Abstract
Pyranoxanthones 6-8 were obtained by dehydrogenation of the respective dihydropyranoxanthones 3-5 with DDQ in dry dioxane. Two prenylated xanthones 10,11 were obtained from the reaction of 1-hydroxyxanthone (9) with prenyl bromide in alkaline medium, or by condensation of xanthone 9 with isoprene in the presence of orthophosphoric acid. The structural elucidation of the two new compounds 6,11, as well as an update of data for the already described prenylated derivatives 7,8,10 were accomplished by IR, UV, HRMS and NMR (1H, 13C, HSQC and HMBC) techniques. The effect of the prenylated xanthone derivatives on the in vitro growth of human tumor cell lines MCF-7 (breast adenocarcinoma) and NCI-H460 (non-small cell lung cancer) is also reported. Compounds 10 and 11 have been found to exhibit a moderate growth inhibitory activity against the MCF-7 cell line.
Keywords: xanthones, prenylation, dehydrogenation, antitumor activity, NMR spectroscopy
1. Introduction
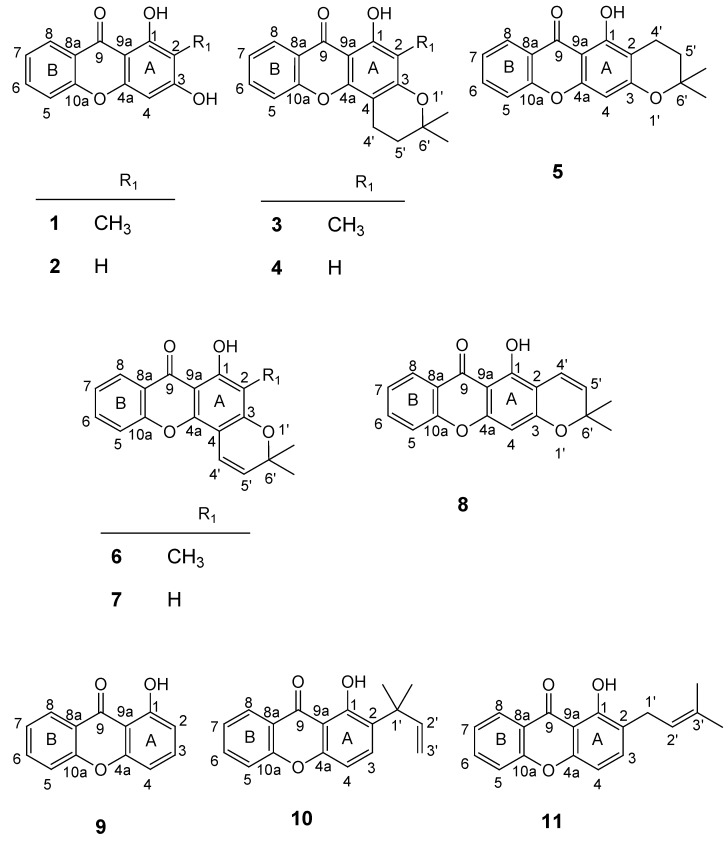
Many naturally occurring xanthones and their prenylated derivatives are found to exhibit significant biological and pharmacological properties, such as antibacterial, antifungal and antitumor activities and it can be inferred that the presence of prenyl groups can be associated with an improvement of potency and selectivity for some of these properties [1,2]. As a large number of biologically active xanthone derivatives with pyran and dihydropyran rings are commonly found in Nature, we were interested in obtaining this type of compounds to evaluate their antitumor activity. For this purpose, molecular modifications of the hit compounds, 1,3-dihydroxy-2-methylxanthone (1) and 1,3-dihydroxyxanthone (2) (Figure 1) were carried out [3].
Figure 1.
Structures of the xanthone building blocks 1-5, 9 and prenylated derivatives 6-8, 10 and 11 (the numbering used refers to the NMR assignments).
Prenylation of xanthones 1 and 2 with prenyl bromide, followed by cyclisation of the respective monoprenylated products furnished dihydropyranoxanthones 3-5 [3] (Figure 1), which were evaluated for their effects on the in vitro growth of three human tumour cell lines (MCF-7, NCI-H460 and SF-268). These compounds were found to be more selective, showing their growth inhibitory effects only against the breast cancer MCF-7 cells when compared with their building blocks, respectively 1 and 2 [3].
The fact that naturally occurring pyranoxanthones are more active than dihydropyranoxanthones in many biological activity assays [1] has led us to resort to a rigidification strategy to improve the antitumor activity of the xanthone derivatives. Thus, unsaturation strategy was applied to the dihydropyran ring of dihydropyranoxanthones 3-5 to give pyranoxanthones 6-8, respectively (Figure 1).
The second approach is to introduce the prenyl side chain to the xanthone nucleus, using a C-prenylation strategy. Thus, two C-prenylated derivatives, 10 and 11 were synthesized by prenylation of xanthone 9 (Figure 1). Though C-prenylated derivatives are not as common in nature as the O-prenylated analogues, they show very interesting properties [1]. Based on this observation, xanthone 9 (Figure 1) was submitted to a C-prenylation strategy to furnish compounds 10 and 11.
The xanthone derivatives 6-8, 10 and 11, were then evaluated for their capacity to inhibit the in vitro growth of MCF-7 (breast adenocarcinoma) and NCI-H460 (non-small cell lung cancer) cells, and their effects were compared with those of their building blocks [3,4].
2. Results and Discussion
2.1. Synthesis of prenylated derivatives
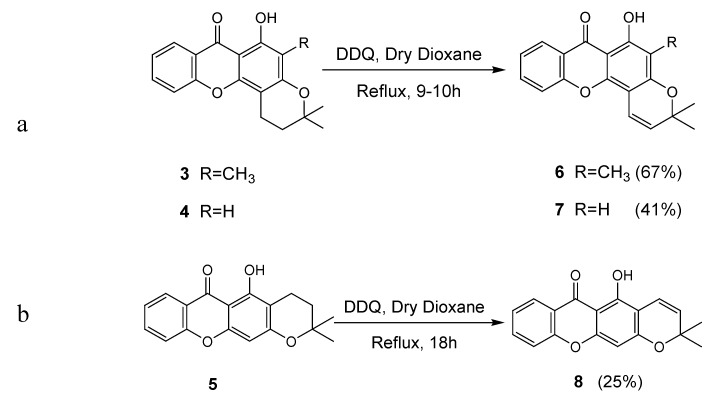
Pyranoxanthones 6-8 were obtained by dehydrogenation of the respective dihydropyranoxanthones 3-5 with DDQ in refluxing dry dioxane [5]. While dihydropyranoxanthone 3 gave pyranoxanthone 6, dihydropyranoxanthone 4 afforded pyranoxanthone 7 (Scheme 1a) and dihydropyranoxanthone 5 gave pyranoxanthone 8 (Scheme 1b) in 67, 41 and 25% yield, respectively.
Scheme 1.
Synthesis of pyranoxanthones 6-8. a). Synthesis of pyranoxanthones 6 and 7; b). Synthesis of pyranoxanthone 8.
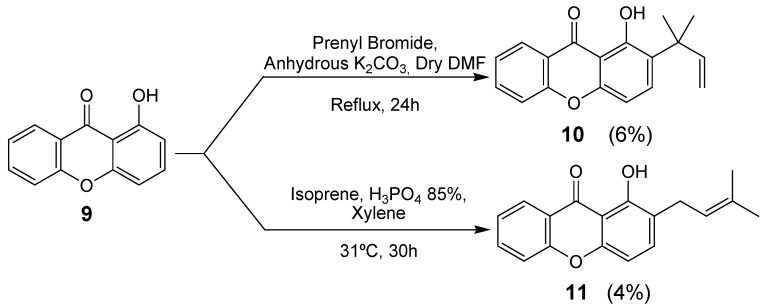
Of these, pyranoxanthone 6, containing a methyl group at C-2 was formed with the highest yield (67%). It can be also observed that the angular pyranoxanthone 7 was obtained in higher yield than the linear counterpart 8. Prenylation of 1-hydroxyxanthone (9), either with prenyl bromide or isoprene, gave 1,1-dimethylallyl- or 3,3-dimethylallyl-derivatives 10 and 11, respectively, in low yields and after long reaction times. Xanthone 10 was obtained by the reaction of 1-hydroxyxanthone (9) with prenyl bromide, in alkaline medium and refluxing N,N-dimethylformamide [6] (DMF) (Scheme 2).
Scheme 2.
Synthesis of prenylated xanthones 10 and 11.
Mechanistically, the formation of xanthone 10 by the described method, can be postulated to occur by prenylation at 1-OH of xanthone 9 with subsequent ortho Claisen rearrangement of the prenyl group to give the 1,1-dimethylallyl substituent on C-2 of the xanthonic scaffold [7].
The prenylated derivative 11 was obtained by condensation of 1-hydroxyxanthone (9) with isoprene, in the presence of catalytic amounts of orthophosphoric acid [8] (Scheme 2). The acid-catalysed condensation of isoprene with the phenol moiety of the xanthonic scaffold may be regarded as the chemical equivalent of the proposed biogenetic pathways [8].
2.2. Structural elucidation of the prenylated xanthones
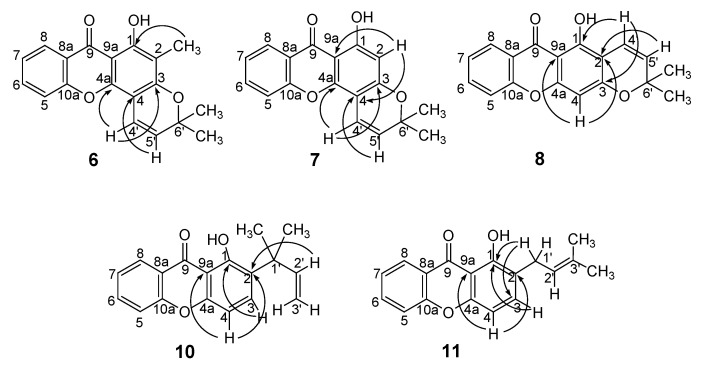
The structures of compounds 6-8 and 10, 11 were established by IR, UV, HRMS and NMR (1H-, 13C-, HSQC and HMBC) techniques, while the spectroscopic data of compounds 1-5 and 9 are in agreement with those reported in the literature [3,9,10,11,12]. Although the spectroscopic data of pyranoxanthones 7 and 8, as well as of prenylated xanthone 10 have been previously described [7,13,14], here we provide an updated and complete structure elucidation of these compounds.
The EI-HRMS of compound 6 gave the accurate molecular mass at 308.1049 and the corresponding molecular formula C19H16O4, indicating that there were two hydrogen atoms less than in its dihydropyranoxanthone precursor 3. The 1H-NMR spectrum of compound 6 was very similar to that of compound 3, except for the two doublets of the olefinic protons at δH 5.62 (J = 10.0 Hz) and δH 6.86 (J = 10.0 Hz), instead of the triplets of the protons of two methylene groups at δH 1.88 (J = 6.8 Hz) and δH 2.89 (J = 6.8 Hz) of the dihydropyran ring [3]. The protons of the geminal methyl groups of the pyran ring in compound 6 appeared as a singlet at δH 1.50. The 13C-NMR spectrum of compound 6 was also similar to that of dihydropyranoxanthone 3 [3], except for the substitution of the two methylene carbons at δC 16.4 and 31.7 with the two olefinic carbon signals at δC 115.3 and δC 126.8.
In turn, the EI-HRMS of compound 7 indicated the accurate molecular mass at 294.0886, corresponding to the molecular formula C18H14O4. The 1H- and 13C-NMR spectra of compound 7 were very similar to those of compound 6, except for the presence of a singlet of the aromatic proton at C-2 at δH 6.28 instead of the singlet of the methyl group at δH 2.12. As in compound 6, the presence of the pyran ring in compound 7 was confirmed by the two doublets of the olefinic protons at δH 5.62 (J = 10.0 Hz) and δH 6.85 (J = 10.0 Hz) in the 1H-NMR spectrum which showed cross peaks with the olefinic carbons at δC 127.2 and δC 115.0, respectively in the HSQC spectrum.
The EI-HRMS of compound 8 gave the accurate molecular mass at 294.0898 and the molecular formula C18H14O4. As expected, the 1H- and 13C-NMR spectra of compound 8 were similar to those of its dihydropyranoxanthone precursor 5 [3], except for the signals of the olefinic protons (δH 6.74, d, J = 10.0 Hz and δH 5.61, d, J = 10.0 Hz) and carbons (δC 115.4 and δC 127.6).
Finally, the EI-HRMS of compounds 10 and 11 indicated their accurate molecular masses at 280.1099 and 280.1096, respectively, and thus, a molecular formula C18H16O3 for both compounds. This molecular formula confirmed the prenylation of xanthone 9. In turn, the 1H-NMR spectra of compounds 10 and 11 showed, besides, the proton signals corresponding to the non substituted aromatic ring of the xanthone nucleus, the signals of another two ortho coupled aromatic protons (δH 6.88, d, J = 8.8 Hz; δH 7.64, d, J = 8.8 Hz and δH 6.76, d, J = 8.4 Hz; δH 7.46, d, J = 8.4 Hz) and 1-OH (δH 13.47, s and δH 12.56, s). The presence of these two ortho coupled aromatic protons indicated that the prenylation occurred at C-2. That the side chain of compound 10 was 2-methylbut-3-en-2-yl was confirmed by the signals of the protons of the vinyl group at δH 5.07, dd (J = 17.0, 1.2 Hz), δH 5.02, dd (J = 11.0, 1.2 Hz) and δH 6.28, dd (J = 17.0, 11.0 Hz) and the methyl groups at δH 1.55, s, respectively. This was corroborated by the correlation between the proton signal at δH 6.28, dd (J = 17.0, 11.0 Hz, H-2’) and the carbon signal at δC 128.9 (C-2). On the other hand, the 3-methylbut-2-enyl side chain of compound 11 was established by the presence of the signals of the allylic proton at δH 5.33, t (J = 7.4 Hz), the methyl protons at δH 1.76, s and δH 1.81, s and the methylene protons at δH 3.53, d (J = 7.4 Hz). The HMBC spectrum of compound 11 also showed the correlation between the signal of the methylene protons (δH 3.53, d) and the signal of C-1 at δC 160.0.
Figure 2.
Main connectivities found in the HMBC of prenylated xanthones 6-8, 10 and 11.
2.3. Biological Activity studies
Though the number of compounds prepared was small, some basic structure-activity relationship trends can be observed. When the effects of the prenylated xanthones 6-8 on the growth of MCF-7 cells are compared with those of their respective xanthonic building blocks 3-5, it was found that the presence of the unsaturation in the pyran ring was associated with a loss of inhibitory activity against MCF-7 (Table 1). It can be presumed that the lack of activity of compounds 6 and 8 could be a consequence of the rigidification of the dihydropyran ring. On the other hand, C-prenylation of the inactive xanthone 9 [4] was found to be associated with the growth of the inhibitory effect against MCF-7 of the prenylated derivatives 10 and 11 (Table 1). The introduction of the lipophilic prenyl group in C-2 of the xanthonic scaffold is probably the reason for the appearance of this activity for xanthones 10 and 11.
Table 1.
Effect of xanthone derivatives 1-11 on the growth of human tumor cell lines.
| Compound | GI50 (µM) | ||
|---|---|---|---|
| MCF-7 | NCI-H460 | SF-268 | |
| Results are given in concentrations that were able to cause 50% of cell growth inhibition (GI50) after a continuous exposure of 48h and represent means of ±SEM of 3 independent experiments performed in duplicate and carry out independently. aResults published elsewhere [3,4]. bResults of one or two experiments performed in duplicate. Doxorubicin was used as positive control, GI50: MCF-7 = 42.8±8.2 nM; NCI-H460 = 94.0±8.7 nM; SF-268 = 93.0±7.0 nM. ND = not determined. | |||
| 1a | 21.9 ± 0.4 | 20.6 ± 0.9 | 33.4 ± 0.2 |
| 2a | 50.8 ± 2.2 | 37.9 ± 2.9 | 61.4 ± 5.2 |
| 3a | 18.4 ± 1.9 | >160 | >160 |
| 4a | >160 | >160 | >160 |
| 5a | 88.6 ± 12.9 | >160 | >160 |
| 6 | >150b | >150b | ND |
| 7 | >150 | >150b | ND |
| 8 | >150b | >150b | ND |
| 9a | >200 | ND | ND |
| 10 | 55b | >150b | ND |
| 11 | 88b | ND | ND |
3. Experimental
3.1. General
Purification of compounds were performed by flash chromatography using Merck silica gel 60 (0.040-0.063 mm) and preparative thin layer chromatography (TLC) using Merck silica gel 60 (GF254) plates. Reactions were monitored by TLC. Melting points were obtained in a Köfler microscope and are uncorrected. IR spectra were measured on an ATI Mattson Genesis series FTIR (software: WinFirst v. 2.10) spectrophotometer in KBr microplates (cm-1). UV spectra were taken in ethanol [15] and were recorded on a Varian CARY 100 spectrophotometer: λmax in nm (software: Cary Win UV v. 3.0). 1H and 13C NMR spectra were taken in CDCl3 at room temperature, on a Bruker Avance 300 instrument. Chemical shifts are expressed in δ (ppm) values relative to tetramethylsilane (TMS) as an internal reference. 1H-NMR spectra were measured at 300.13 MHz and assignment abbreviations are the following: singlet (s), doublet (d), triplet (t), quartet (q), multiplet (m), doublet of doublets (dd), and double doublet of doublets (ddd). 13C-NMR spectra were measured at 75.47 MHz. 13C-NMR assignments were made by 2D HSQC and HMBC experiments (long-range C, H coupling constants were optimized to 7 Hz). HRMS spectra were recorded as EI (electronic impact) mode on a VG Autospec M spectrometer (m/z) at CACTI, Vigo, Spain. Prenyl bromide, Isoprene and DDQ were purchased from Sigma Aldrich. Compounds 1-5 and 9 were obtained and characterized according to the described procedures [3,9,10,11,12]. The following materials were synthesized and purified by the described procedures.
3.2. General Procedure for the Synthesis of Pyranoxanthones 6-8
To a solution of dihydropyranoxanthones 3-5 (0.06 mmol) in dry dioxane (10 mL) was added DDQ (0.12 mmol) and the reaction mixture was refluxed (100ºC) for 9-18 h. After cooling, the precipitate was filtered off and the filtrate evaporated. The crude product was purified by preparative TLC (SiO2; hexane/EtOAc 95:5 or light petroleum/CHCl3 5:5). Compounds 6, 7 [13] and 8 [14] were identified by their spectroscopic and analytical data.
6-Hydroxy-3,3,5-trimethylpyrano[2,3-c]xanthen-7(3H)-one (6): The compound was obtained (67%) as yellow solid; m.p. 196-198ºC (EtOH); λmax (ε): 327, 273, 250 (6821, 25216, 22253); (EtOH + NaOH): 426, 296, 217 (3056, 30278, 95370); (EtOH + AlCl3): 330, 275, 235 (6481, 24753, 17685); νmax (KBr): 3431, 2959, 2917, 1644, 1606, 1564, 1471, 1431, 1320, 1145, 1108, 742 cm-1; 1H-NMR: δ =13.22 (s, 1H, 1-OH), 8.25 (dd, 1H, J = 8.0, 1.6 Hz, 8-H), 7.70 (ddd, 1H, J = 8.4, 7.1, 1.6 Hz, 6-H), 7.44 (d, 1H, J = 8.4 Hz, 5-H), 7.36 (dd, 1H, J = 8.0, 7.1 Hz, 7-H), 6.86 (d, 1H, J = 10.0 Hz, 4’-H), 5.62 (d, 1H, J= 10.0 Hz, 5’-H), 2.12 (s, 3H, 2-CH3), 1.50 (s, 6H, 6’-CH3) ppm; 13C-NMR: δ =180.8 (C-9), 160.5 (C-1), 158.8 (C-3), 155.8 (C-10a), 149.8 (C-4a), 134.7 (C-6), 126.8 (C-5’), 125.9 (C-8), 123.8 (C-7), 120.6 (C-8a), 117.5 (C-5), 115.3 (C-4’), 107.8 (C-2), 103.1 (C-9a), 100.5 (C-4), 78.0 (C-6’), 28.4 (6’-CH3, 2C), 7.0 (2-CH3) ppm; EI-MS m/z (%): 308 (6, M+.), 293 (100), 267 (4), 149 (5), 137 (4), 121 (4), 109 (5), 95 (6), 81 (11), 69 (12); EI-HR-MS m/z: Anal. Calc. for C19H16O4: 308.1049; found: 308.1049.
6-Hydroxy-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-one (7): The compound was obtained (41%) as yellow crystals; m.p. 164-168ºC (Acetone); λmax (ε): 271, 244, 201 (8471, 7588, 5603); (EtOH + NaOH): 293, 216 (9103, 45176); (EtOH + AlCl3): 335, 285, 228, 201 (2838, 9412, 6941, 6147); νmax (KBr): 3432, 2956, 2921, 2853, 1650, 1596, 1465, 1279, 1142, 1102, 1073, 804, 749 cm-1; 1H-NMR: δ =12.97 (s, 1H, 1-OH), 8.25 (d, 1H, J = 7.8 Hz, 8-H), 7.72 (dd, 1H, J = 8.4, 7.4 Hz, 6-H), 7.46 (d, 1H, J = 8.4 Hz, 5-H), 7.38 (dd, 1H, J = 7.8, 7.4 Hz, 7-H), 6.85 (d, 1H, J = 10.0 Hz, 4’-H), 6.28 (s, 1H, 2-H), 5.62 (d, 1H, J= 10.0 Hz, 5’-H), 1.49 (s, 6H, 6’-CH3) ppm; 13C-NMR: δ =180.9 (C-9), 163.2 (C-1), 161.0 (C-4a), 155.8 (C-10a), 151.8 (C-3), 135.0 (C-6), 127.2 (C-5’), 125.9 (C-8), 124.1 (C-7), 120.6 (C-8a), 117.6 (C-5), 115.0 (C-4’), 103.8 (C-9a), 101.1 (C-4), 99.4 (C-2), 78.3 (C-6’), 28.3 (6’-CH3, 2C) ppm; EI-MS m/z (%): 294 (2, M+.), 279 (22), 183 (74), 181 (78), 171 (60), 169 (66), 163 (100), 149 (20), 145 (25), 117 (40), 115 (37), 104 (30), 103 (46), 91 (35), 90 (50), 89 (53), 77 (26); EI-HR-MS m/z: Anal. Calc. for C18H14O4: 294.0892; found: 294.0886.
5-Hydroxy-2,2-dimethylpyrano[3,2-b]xanthen-6(2H)-one (8): The compound was obtained (25%) as yellow crystals; m.p. 170-173ºC (Acetone); λmax (ε): 289, 237, 201 (17059, 14676, 11853); (EtOH + NaOH): 405, 309, 215 (1647, 14471, 88324); (EtOH + AlCl3): 293, 237, 201 (16471, 14765, 13324); νmax (KBr): 3410, 2963, 2921, 2855, 1646, 1609, 1567, 1453, 1301, 1212, 1139, 1081, 749 cm-1; 1H-NMR: δ =13.17 (s, 1H, 1-OH), 8.24 (dd, 1H, J = 8.0, 1.6 Hz, 8-H), 7.70 (ddd, 1H, J = 8.6, 7.1, 1.6 Hz, 6-H), 7.43 (d, 1H, J = 8.6 Hz, 5-H), 7.37 (dd, 1H, J = 8.0, 7.1 Hz, 7-H), 6.74 (d, 1H, J = 10.0 Hz, 4’-H), 6.36 (s, 1H, 4-H), 5.61 (d, 1H, J= 10.0 Hz, 5’-H), 1.49 (s, 6H, 6’-CH3) ppm; 13C-NMR: δ =180.8 (C-9), 160.9 (C-3), 157.7 (C-1), 157.1 (C-4a), 155.9 (C-10a), 134.9 (C-6), 127.6 (C-5’), 125.8 (C-8), 124.0 (C-7), 120.5 (C-8a), 117.6 (C-5), 115.4 (C-4’), 107.1 (C-4), 104.6 (C-2), 103.8 (C-9a), 78.3 (C-6’), 28.4 (6’-CH3, 2C) ppm; EI-MS m/z (%): 294 (9, M+.), 279 (100), 69 (7); EI-HR-MS m/z: Anal. Calc. for C18H14O4: 294.0892; found: 294.0898.
3.3. Synthesis of Prenylated Xanthone 10
A mixture of 1-hydroxyxanthone (9) (0.10 g; 0.47 mmol), prenyl bromide (110 μL; 0.95 mmol) and anhydrous K2CO3 (0.22 g, 1.58 mmol) in dry DMF (7 mL), was refluxed at 150ºC for 24 h. After cooling, the solid was filtered and the solvent removed under reduced pressure, affording the crude product that was purified by flash chromatography (SiO2; Hexane/EtOAc 95:5) and by preparative TLC (SiO2; Hexane/CHCl3 9:1). The product, 1-hydroxy-2-(2-methylbut-3-en-2-yl)-9H-xanthen-9-one (10) [7] was obtained in 6% yield as yellow crystals, and identified by spectroscopic and analytical data; m.p. 99-102ºC (acetone); λmax (ε): 281, 258, 230, 203 (2511, 10196, 9453, 6858); (EtOH + NaOH): 426, 309, 216 (1641, 3815, 43184); (EtOH + AlCl3): 259, 231, 206 (7475, 9341, 6466); νmax (KBr): 3432, 2954, 2919, 2858, 1632, 1608, 1462, 1433, 1374, 1285, 1213, 1057, 752 cm-1; 1H-NMR: δ =13.47 (s, 1H, 1-OH), 8.29 (dd, 1H, J = 8.0, 1.6 Hz, 8-H), 7.74 (ddd, 1H, J = 8.7, 7.0, 1.6 Hz, 6-H), 7.64 (d, 1H, J = 8.8 Hz, 3-H), 7.45 (d, 1H, J = 8.7 Hz, 5-H), 7.38 (dd, 1H, J = 8.0, 7.0 Hz, 7-H), 6.88 (d, 1H, J = 8.8 Hz, 4-H), 6.28 (dd, 1H, J = 17.0, 11.0 Hz, 2’-H), 5.07 (dd, 1H, J= 17.0, 1.2 Hz, 3’-H), 5.02 (dd, 1H, J= 11.0, 1.2 Hz, 3’-H), 1.55 (s, 6H, 1’-CH3) ppm; 13C-NMR: δ =182.9 (C-9), 160.5 (C-1), 156.1 (C-10a), 154.8 (C-4a), 147.0 (C-2’), 135.4 (C-6), 135.0 (C-3), 128.9 (C-2), 126.0 (C-8), 123.8 (C-7), 120.5 (C-8a), 117.7 (C-5), 110.6 (C-3’), 108.8 (C-9a), 105.6 (C-4), 40.3 (C-1’), 26.7 (1’-CH3, 2C) ppm; EI-MS m/z (%): 280 (20, M+.), 265 (100), 251 (17), 250 (16), 239 (16), 237 (20), 225 (35), 69 (11); EI-HR-MS m/z: Anal. Calc. for C18H16O3: 280.1100; found: 280.1099.
3.4. Synthesis of prenylated xanthone 11
A solution of isoprene (200 μL; 2.00 mmol) in xylene (1 mL) was added to a stirred mixture of 1-hydroxyxanthone (9, 0.20 mg; 0.96 mmol), orthophosphoric acid (85%, 1 mL) and xylene (4 mL), with constant stirring at 31ºC during 2 h. The mixture was stirred for a further 28 h and then neutralised with hydrogen carbonate solution (5%). The mixture thus obtained, was extracted with diethyl ether. The extract was washed with water, dried (Na2SO4) and the solvent evaporated under reduced pressure. The crude product thus obtained was purified by flash chromatography (SiO2; Hexane/EtOAc 98:2) and preparative TLC (SiO2; EP/Et2O 9:1). 1-Hydroxy-2-(3-methylbut-2-enyl)-9H-xanthen-9-one (11) was identified by its spectroscopic and analytical data. Yield: 4%, as yellow crystals; m.p. 68-71ºC (acetone); λmax (ε): 368, 300, 257, 232, 203 (3240, 5526, 23689, 24109, 18794); (EtOH + NaOH): 416, 308, 265, 217 (4600, 9257, 16157, 49130); (EtOH + AlCl3): 445, 316, 275, 231, 205 (3394, 7798, 21837, 26452, 20084); νmax (KBr): 3448, 2963, 2917, 2853, 1642, 1604, 1472, 1369, 1279, 1227, 763 cm-1; 1H-NMR: δ =12.56 (s, 1H, 1-OH), 8.29 (dd, 1H, J = 8.0, 1.6 Hz, 8-H), 7.76 (ddd, 1H, J = 8.4, 7.1, 1.6 Hz, 6-H), 7.51 (d, 1H, J = 8.4 Hz, 5-H), 7.46 (d, 1H, J = 8.4 Hz, 3-H), 7.40 (dd, 1H, J = 8.0, 7.1 Hz, 7-H), 6.76 (d, 1H, J = 8.4 Hz, 4-H), 5.33 (t, 1H, J = 7.4 Hz, 2’-H), 3.53 (d, 2H, J= 7.4 Hz, 1’-H), 1.81 and 1.76 (2s, 2´3H, 3’-CH3) ppm; 13C-NMR: δ =182.6 (C-9), 160.0 (C-1), 156.1 (C-10a), 153.4 (C-4a), 137.0 (C-3), 135.4 (C-6), 133.3 (C-3’), 126.0 (C-8), 124.0 (C-7), 121.7 (C-2’), 120.5 (C-8a), 119.3 (C-2), 117.9 (C-5), 110.0 (C-4), 108.9 (C-9a), 27.6 (C-1’), 25.8 and 17.9 (3’-CH3, 2C) ppm; EI-MS m/z (%): 280 (15, M+.), 265 (33), 225 (12), 149 (11), 137 (18), 121 (18), 109 (12), 107 (12), 95 (26), 81 (69), 69 (100); EI-HR-MS m/z: Anal. Calc. for C18H16O3: 280.1100; found: 280.1096.
3.5. Tumor cell growth assay
Stock solutions of compounds 6-8, 10 and 11 and doxorubicin were prepared in DMSO (Sigma Chemical Co) and stored at –20 ºC. The frozen samples were freshly diluted with culture medium just prior the assays. Final concentrations of DMSO (0.25%) did not interfere with the growth of cell lines.
The human tumor cell lines MCF-7 (breast adenocarcinoma) and NCI-H460 (non-small cell lung cancer) were used. Cells growing as monolayer, were routinely maintained in RPMI-1640 medium (Gibco BRL) supplemented with 5% heat-inactivated fetal bovine serum (Gibco BRL), 2 mM glutamine (Sigma Chemical Co.), penicillin 100 U/mL and 100 μg/mL streptomycin (Gibco BRL), at 37 ºC in an humidified atmosphere containing 5% CO2. The optimal plating density of each cell line, that ensure exponential growth throughout all the experimental period was respectively 1.5 × 105 cells/ml to MCF-7 and 7.5 × 104 cells/ml for NCI-H460.
The effects of compounds on the growth of the human tumor cell lines were evaluated according to the procedure adopted by the National Cancer Institute (NCI, USA) for the “In vitro Anticancer Drug Discovery Screen” that uses the protein-binding dye sulforhodamine B (SRB) (Sigma Chemical Co.) to assess cell growth [16,17]. Briefly, exponentially growing cells were exposed for 48 h to five serial concentrations (1:2 or 1:3 dilution) of each compound, starting from a maximum concentration of 150 μM. Following this exposure period adherent cells were fixed in situ with 50% TCA, washed with distillate water and stained with 0.4% SRB solubilized in 1% acetic acid. The bound stain was solubilized and the absorbance was measured at 492 nm in a microplate reader (Bio-tek Instruments Inc., PowerWave XS, Winooski, USA). For each cell line a dose-response curve was obtained and the growth inhibition of 50% (GI50), corresponding to the concentration of compound that inhibited 50% of the net cell growth, was determined as described elsewhere [16]. Doxorubicin used as a positive control, was tested in the same manner. Moreover the effect of the vehicle solvent (DMSO) on the growth of these cell lines was evaluated in all experiments by exposing untreated control cells to the maximum concentration (0.25%) of DMSO used in each assay.
4. Conclusions
In contrast to their dihydropyranoxanthone precursors 3-5, the pyranoxanthones 6-8 did not exhibit growth inhibitory effect against the breast adenocarcinoma MCF-7 cells. On the other hand, C-prenylation of the inactive hydroxyxanthone 9, led to prenylated derivatives 10 and 11 which exhibited moderate growth inhibitory activity against the MCF-cells. From these results, we can conclude that introduction of an unsaturation on the extra ring was not effective in improving the biological activity of these compounds. On the contrary, the introduction of the prenyl side chain on an appropriate position of the xanthonic scaffold was found to improve the antitumor activity of compounds 10 and 11. The increase of the lipophilicity of the molecule and/or an extra molecular motif to interact with biological targets furnished by the prenyl group can be a key to explain the improvement this activity.
Acknowledgements
The authors thank to Fundação para a Ciência e a Tecnologia (FCT), I&D Units 226/2003 (CEQOFFUP), 4040/2007 (CEQUIMED-UP) and 62/94 (QOPNA), FEDER, POCI for financial support and to FCT for the Ph.D. grant to Raquel Castanheiro (SFRH/BD/13167/2003). The authors are also indebted to the National Cancer Institute, Bethesda, MD, USA for the generous provision of the human tumor cell lines. We thank Sara Cravo for technical support.
References and Notes
- 1.Pinto M., Castanheiro R. Natural Prenylated Xanthones: Chemistry and Biological Activities. In: Brahmachari G., editor. Natural Products: Chemistry, Biochemistry and Pharmacology. 1st. Narosa Publishing House PVT. LTD; New Dehli, India: 2009. pp. 520–676. [Google Scholar]
- 2.Pinto M.M.M., Sousa M.E., Nascimento M.S.J. Xanthone Derivatives: New Insights in Biological Activities. Curr. Med. Chem. 2005;12:2517–2538. doi: 10.2174/092986705774370691. [DOI] [PubMed] [Google Scholar]
- 3.Castanheiro R.A.P., Pinto M.M.M., Silva A.M.S., Cravo S.M.M., Gales L., Damas A.M., Nazareth N., Nascimento M.S.J., Eaton G. Dihydroxyxanthones Prenylated Derivatives: Synthesis, Structure Elucidation and Growth Inhibitory Activity on Human Tumor Cell Lines with Improvement of Selectivity for MCF-7. Bioorg. Med. Chem. 2007;15:6080–6088. doi: 10.1016/j.bmc.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 4.Pedro M.M., Cerqueira F., Sousa M.E., Nascimento M.S.J., Pinto M.M.M. Xanthones as Inhibitors of Growth of Human Cancer Cell Lines and Their Effects on the Proliferation of Human Lymphocytes In Vitro. Bioorg. Med. Chem. 2002;10:3725–3730. doi: 10.1016/S0968-0896(02)00379-6. [DOI] [PubMed] [Google Scholar]
- 5.Ho L.-K., Yu H.-J., Ho C.-T., Don M.-J. Synthesis of Naturally Occurring Rubilactone, Mollugin, and Dihydromollugin of Rubia cordifolia. J. Chin. Chem. Soc. 2001;48:77–79. [Google Scholar]
- 6.Pisco L., Kordian M., Peseke K., Feist H., Michalik D., Estrada E., Carvalho J., Hamilton G., Rando D., Quincoces J. Synthesis of compounds with antiproliferative activity as analogues of prenylated natural products existing in Brazilian propolis. Eur. J. Med. Chem. 2006;41:401–407. doi: 10.1016/j.ejmech.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 7.Helboe P., Arends P. Xanthone Studies VI. Synthesis of Jacareubin, Isojacareubin and some hydroxyxanthones with allylic substituents. Arch. Pharm. Chemi. Sci. Ed. 1973;1:69–75. [Google Scholar]
- 8.Ahluwalia V.K., Arora K.K., Jolly R.S. Acid-catalysed Condensation of Isoprene with Phenols. Formation of 2,2-Dimethylchromans. J. Chem. Soc. Perkin Trans. I. 1982:335–338. [Google Scholar]
- 9.Fernandes E.G.R., Silva A.M.S., Cavaleiro J.A.S., Silva F.M., Borges M.F.M., Pinto M.M. 1H and 13C NMR Spectroscopy of Mono-, Di-, Tri-, and Tetrasubstituted Xanthones. Magn. Reson. Chem. 1998;36:305–309. [Google Scholar]
- 10.Pinto M.M.M., Polónia J. Synthesis of New Xanthones, I. Helv. Chim. Acta. 1974;57:2613–2618. [Google Scholar]
- 11.Grover P.K., Shah G.D., Shah R.C. Xanthones. Part IV. A New Synthesis of Hydroxyxanthones and Hydroxybenzophenones. J. Chem. Soc. 1955:3982–3985. [Google Scholar]
- 12.Pankajamani K.S., Seshadri T.R. Synthetic Experiments in the Benzopyrone Series: Part XLVI- Application on the Nencki Reaction in the Synthesis of Xanthones. J. Sci. Industr. Res. 1954;13B:396–400. [Google Scholar]
- 13.Kolokythas G., Kostakis I.K., Pouli N., Marakos P., Kousidou O.C., Tzanakakis G.N., Karamanos N.K. Design and synthesis of new pyranoxanthenones bearing a nitro group or an aminosubstituted side chain on the pyran ring. Evaluation of their growth inhibitory activity in breast cancer cells. Eur. J. Med. Chem. 2007;42:307–319. doi: 10.1016/j.ejmech.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Subba Rao G.S.R., Raghavan S. Synthetic studies on morellin. Part 4: Synthesis of 2,2-dimethyl- 12-[3-methylbut-2-enyl]-2H,6H-pyrano[3,2-b]xanthen-6-one. J. Indian Inst. Sci. 2001;81:393–401. [Google Scholar]
- 15.Mesquita A.A.L., Corrêa D.B., Gottlieb O.R, Magalhães M.T. Methods for the Structural Investigation of Xanthones. Part II. Location of the Hydroxyl Groups by Ultra-Violet and Visible Spectroscopy. Anal. Chim. Acta. 1968;42:311–323. doi: 10.1016/S0003-2670(01)80312-3. [DOI] [Google Scholar]
- 16.Monks A., Scudiero D., Skehan P., Shoemaker R., Paull K., Vistica D., Hose C., Langley J., Cronise P., Vaigrowolff A., Graygoodrich M., Campbell H., Mayo J., Boyd M. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer I. 1991;83:757–766. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- 17.Skehan P., Storeng R., Scudiero D., Monks A., McMahon J., Vistica D., Warren J.T., Bokessch H., Kenney S., Boyd M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer I. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]