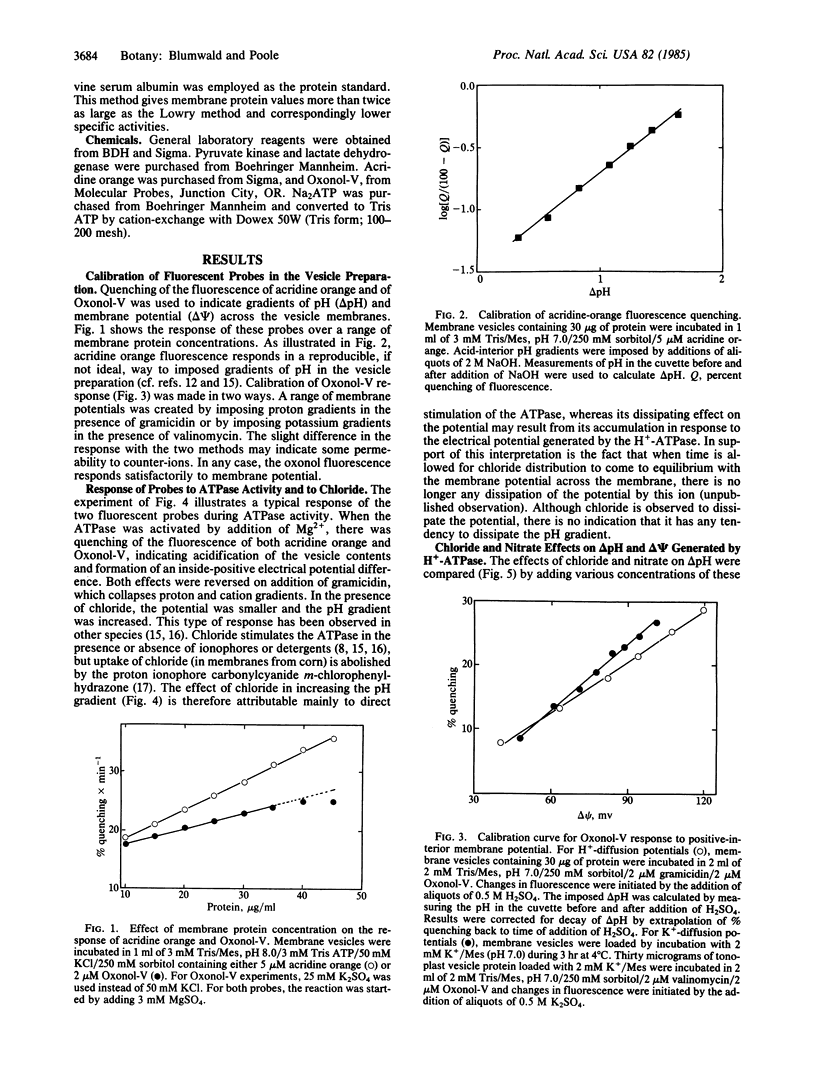
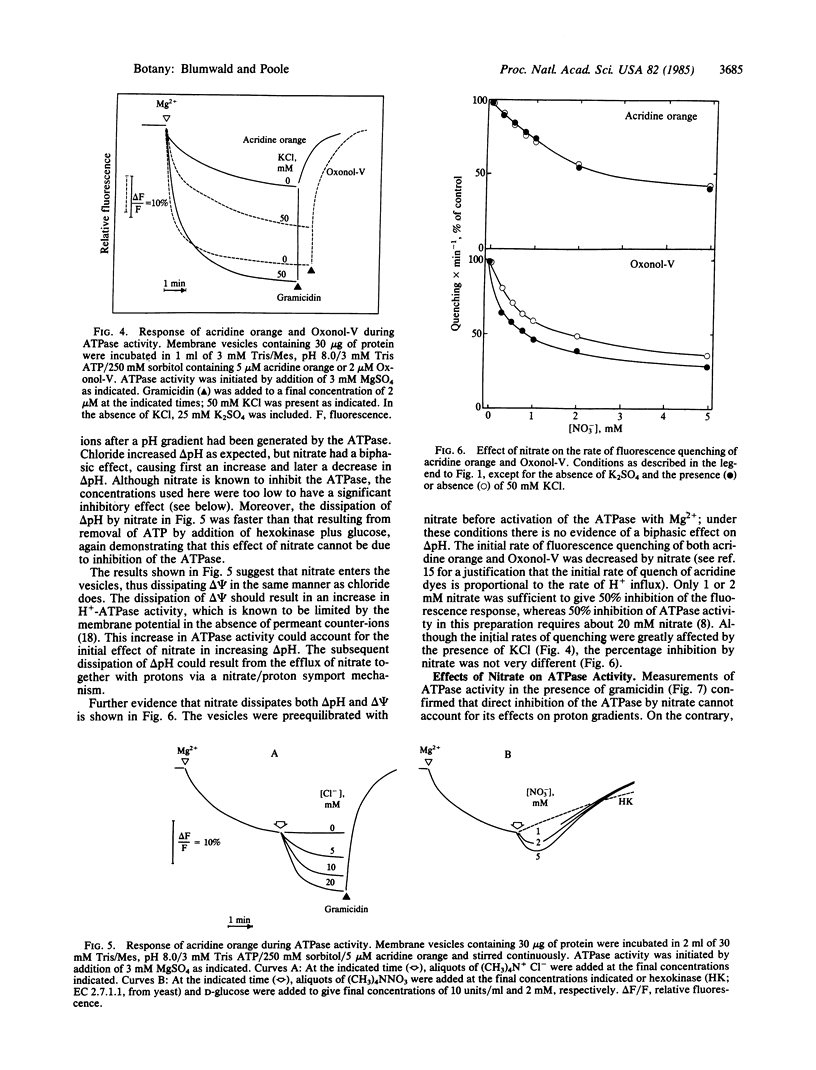
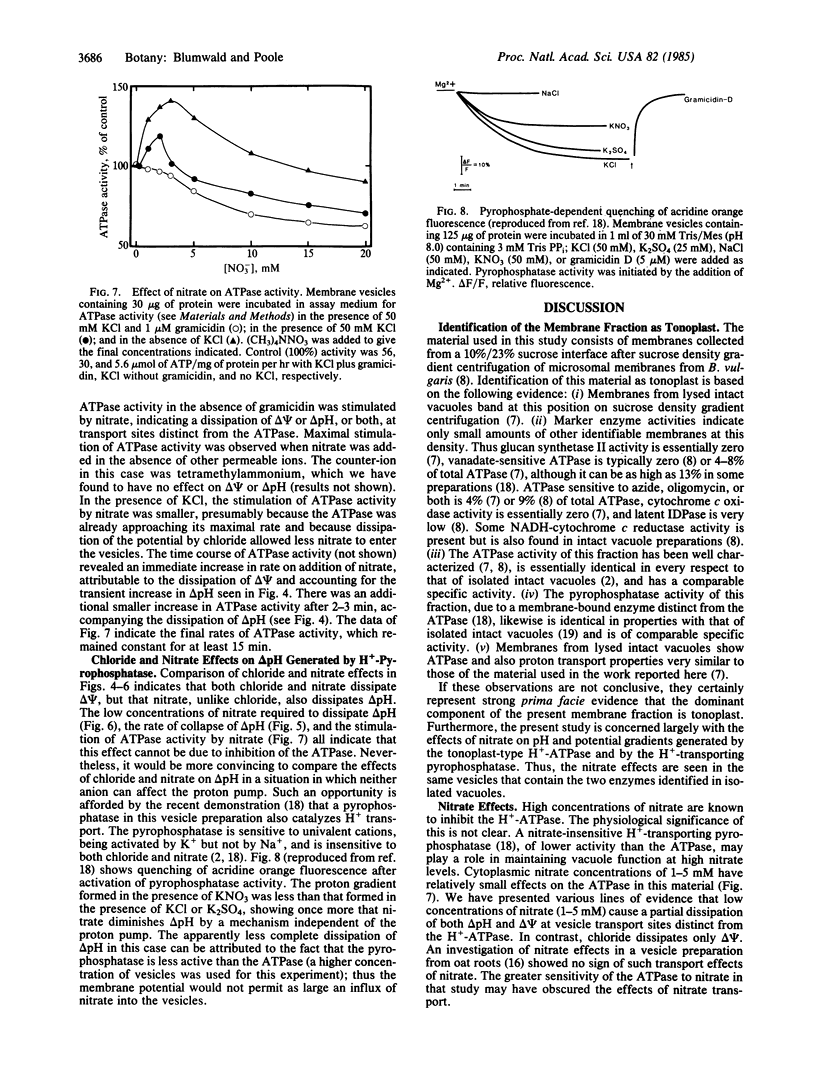
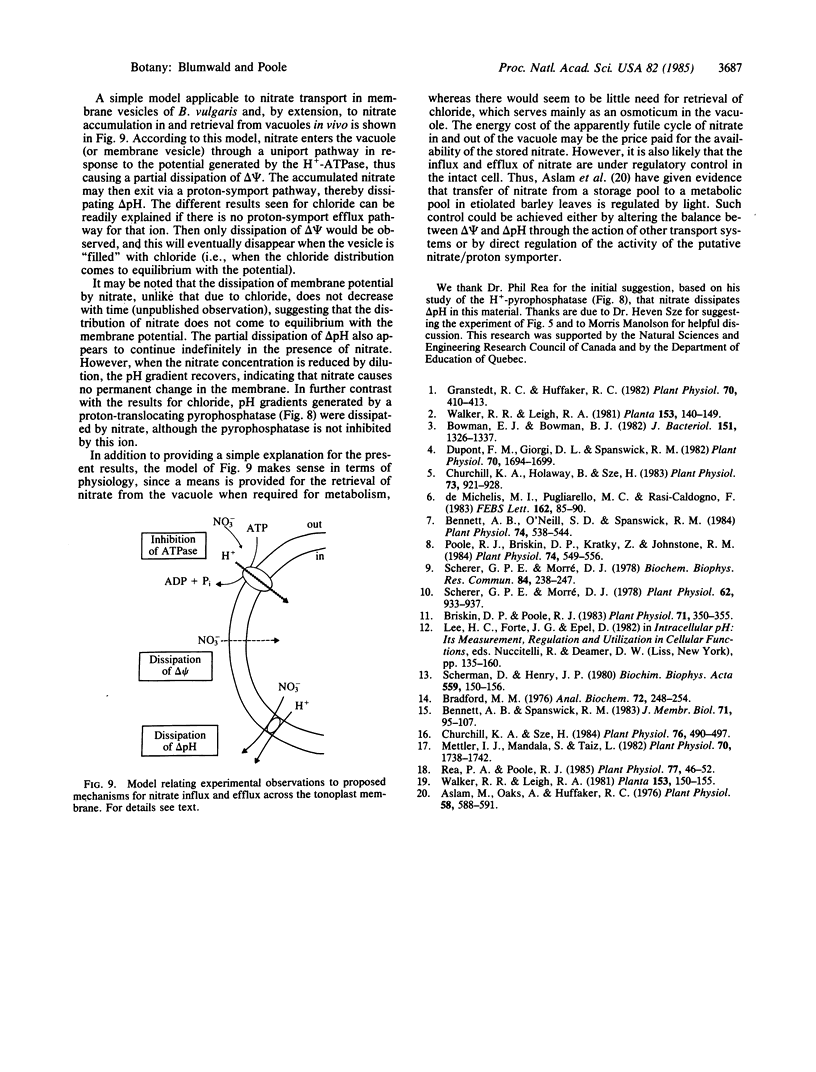
Abstract
The fluorescent probes acridine orange and oxonol-V were used as indicators of pH gradients (ΔpH) and membrane potential differences (ΔΨ), respectively, in membrane vesicles believed to be derived from the tonoplast of Beta vulgaris L. Low concentrations of nitrate (1-5 mM) caused a partial dissipation of both ΔpH and ΔΨ at vesicle transport sites distinct from the H+-ATPase. In contrast, chloride dissipated only ΔΨ. A model is proposed in which nitrate and chloride enter the plant cell vacuole in response to a potential generated by the tonoplast H+-ATPase. Nitrate but not chloride may then be retrieved for metabolic use by the operation of a nitrate/proton symport at the tonoplast.
Keywords: transport, vacuoles, fluorescence quenching, nitrate, proton symport, chloride
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aslam M., Oaks A., Huffaker R. C. Effect of light and glucose on the induction of nitrate reductase and on the distribution of nitrate in etiolated barley leaves. Plant Physiol. 1976 Oct;58(4):588–591. doi: 10.1104/pp.58.4.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A. B., O'neill S. D., Spanswick R. M. H-ATPase Activity from Storage Tissue of Beta vulgaris: I. Identification and Characterization of an Anion-Sensitive H-ATPase. Plant Physiol. 1984 Mar;74(3):538–544. doi: 10.1104/pp.74.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman E. J., Bowman B. J. Identification and properties of an ATPase in vacuolar membranes of Neurospora crassa. J Bacteriol. 1982 Sep;151(3):1326–1337. doi: 10.1128/jb.151.3.1326-1337.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Briskin D. P., Poole R. J. Characterization of a k-stimulated adenosine triphosphatase associated with the plasma membrane of red beet. Plant Physiol. 1983 Feb;71(2):350–355. doi: 10.1104/pp.71.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill K. A., Holaway B., Sze H. Separation of two types of electrogenic h-pumping ATPases from oat roots. Plant Physiol. 1983 Dec;73(4):921–928. doi: 10.1104/pp.73.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill K. A., Sze H. Anion-Sensitive, H-Pumping ATPase of Oat Roots : Direct Effects of Cl, NO(3), and a Disulfonic Stilbene. Plant Physiol. 1984 Oct;76(2):490–497. doi: 10.1104/pp.76.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont F. M., Giorgi D. L., Spanswick R. M. Characterization of a proton-translocating ATPase in microsomal vesicles from corn roots. Plant Physiol. 1982 Dec;70(6):1694–1699. doi: 10.1104/pp.70.6.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granstedt R. C., Huffaker R. C. Identification of the leaf vacuole as a major nitrate storage pool. Plant Physiol. 1982 Aug;70(2):410–413. doi: 10.1104/pp.70.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettler I. J., Mandala S., Taiz L. Characterization of in vitro proton pumping by microsomal vesicles isolated from corn coleoptiles. Plant Physiol. 1982 Dec;70(6):1738–1742. doi: 10.1104/pp.70.6.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. J., Briskin D. P., Krátký Z., Johnstone R. M. Density gradient localization of plasma membrane and tonoplast from storage tissue of growing and dormant red beet : characterization of proton-transport and ATPase in tonoplast vesicles. Plant Physiol. 1984 Mar;74(3):549–556. doi: 10.1104/pp.74.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea P. A., Poole R. J. Proton-Translocating Inorganic Pyrophosphatase in Red Beet (Beta vulgaris L.) Tonoplast Vesicles. Plant Physiol. 1985 Jan;77(1):46–52. doi: 10.1104/pp.77.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer G. F., Morré D. J. Action and Inhibition of Endogenous Phospholipases during Isolation of Plant Membranes. Plant Physiol. 1978 Dec;62(6):933–937. doi: 10.1104/pp.62.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer G. F., Morré D. J. In vitro stimulation by 2,4-dichlorophenoxyacetic acid of an ATPase and inhibition of phosphatidate phosphatase of plant membranes. Biochem Biophys Res Commun. 1978 Sep 14;84(1):238–247. doi: 10.1016/0006-291x(78)90288-7. [DOI] [PubMed] [Google Scholar]
- Scherman D., Henry J. P. Oxonol-V as a probe of chromaffin granule membrane potentials. Biochim Biophys Acta. 1980 Jun 20;599(1):150–166. doi: 10.1016/0005-2736(80)90064-4. [DOI] [PubMed] [Google Scholar]


