diffusion, binding, and endocytic consumption (Graff and Wittrup, 2003; Thurber and Wittrup, 2008). The critical role for tumor metabolism of antibodies in determining penetration has recently been confirmed experimentally in xenografted tumor models (Rudnick et al., 2011). In this study, it was concluded that “high-density, rapidly internalizing antigens subject high-affinity antibodies to greater internalization and degradation, thereby limiting their penetration of tumors.” In essence, the depth an antibody penetrates into tumor tissue is a dynamic balance between degradation and diffusion (Thurber et al., 2008a,b), and since diffusion is driven by a concentration gradient, one can in principle “dose through” the internalization-driven limitation of penetration. In fact, the Weinstein group that coined the “barrier” nomenclature also demonstrated its dynamic and flexible nature by overcoming poor microdistribution by raising antibody doses (Blumenthal et al., 1991; Saga et al., 1995).
Quantitative comparison of key timescales can be gainfully employed to determine which rate processes dominate observed system behaviors. This type of scaling analysis is widely applied in engineering—for example, the Reynolds number Re=Dvρ/μprovides a dimensionless ratio of inertial and viscous forces in a fluid flow (D is the pipe diameter, v the fluid velocity, ρ the density, and μ the viscosity). The units in the numerator and denominator cancel out, resulting in a fundamental dimensionless ratio. When Re>2100, the flow is turbulent; when Re<2100, the flow is laminar. We have applied this flavor of dimensional analysis to tumor-targeting agents and found that there are two such dimensionless numbers of particular value in understanding tumor penetration (Thurber et al., 2007): a clearance modulus and the Thiele modulus.
The clearance modulus Γ compares systemic clearance time to diffusion time within the tumor. For rapidly clearing agents smaller than the renal filtration threshold, it is possible for systemic clearance to remove the agent from circulation before the moving diffusion front within the tumor has fully penetrated the tumor. The clearance modulus is defined as follows:
where AUCplasma is the area under curve in plasma for the agent, that is, the integral of plasma concentration versus time. When Γ>1, the agent clears from plasma before substantial penetration can occur. Consequently, Γ<1 is a necessary (but not sufficient) criterion to achieve penetration of the agent throughout the tumor volume. scFv-sized agents very often fail this criterion due to their rapid clearance rate. In contrast, IgGs are typically not limited by clearance due to their relatively long serum half-life.
For IgGs and other agents that are not limited by systemic clearance, saturation can be predicted from the balance between extravasation rate and endocytic consumption. The dimensionless ratio of these two rates is the Thiele modulus, named after Ernest Thiele who first derived it in order to understand why industrial catalysts varied in their activity with size (Thiele, 1939). Substituting endocytic metabolism for surface catalytic rate, and vascular extravasation rate for diffusion through ceramic pores, one obtains a Thiele modulus describing tumor penetration (Thurber et al., 2007, 2008a,b):
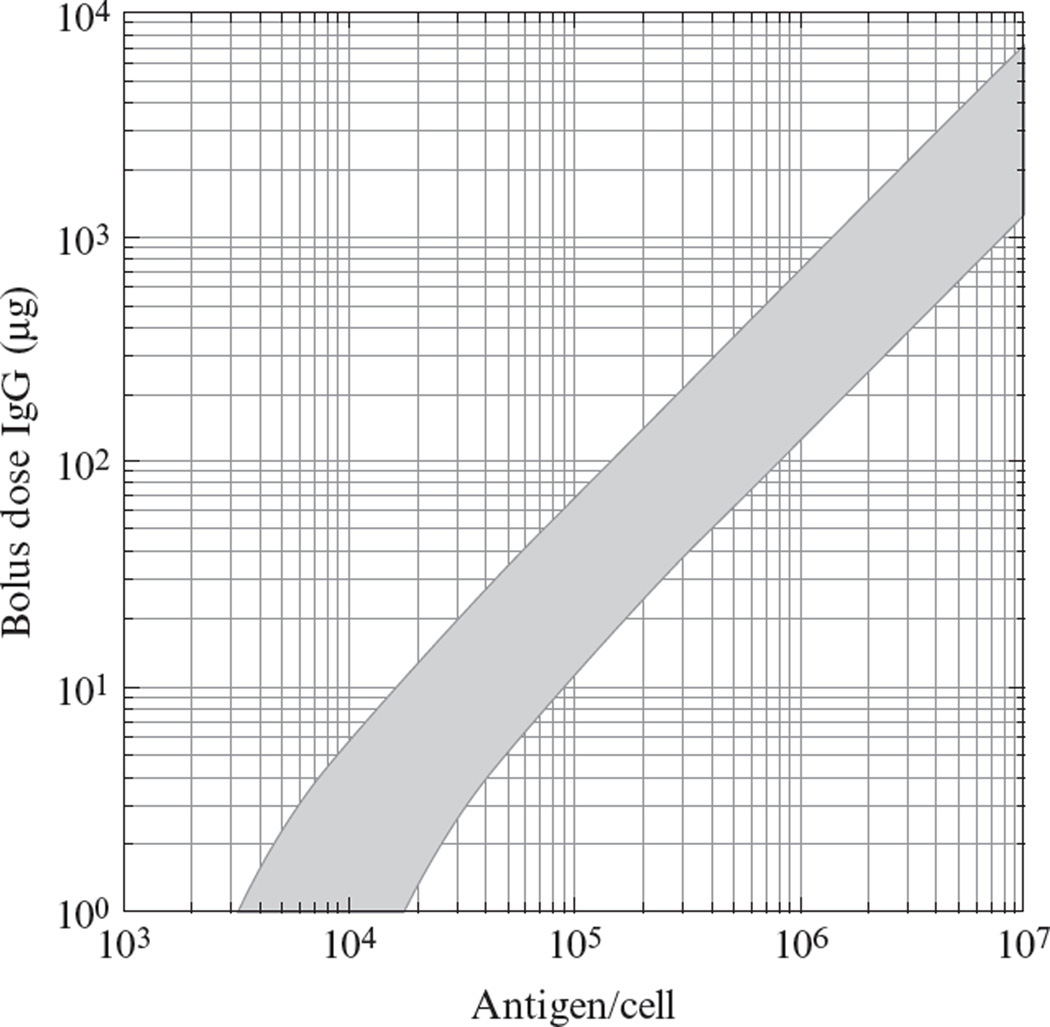
where ke is the net endocytosis rate constant for degradation of bound antibody. For typical parameter values, this relationship predicts that for affinities of Kd>1nM, poor spatial distribution is unlikely to occur. For pM affinities, one can predict the required dosage in a mouse xenograft experiment by assuming constitutive membrane turnover rates, as in Fig. 10.4. For example, for a tumor cell line expressing 105 antigens/cell, a bolus of at least 10–70µg of IgG should be sufficient to permeate throughout a tumor. However, for a more highly expressed antigen at 106/cell, one would require at least 100–700µg doses for saturation. Since the Thiele modulus is inversely proportional to the surface half-life of bound antibody, one could expect to encounter significant difficulty with permeation of antibody–drug conjugates targeted to rapidly internalized antigens with half-lives under an hour.
Figure 10.4.
Predicted bolus dose required to achieve tumor saturation in a mouse. The gray band represents the combination of doses and expression levels for which ϕ2 = 1, the threshold at which degradative consumption equals extravasation, when the degradation half-life for antibody/antigen complexes is in the range 10–54h, typical for constitutive turnover. The high-affinity limit for (Kd = 10pM) is represented. Other parameters are (Schmidt and Wittrup, 2009): ε = 0.24, D = 2.5 × 10−7cm2/s, P = 3.9 × 10−7 cm2/s, Rcap = 8µm, RKrogh = 60µm, mouse blood volume = 2ml, and tumor density = 3 × 108 cells/ml (Schmidt et al., 2008).
6. Conclusions
In this chapter, we have provided simple theoretic relationships that provide predictions of tumor uptake and penetration as a function of targeting agent size and binding affinity. The particular value of these relationships lies not in their quantitative accuracy, but rather in predicting trends in expected outcomes as these key design variables are adjusted. For example, greatest tumor accumulation is expected for proteins ~200 kDa in size—favoring IgG-like constructs. Nanoparticles 100nm in diameter are not predicted (and have not been observed) to accumulate to a greater extent in tumors when targeting ligands are attached to their surfaces. The dynamically poor microdistribution termed the “binding site barrier” can be overcome by predictable increases in bolus dosing. These relationships may assume particular importance in the design of antibody–drug conjugates, which are often selected for rapid endocytosis, and must be dosed at lower levels due to toxicity—conditions particularly conducive to poor microdistribution.
REFERENCES
- Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc. Natl. Acad. Sci. USA. 2007;104(39):15549–15554. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal RD, Fand I, Sharkey RM, Boerman OC, Kashi R, Goldenberg DM. The effect of antibody protein dose on the uniformity of tumor distribution of radioantibodies: An autoradiographic study. Cancer Immunol. Immunother. 1991;33(6):351–358. doi: 10.1007/BF01741594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coliva A, Zacchetti A, Luison E, Tomassetti A, Bongarzone I, Seregni E, Bombardieri E, Martin F, Giussani A, Figini M, Canevari S. 90Y Labeling of monoclonal antibody MOv18 and preclinical validation for radioimmunotherapy of human ovarian carcinomas. Cancer Immunol. Immunother. 2005;54(12):1200–1213. doi: 10.1007/s00262-005-0693-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo SJ, DeNardo GL, Miers LA, Natarajan A, Foreman AR, Gruettner C, Adamson GN, Ivkov R. Development of tumor targeting bioprobes ((111)In-chimeric L6 monoclonal antibody nanoparticles) for alternating magnetic field cancer therapy. Clin. Cancer Res. 2005;11(19 Pt 2):7087s–7092s. doi: 10.1158/1078-0432.CCR-1004-0022. [DOI] [PubMed] [Google Scholar]
- Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J. Natl. Cancer Inst. 2006;98(5):335–344. doi: 10.1093/jnci/djj070. [DOI] [PubMed] [Google Scholar]
- Fujimori K, Covell DG, Fletcher JE, Weinstein JN. Modeling analysis of the global and microscopic distribution of immunoglobulin G, F(ab’)2, and Fab in tumors. Cancer Res. 1989;49(20):5656–5663. [PubMed] [Google Scholar]
- Gabizon A, Horowitz AT, Goren D, Tzemach D, Shmeeda H, Zalipsky S. In vivo fate of folate-targeted polyethylene-glycol liposomes in tumor-bearing mice. Clin. Cancer Res. 2003;9(17):6551–6559. [PubMed] [Google Scholar]
- Goldrosen MH, Biddle WC, Pancook J, Bakshi S, Vanderheyden JL, Fritzberg AR, Morgan AC, Jr, Foon KA. Biodistribution, pharmacokinetic, and imaging studies with 186Re-labeled NR-LU-10 whole antibody in LS174T colonic tumor-bearing mice. Cancer Res. 1990;50(24):7973–7978. [PubMed] [Google Scholar]
- Graff CP, Wittrup KD. Theoretical analysis of antibody targeting of tumor spheroids: Importance of dosage for penetration, and affinity for retention. Cancer Res. 2003;63(6):1288–1296. [PubMed] [Google Scholar]
- Gu F, Zhang L, Teply BA, Mann N, Wang A, Radovic-Moreno AF, Langer R, Farokhzad OC. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc. Natl. Acad. Sci. USA. 2008;105(7):2586–2591. doi: 10.1073/pnas.0711714105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington KJ, Mohammadtaghi S, Uster PS, Glass D, Peters AM, Vile RG, Stewart JS. Effective targeting of solid tumors in patients with locally advanced cancers by radiolabeled pegylated liposomes. Clin. Cancer Res. 2001;7(2):243–254. [PubMed] [Google Scholar]
- Hussain S, Pluckthun A, Allen TM, Zangemeister-Wittke U. Antitumor activity of an epithelial cell adhesion molecule targeted nanovesicular drug delivery system. Mol. Cancer Ther. 2007;6(11):3019–3027. doi: 10.1158/1535-7163.MCT-07-0615. [DOI] [PubMed] [Google Scholar]
- Kelly KA, Setlur SR, Ross R, Anbazhagan R, Waterman P, Rubin MA, Weissleder R. Detection of early prostate cancer using a hepsin-targeted imaging agent. Cancer Res. 2008;68(7):2286–2291. doi: 10.1158/0008-5472.CAN-07-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, Marks JD, Benz CC, Park JW. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66(13):6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- Kukowska-Latallo JF, Candido KA, Cao Z, Nigavekar SS, Majoros IJ, Thomas TP, Balogh LP, Khan MK, Baker JR., Jr Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res. 2005;65(12):5317–5324. doi: 10.1158/0008-5472.CAN-04-3921. [DOI] [PubMed] [Google Scholar]
- Lopes de Menezes DE, Pilarski LM, Allen TM. In vitro and in vivo targeting of immunoliposomal doxorubicin to human B-cell lymphoma. Cancer Res. 1998;58(15):3320–3330. [PubMed] [Google Scholar]
- Lub-de Hooge MN, Kosterink JG, Perik PJ, Nijnuis H, Tran L, Bart J, Suurmeijer AJ, de Jong S, Jager PL, de Vries EG. Preclinical characterisation of 111In-DTPA-trastuzumab. Br. J. Pharmacol. 2004;143(1):99–106. doi: 10.1038/sj.bjp.0705915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamot C, Drummond DC, Noble CO, Kallab V, Guo Z, Hong K, Kirpotin DB, Park JW. Epidermal growth factor receptor-targeted immunoliposomes significantly enhance the efficacy of multiple anticancer drugs in vivo. Cancer Res. 2005;65(24):11631–11638. doi: 10.1158/0008-5472.CAN-05-1093. [DOI] [PubMed] [Google Scholar]
- Mattes MJ, Griffiths GL, Diril H, Goldenberg DM, Ong GL, Shih LB. Processing of antibody-radioisotope conjugates after binding to the surface of tumor cells. Cancer. 1994;73(Suppl. 3):787–793. doi: 10.1002/1097-0142(19940201)73:3+<787::aid-cncr2820731307>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Orlova A, Magnusson M, Eriksson TL, Nilsson M, Larsson B, Hoiden-Guthenberg I, Widstrom C, Carlsson J, Tolmachev V, Stahl S, Nilsson FY. Tumor imaging using a picomolar affinity HER2 binding affibody molecule. Cancer Res. 2006;66(8):4339–4348. doi: 10.1158/0008-5472.CAN-05-3521. [DOI] [PubMed] [Google Scholar]
- Ping Li W, Meyer LA, Capretto DA, Sherman CD, Anderson CJ. Receptor-binding, biodistribution, and metabolism studies of 64Cu-DOTA-cetuximab, a PET-imaging agent for epidermal growth-factor receptor-positive tumors. Cancer Biother. Radiopharm. 2008;23(2):158–171. doi: 10.1089/cbr.2007.0444. [DOI] [PubMed] [Google Scholar]
- Rudnick SI, Lou J, Shaller CC, Tang Y, Klein-Szanto AJ, Weiner LM, Marks JD, Adams GP. Influence of affinity and antigen internalization on the uptake and penetration of anti-HER2 antibodies in solid tumors. Cancer Res. 2011;71(6):2250–2259. doi: 10.1158/0008-5472.CAN-10-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saga T, Neumann RD, Heya T, Sato J, Kinuya S, Le N, Paik CH, Weinstein JN. Targeting cancer micrometastases with monoclonal antibodies: A binding-site barrier. Proc. Natl. Acad. Sci. USA. 1995;92(19):8999–9003. doi: 10.1073/pnas.92.19.8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapra P, Allen TM. Internalizing antibodies are necessary for improved therapeutic efficacy of antibody-targeted liposomal drugs. Cancer Res. 2002;62(24):7190–7194. [PubMed] [Google Scholar]
- Schmidt MM, Wittrup KD. A modeling analysis of the effects of molecular size and binding affinity on tumor targeting. Mol. Cancer Ther. 2009;8(10):2861–2871. doi: 10.1158/1535-7163.MCT-09-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MM, Thurber GM, Wittrup KD. Kinetics of anti-carcinoembryonic antigen antibody internalization: Effects of affinity, bivalency, and stability. Cancer Immunol. Immunother. 2008;57(12):1879–1890. doi: 10.1007/s00262-008-0518-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Jones PM, Vallabhajosula S, Navarro V, Bastidas D, Goldsmith SJ, Bander NH. Radiolabeled monoclonal antibodies specific to the extracellular domain of prostate-specific membrane antigen: Preclinical studies in nude mice bearing LNCaP human prostate tumor. J. Nucl. Med. 2003;44(4):610–617. [PubMed] [Google Scholar]
- Tang Y, Lou J, Alpaugh RK, Robinson MK, Marks JD, Weiner LM. Regulation of antibody-dependent cellular cytotoxicity by IgG intrinsic and apparent affinity for target antigen. J. Immunol. 2007;179(5):2815–2823. doi: 10.4049/jimmunol.179.5.2815. [DOI] [PubMed] [Google Scholar]
- Thiele EW. Relation between catalytic activity and size of particle. Ind. Eng. Chem. 1939;31(7):916–920. [Google Scholar]
- Thurber GM, Wittrup KD. Quantitative spatiotemporal analysis of antibody fragment diffusion and endocytic consumption in tumor spheroids. Cancer Res. 2008;68(9):3334–3341. doi: 10.1158/0008-5472.CAN-07-3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurber GM, Zajic SC, Wittrup KD. Theoretic criteria for antibody penetration into solid tumors and micrometastases. J. Nucl. Med. 2007;48(6):995–999. doi: 10.2967/jnumed.106.037069. [DOI] [PubMed] [Google Scholar]
- Thurber GM, Schmidt MM, Wittrup KD. Antibody tumor penetration: Transport opposed by systemic and antigen-mediated clearance. Adv. Drug Deliv. Rev. 2008a;60(12):1421–1434. doi: 10.1016/j.addr.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurber GM, Schmidt MM, Wittrup KD. Factors determining antibody distribution in tumors. Trends Pharmacol. Sci. 2008b;29(2):57–61. doi: 10.1016/j.tips.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan F, Leunig M, Huang SK, Berk DA, Papahadjopoulos D, Jain RK. Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res. 1994;54(13):3352–3356. [PubMed] [Google Scholar]
- Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, Jain RK. Vascular permeability in a human tumor xenograft: Molecular size dependence and cutoff size. Cancer Res. 1995;55(17):3752–3756. [PubMed] [Google Scholar]
- Zahnd C, Kawe M, Stumpp MT, de Pasquale C, Tamaskovic R, Nagy-Davidescu G, Dreier B, Schibli R, Binz HK, Waibel R, Pluckthun A. Efficient tumor targeting with high-affinity designed ankyrin repeat proteins: Effects of affinity and molecular size. Cancer Res. 2010;70(4):1595–1605. doi: 10.1158/0008-5472.CAN-09-2724. [DOI] [PubMed] [Google Scholar]